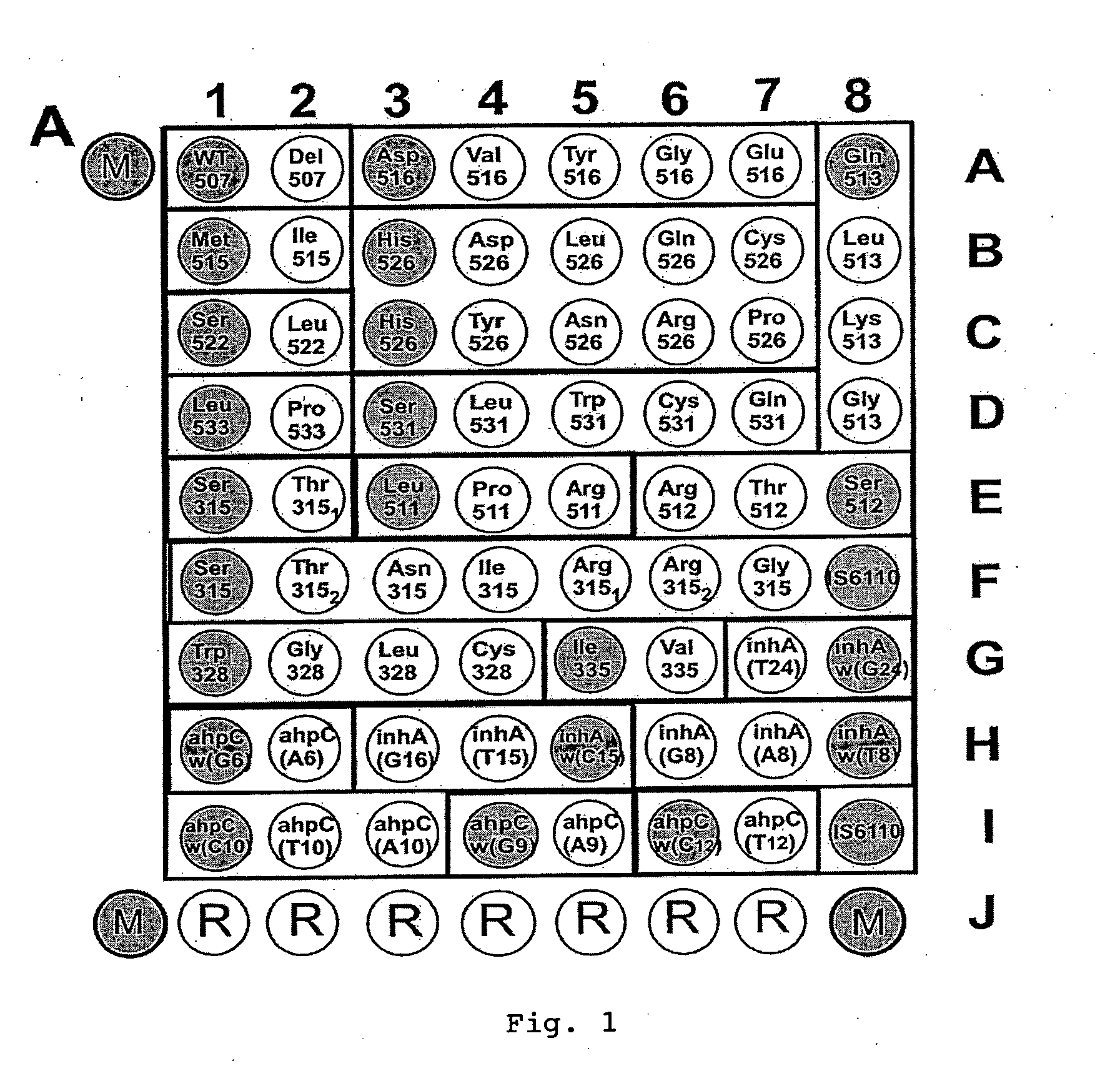
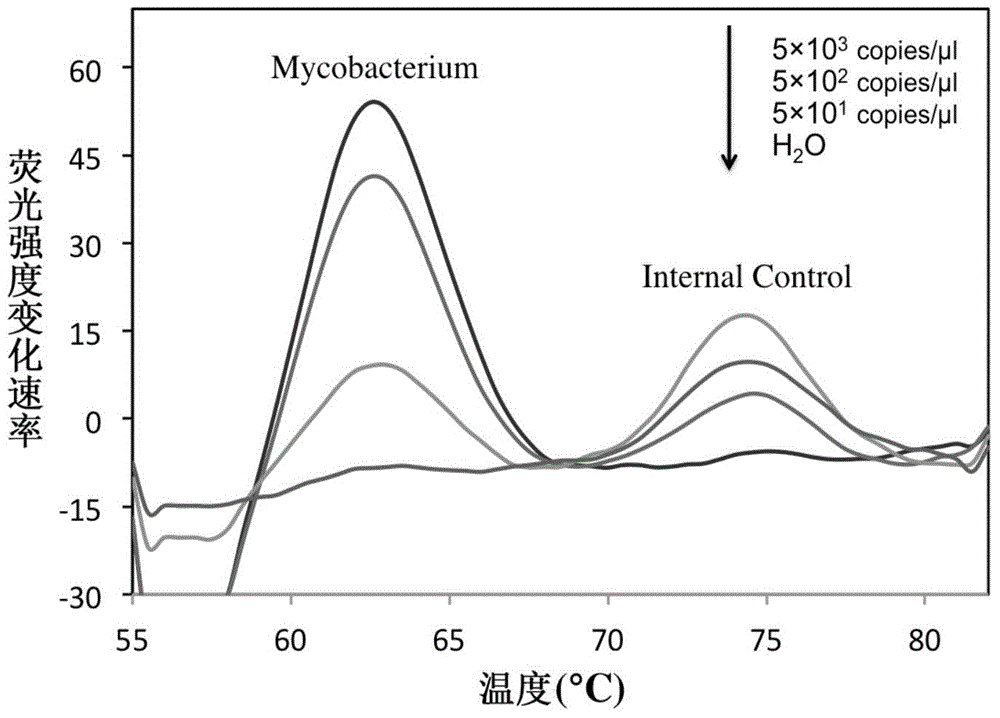
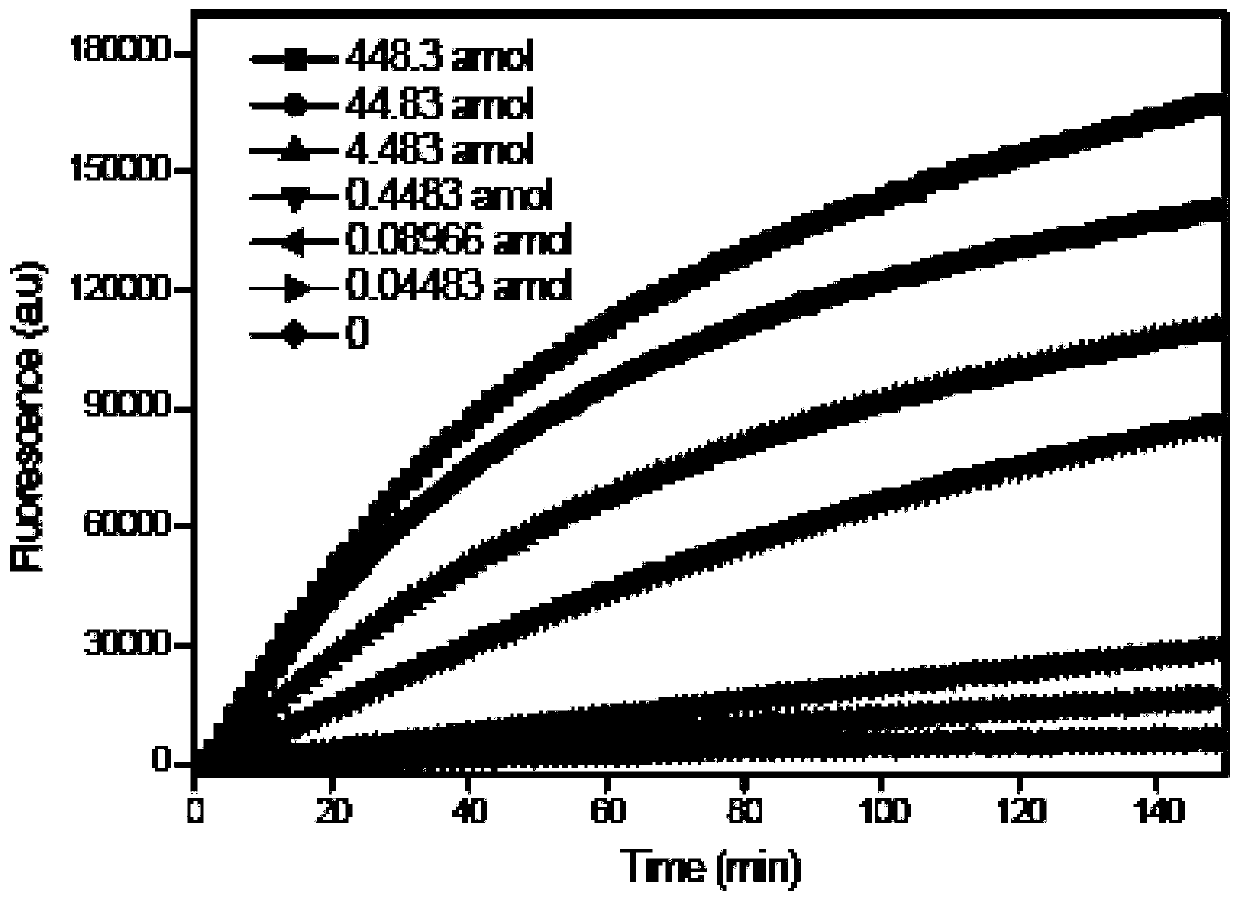
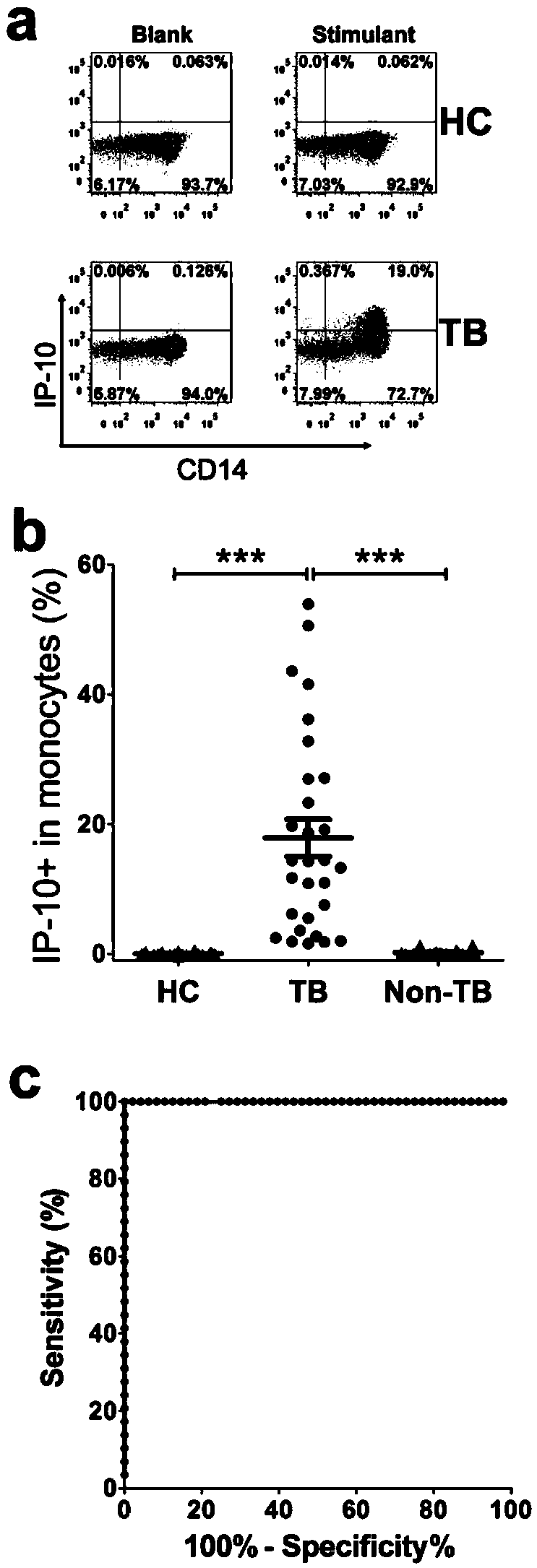
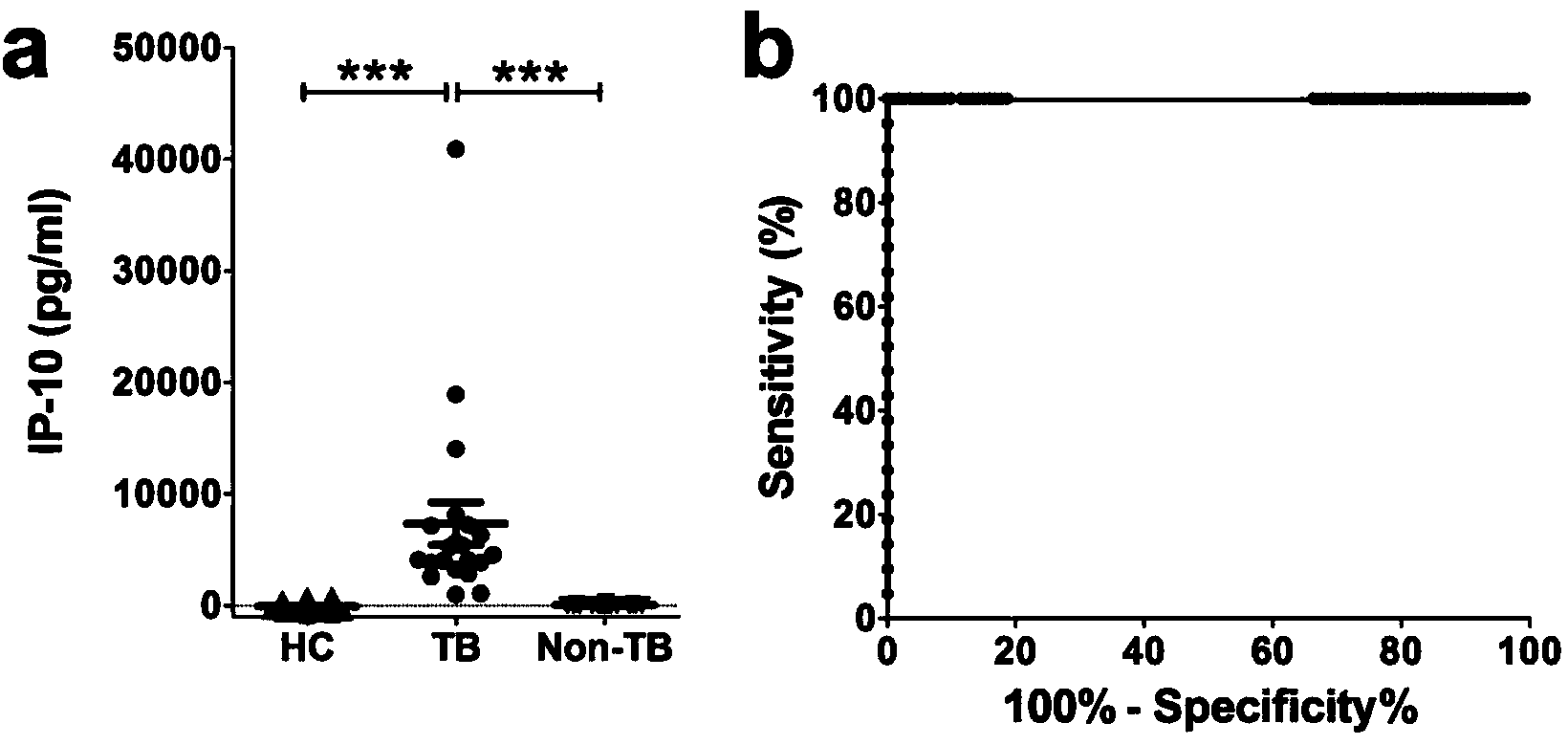
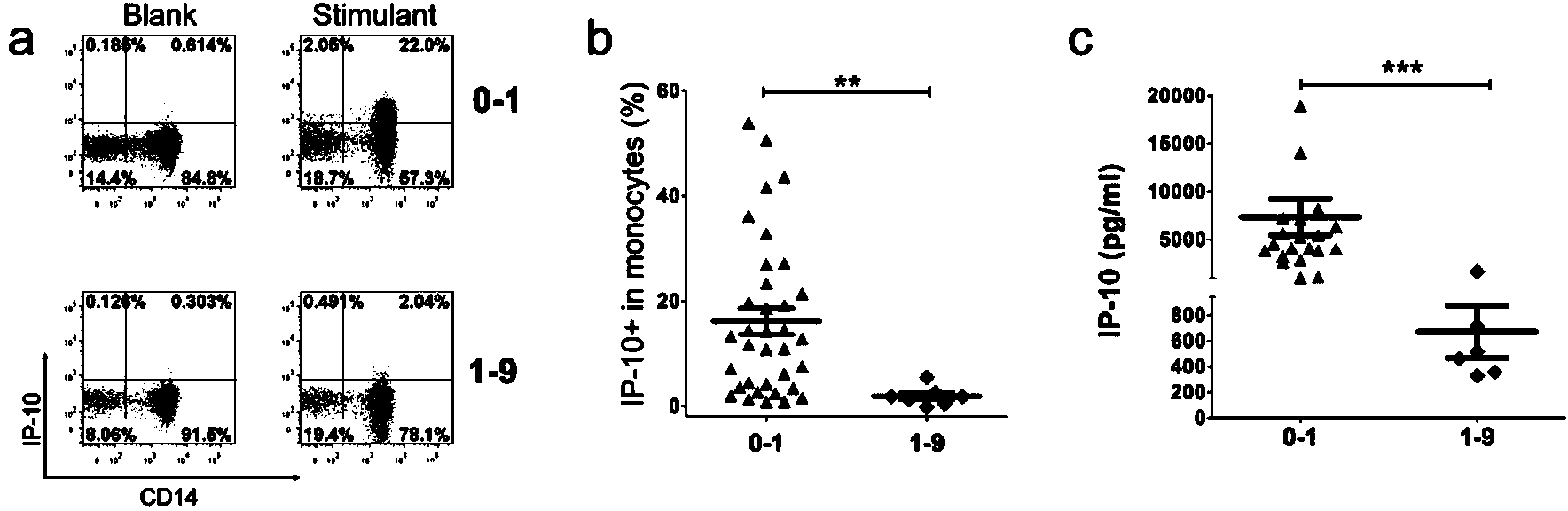
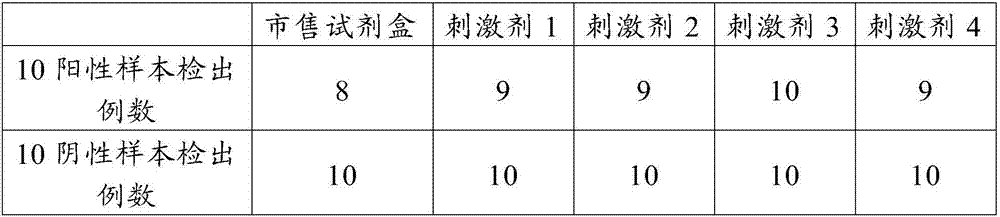
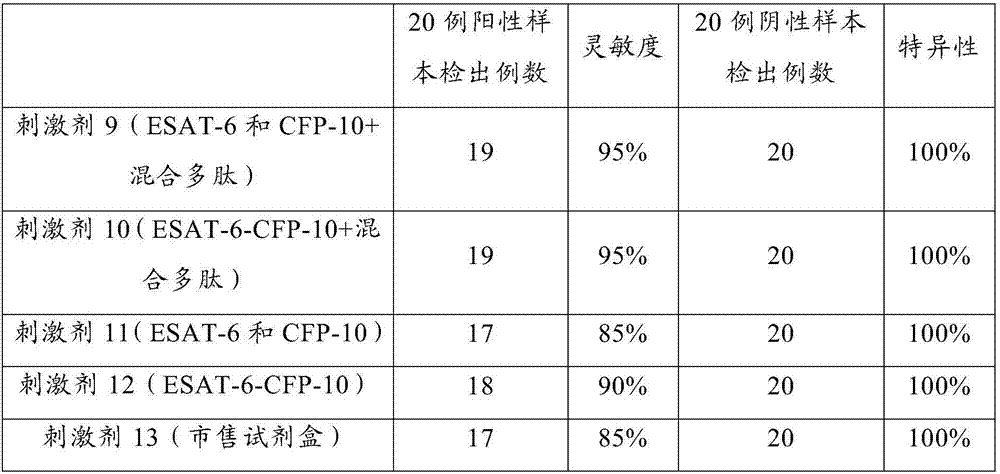
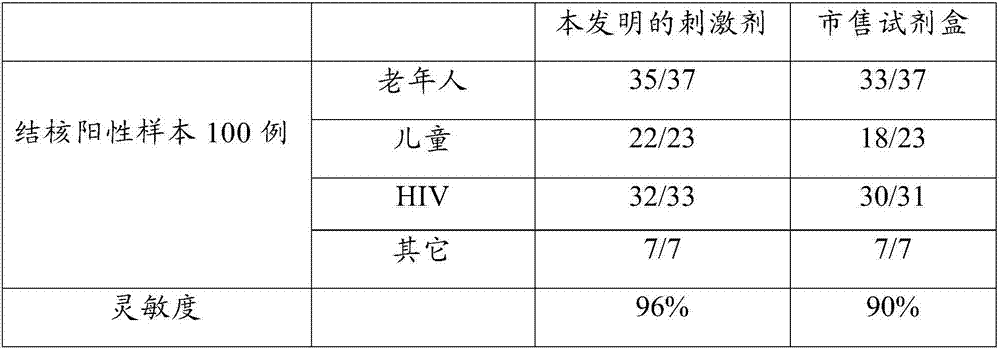
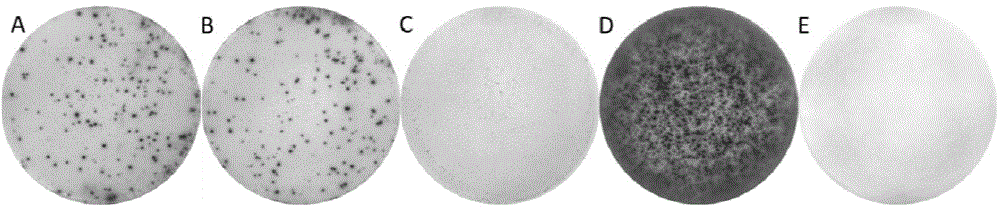
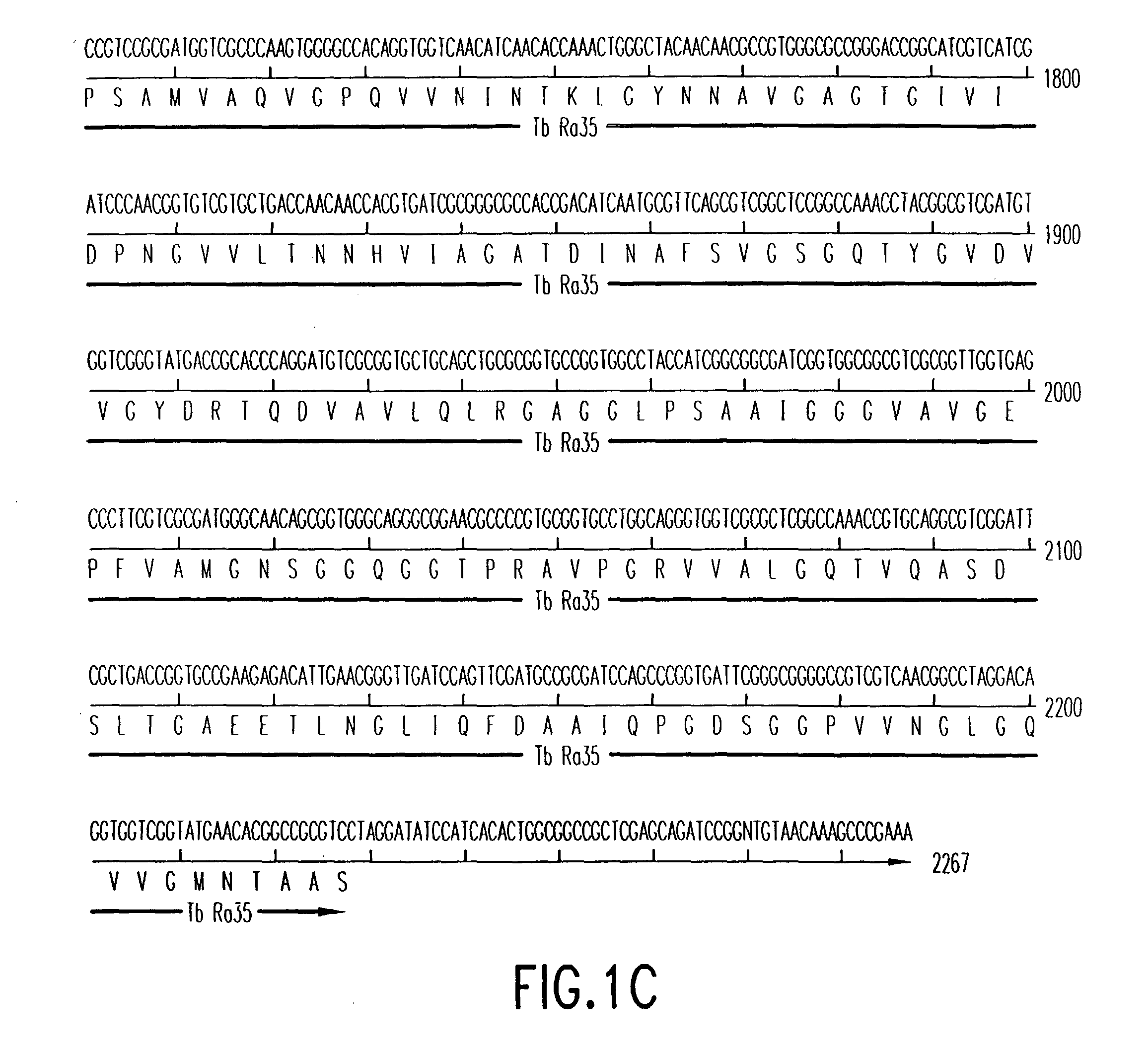
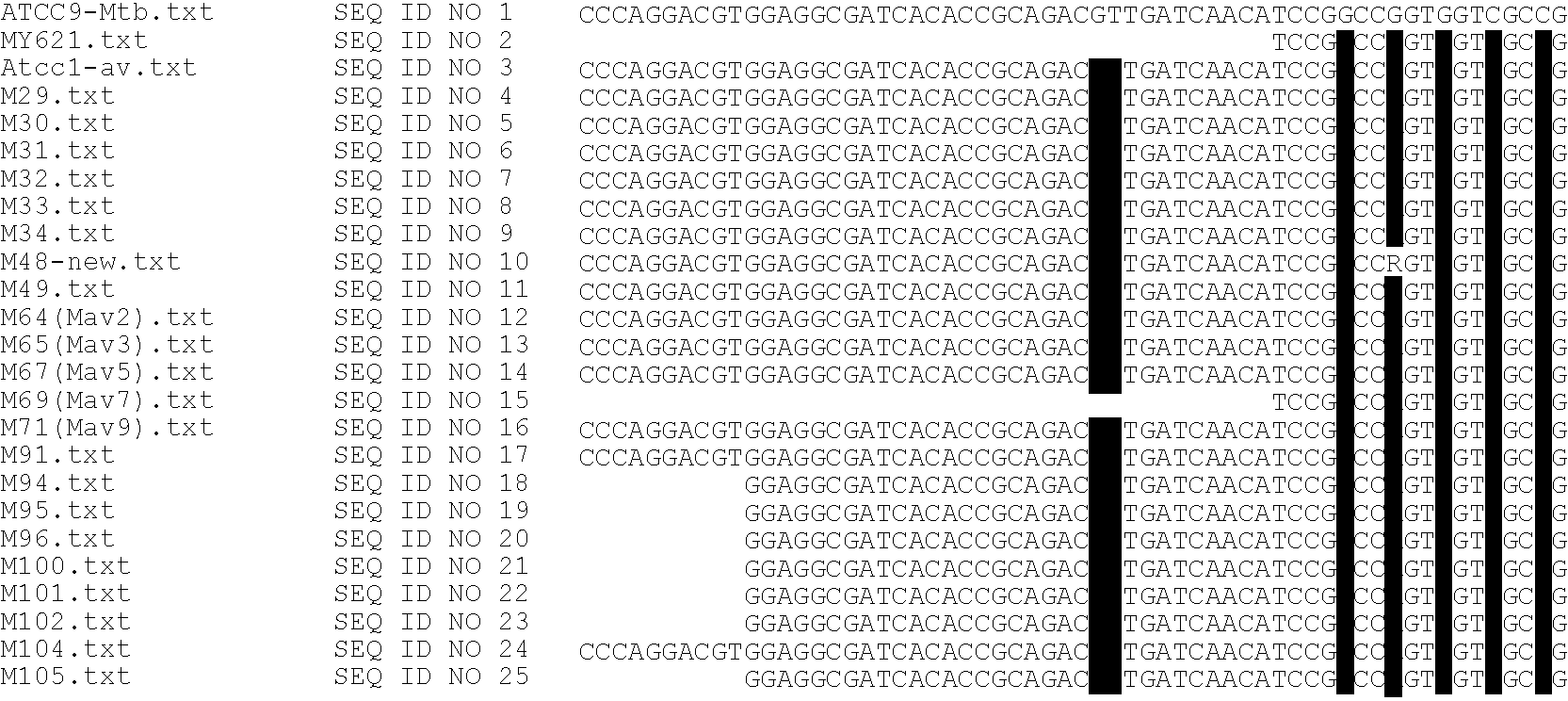Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
204 results about "Mycobacterium tuberculosis complex" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The Mycobacterium tuberculosis complex (MTC or MTBC) is a genetically related group of Mycobacterium species that can cause tuberculosis in humans or other animals.
Rapid lateral flow assay for determining exposure to Mycobacterium tuberculosis and other mycobacteria
InactiveUS6841159B2Auxiliary diagnosisAuxiliary judgmentBacterial antigen ingredientsMicrobiological testing/measurementMycobacterial antigenImmunization status
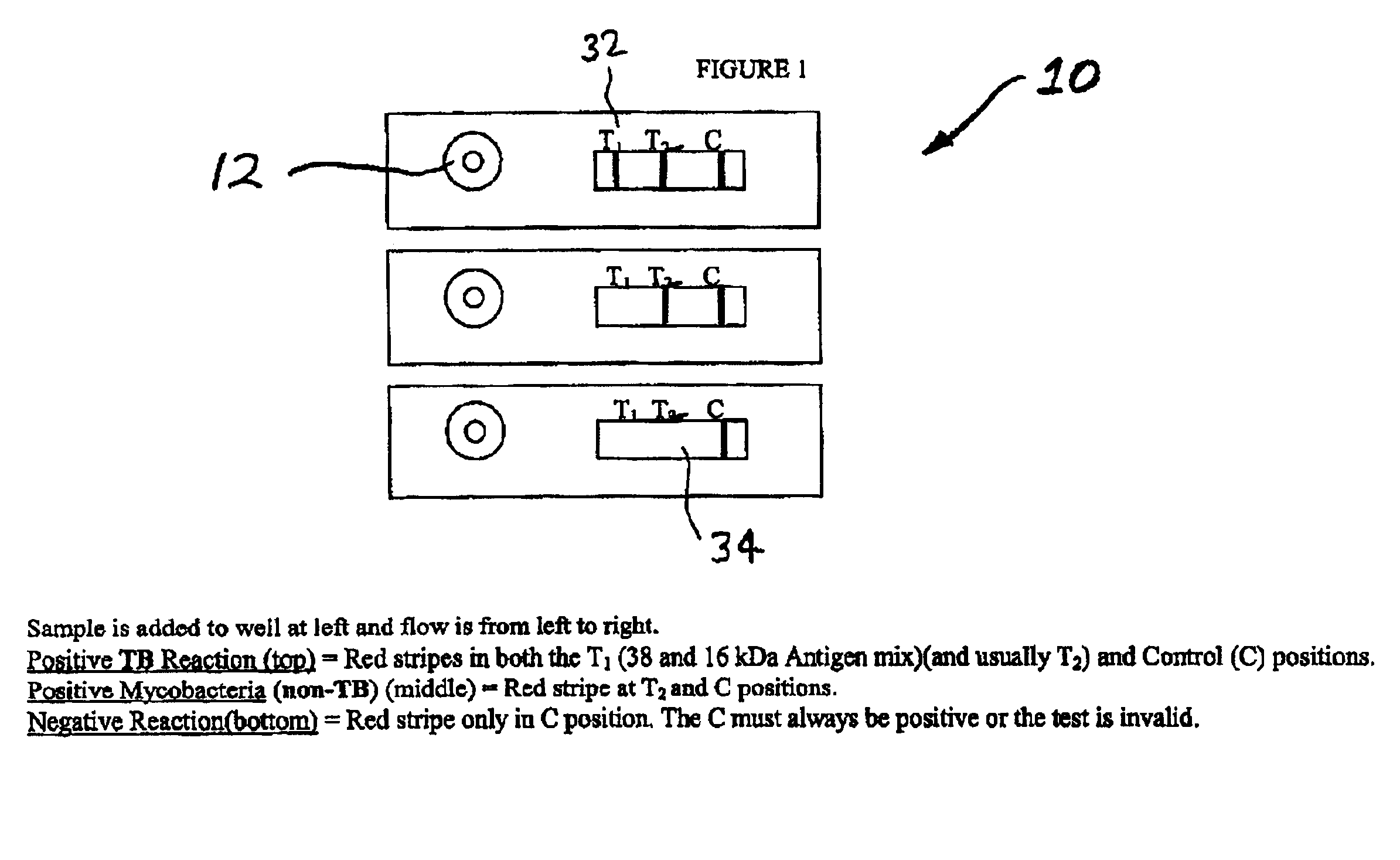
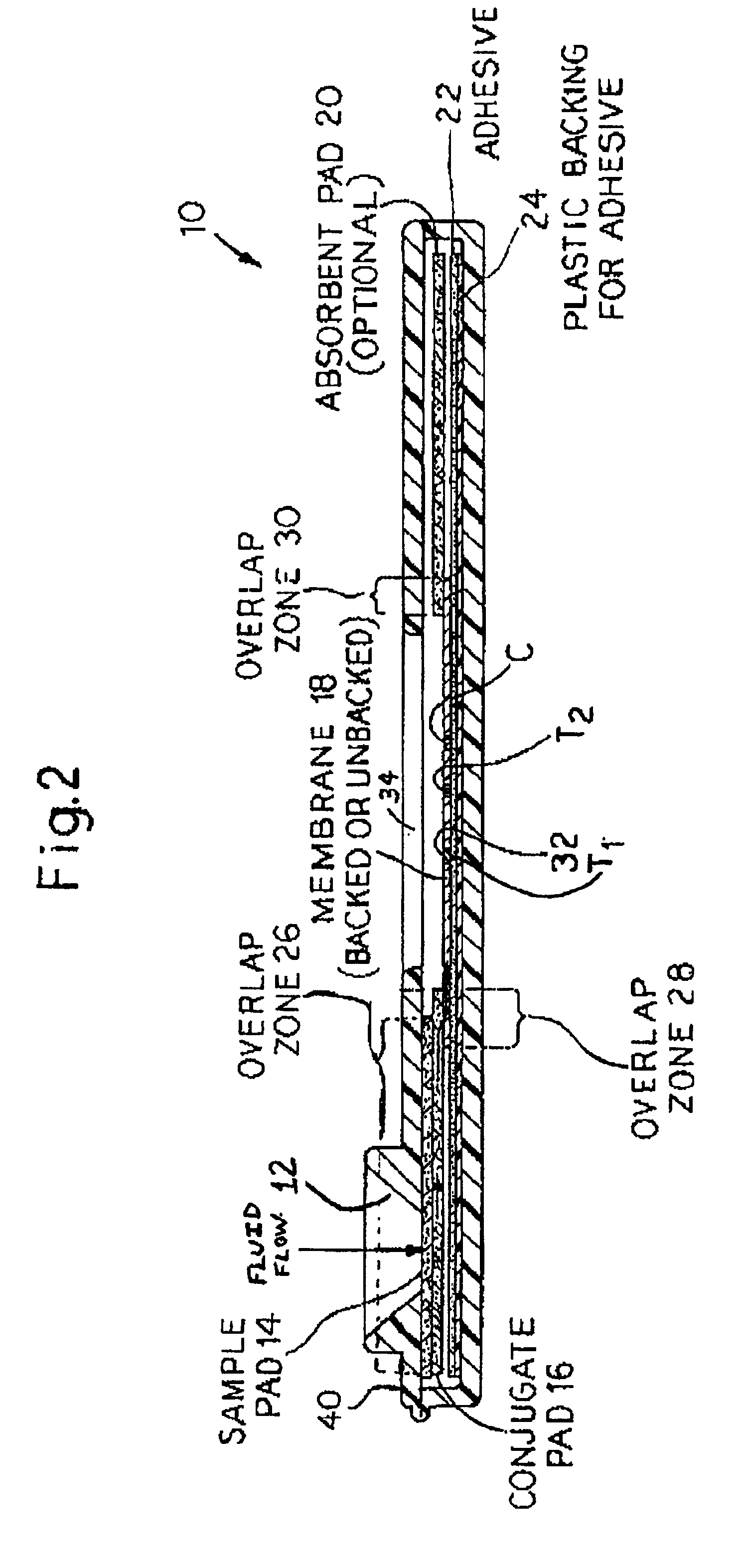
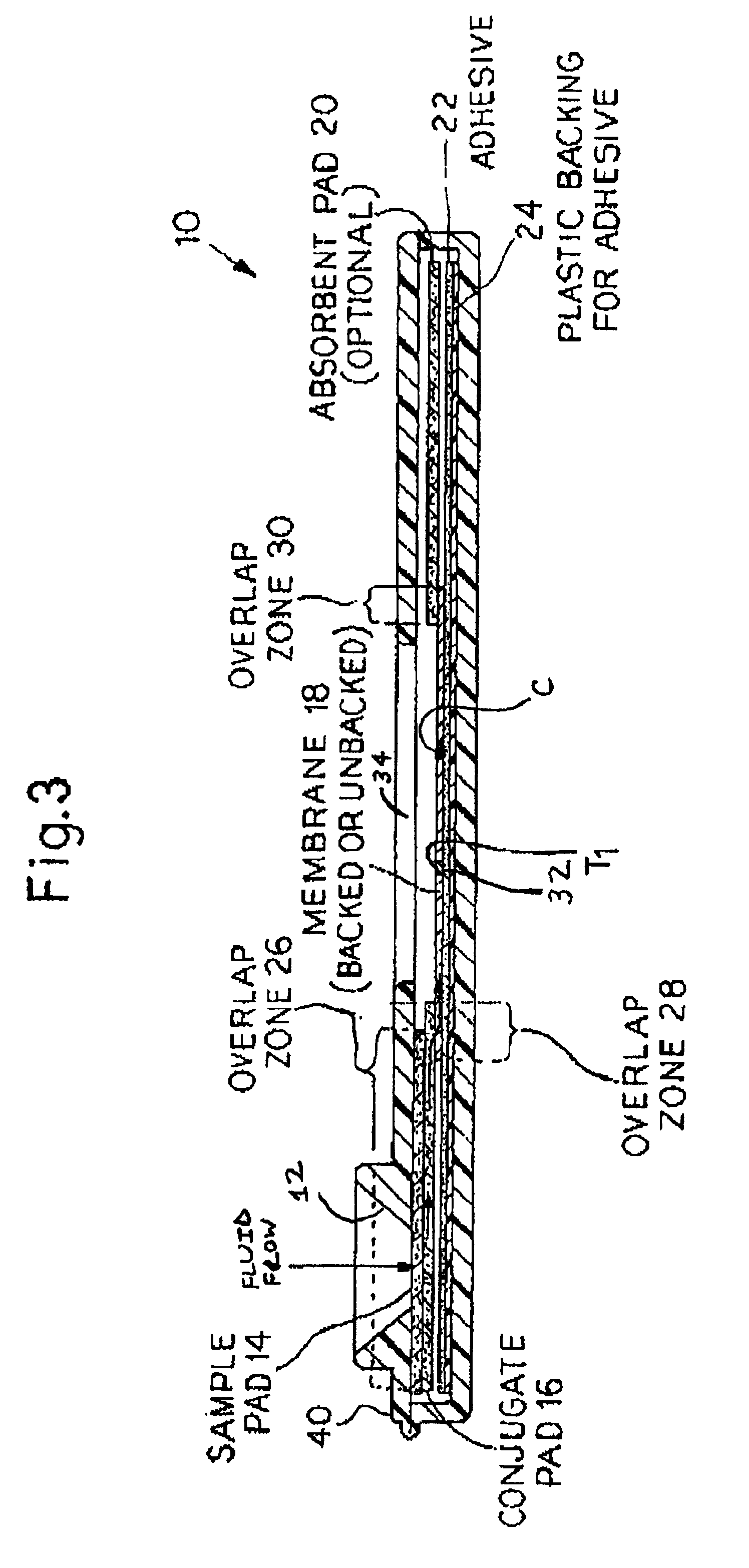
An assay method and kit is disclosed for detecting the presence of at least one predesignated, target antibody to a mycobacterium in a sample selected from one or more patient bodily fluids. The method comprises the following steps: (a) contacting the sample of one or more patient bodily fluids with at least one mycobacterium antigen on a lateral-flow assay membrane to bind to the target antibody in the sample; (b) previously, simultaneously or subsequently to step (a), binding the at least one mycobacterium antigen with a conjugated label producing a detectable signal; and (c) detecting the signal whereby the presence of the target antibody is determined in the sample by the intensity or presence of the signal. The method can further comprise the step of evaluating immunization status of the patient from whom the sample came by comparing the signal or lack thereof with immunizations previously received by the patient and in comparison to a known standard control. In a preferred embodiment, the mycobacterium antigen specifically binds to Mycobacterium tuberculosis specific antibodies. Preferably, the immunoassay of the present invention comprises a lateral-flow assay comprising a membrane, a conjugated label pad, and at least one mycobacterium antigen bound to the membrane. In a preferred embodiment, the at least one mycobacterium antigen is selected from the group consisting of 38 kDa and 16 kDa antigens.
Owner:THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SECRETARY OF THE NAVY +1
Fusion proteins of mycobacterium tuberculosis
InactiveUS7083796B2Good antigenicityHigh sensitivityAntibacterial agentsPeptide/protein ingredientsSerum igeBiology
The present invention relates to compositions and fusion proteins containing at least two Mycobacterium sp. antigens, and nucleic acids encoding such compositions and fusion proteins. The compositions of the invention increase serological sensitivity of sera from individuals infected with tuberculosis, and methods for their use in the diagnosis, treatment, and prevention of tuberculosis infection.
Owner:CORIXA CORP
Fusion proteins of Mycobacterium tuberculosis
InactiveUS7026465B2More immunogenicImprove the level ofAntibacterial agentsBacteriaSerum igeMycobacterium
The present invention relates to compositions and fusion proteins containing at least two Mycobacterium sp. antigens, and nucleic acids encoding such compositions and fusion proteins. The compositions of the invention increase serological sensitivity of sera from individuals infected with tuberculosis, and methods for their use in the diagnosis, treatment, and prevention of tuberculosis infection.
Owner:CORIXA CORP
Kit and method for detecting mycobacterium tuberculosis infection and application
ActiveCN102004155ALow costQuality assuranceBiological testingMycobacterium InfectionsLatent tuberculosis
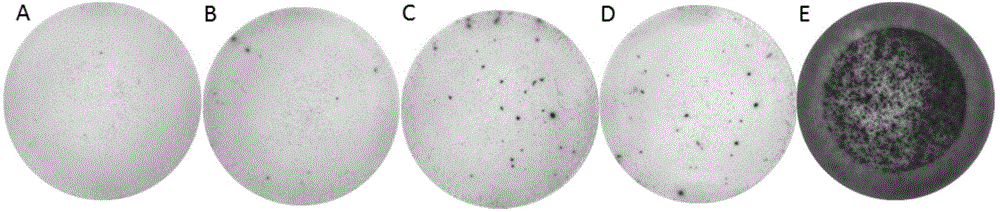
The invention belongs to the field of biomedicine examination, and particularly relates to a kit and a method for detecting mycobacterium tuberculosis infection and application. The invention discloses a novel mycobacterium tuberculosis detection reagent by screening specific T cell epitope of mycobacterium tuberculosis, wherein the reagent contains polypeptide or analog thereof represented by SEQ ID No.1-10. The method detects cell factors released from T cells by using single or more SEQ ID No.1-10 polypeptides to contact the T cells of mycobacterium tuberculosis infected individuals. The method can effectively detect active tuberculosis or latent tuberculosis infection, and is free from disturbance of Bacilli Calmette Guerin (BCG) inoculation vaccines. The invention also discloses a diagnostic kit and other application based on the polypeptide and the method. Compared with the gamma interferon release experiments in the prior art, the method can obviously improve the detection rate without reducing the specificity and has high clinical application value.
Owner:AFFILIATED HUSN HOSPITAL OF FUDAN UNIV
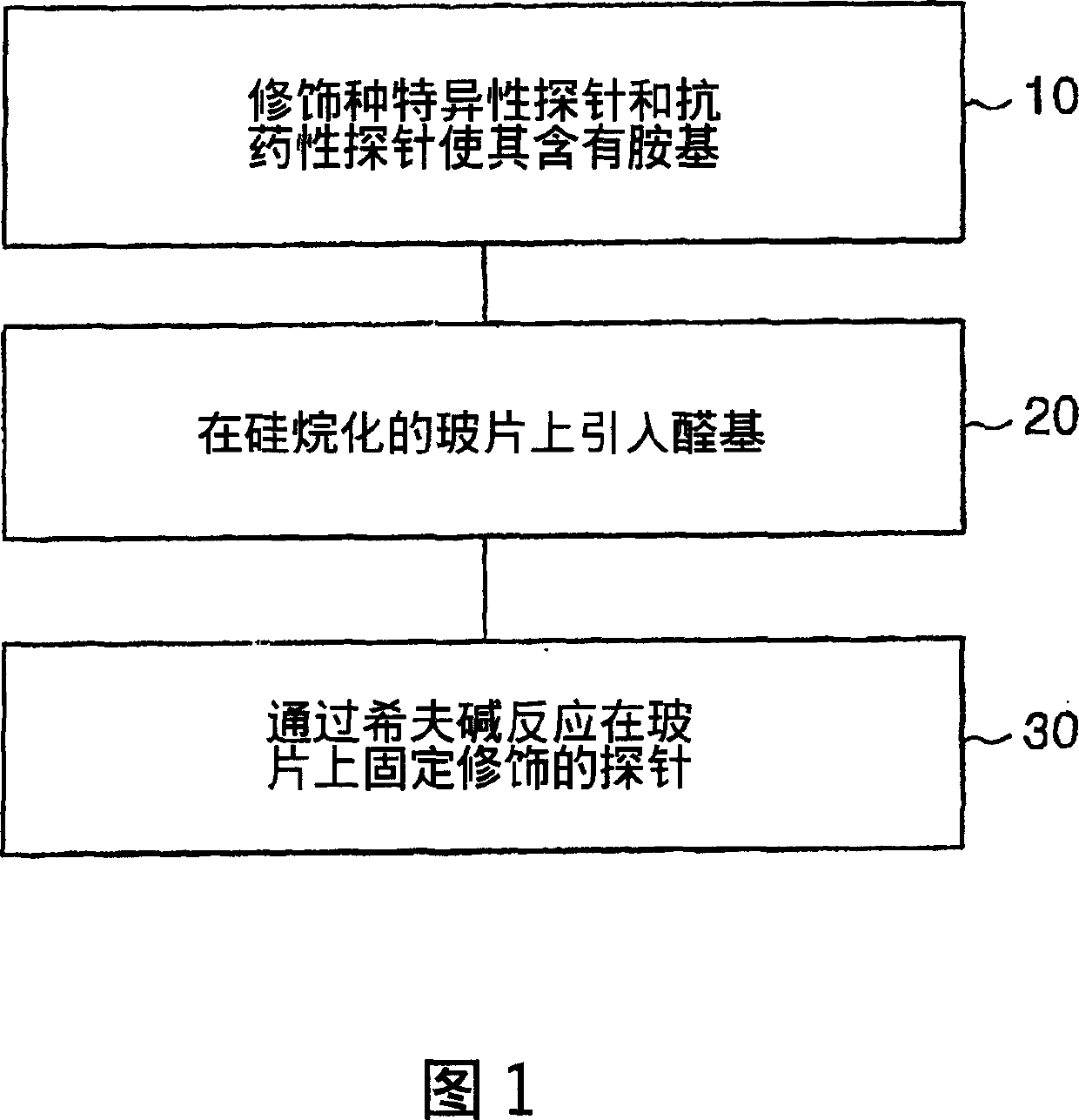
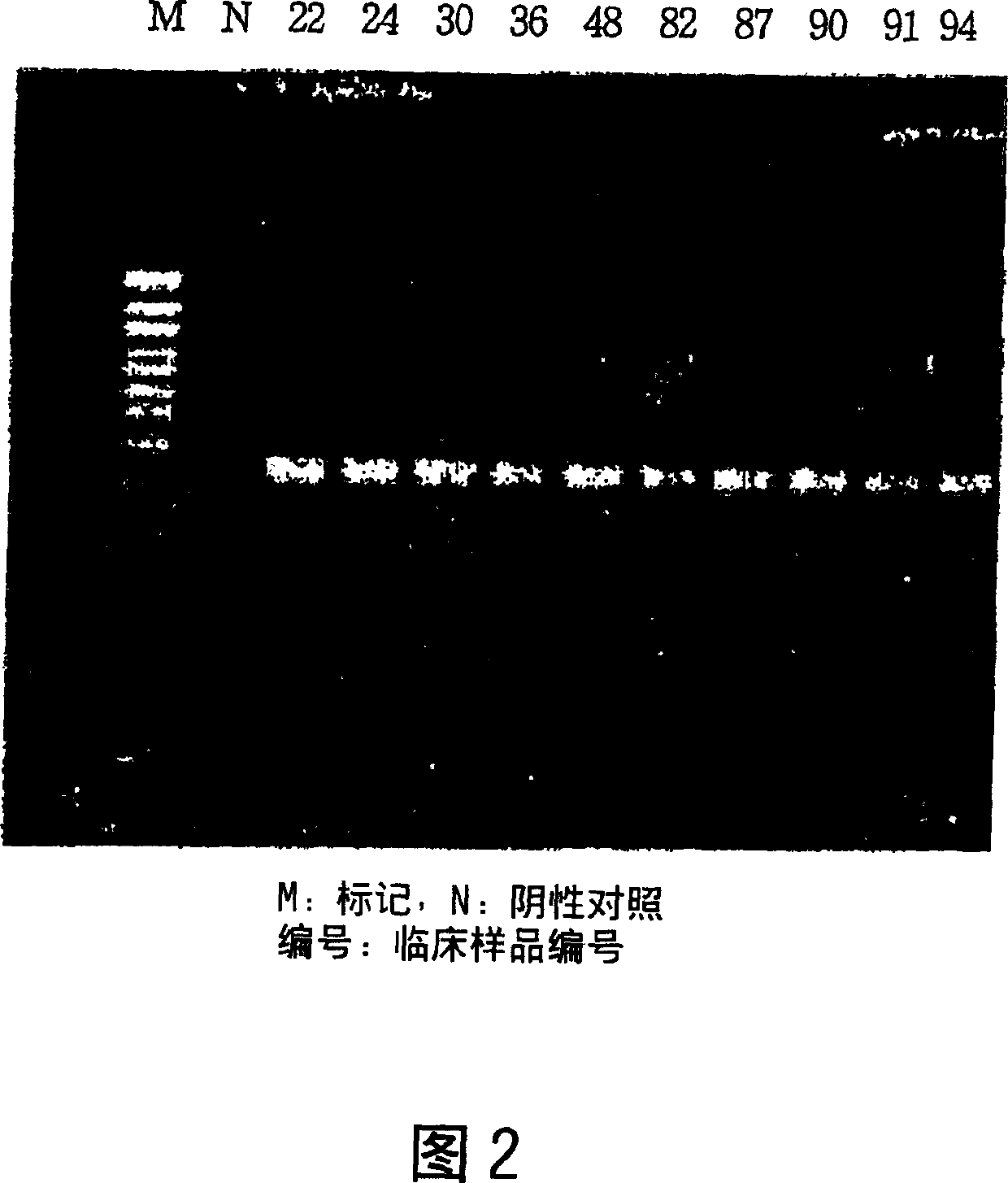
Method for simultaneous detection of Mycobacterium tuberculosis complex and identification of mutations in mycobacterial DNA resulting in the resistance of microorganisms to rifampicin and isoniazid on biological microarrays, set of primers, biochip, and set of oligonucleotide probes used in the method
InactiveUS20100261163A1Low costShort timeBioreactor/fermenter combinationsBiological substance pretreatmentsIsoniazidNucleotide
The present invention relates to molecular biology, microbiology, and medicine and provides the method for detection of Mycobacterium tuberculosis complex with simultaneous evaluation of sensitivity of the strains to rifampicin and isoniazid in clinical sample on differentiating biochip. The method is based on two-stage multiplex PCR to obtain fluorescent DNA fragments followed by hybridization of these fragments on microarray containing the set of specific discriminating oligonucleotides. The determination of the resistance of Mycobacterium tuberculosis to rifampicin and isoniazid is carried out by evaluation of point nucleotide substitutions in DNA of microorganism. The present invention allows conduct analysis directly in clinical sample, to evaluate a number of mutations simultaneously, to decrease the cost price of analysis, and to reduce the time of its conducting. The present invention also relates to set of primers, biochip, and set of oligonucleotide probes used in realization of the method.
Owner:UCHREZHDENIE ROSSIISKOI AKADI NAUK INST MOLEKULYARNOI BLOLOGII IM V A ENGELGARDTA RAN IMB RAN
Rapid identification method and kit for MTBC (mycobacterium tuberculosis complex)
InactiveCN104561245AShorten diagnostic timeSimple and fast operationMicrobiological testing/measurementFluorescence/phosphorescenceFluorescenceRapid identification
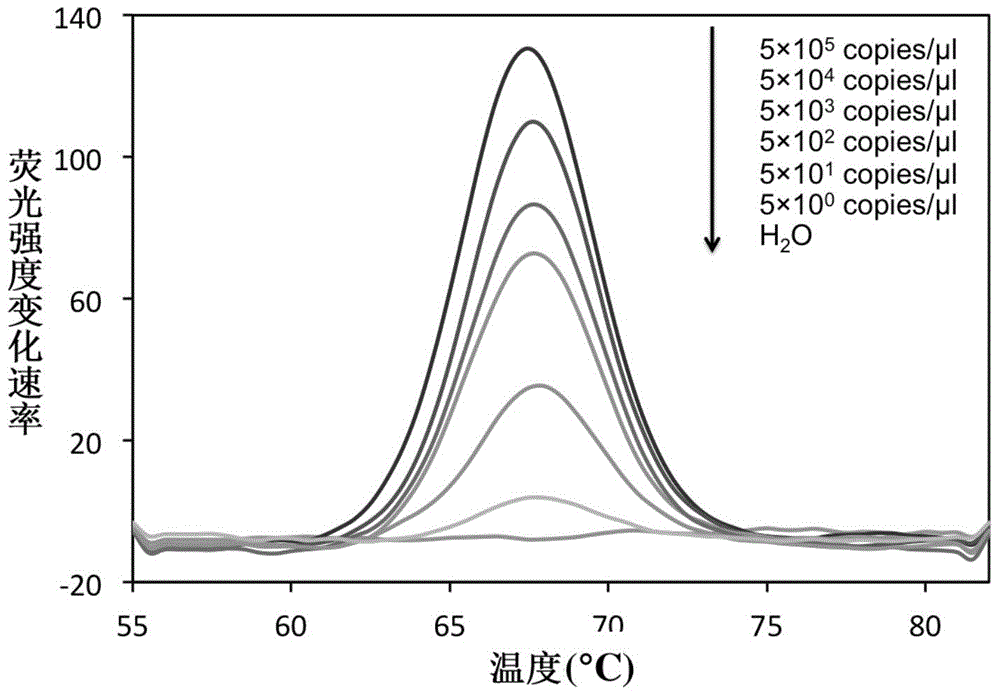
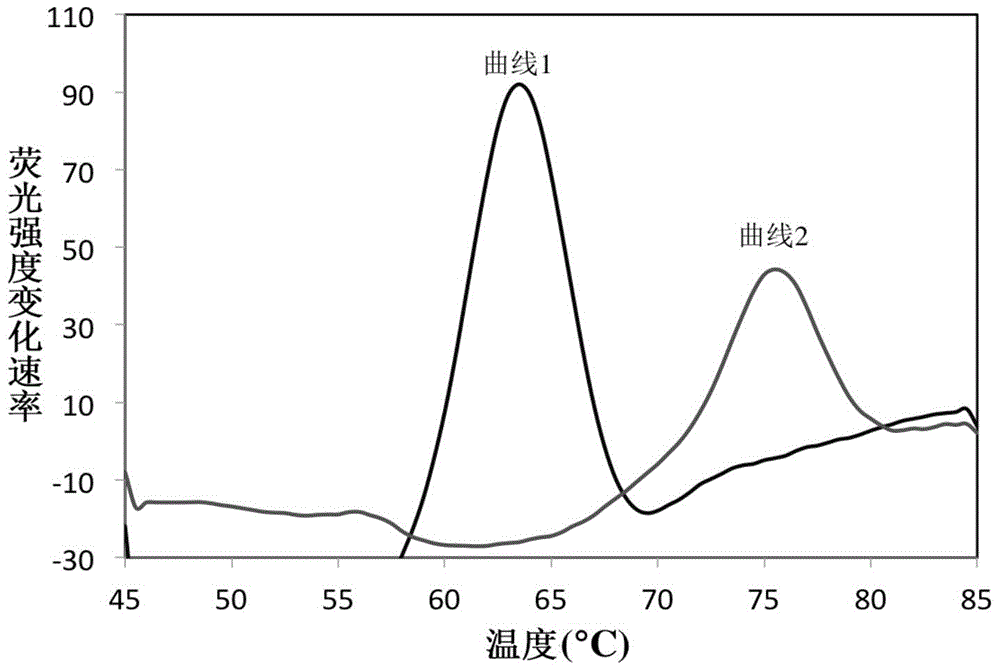
The invention belongs to the field of a detection reagent, and relates to a detection and identification kit for MTBC (mycobacterium tuberculosis complex) and an identification method of the MTBC. According to the method, fluorescence probes and amplification primers for detecting the MTBC are designed through sequence alignment, mycobacterium and the MTBC specific SNP (single nucleotide polymorphism) loci on an rrs gene are detected based on a real-time fluorescent quantitative PCR (polymerase chain reaction) platform and with an asymmetric PCR amplification technology and a probe melting curve analysis technology, so that mycobacteria are identified, and the MTBC and NTM (nontuberculosis mycobacteria) are further identified. The method has the characteristics of convenience in operation, short detection time and high specificity and sensitivity.
Owner:FUDAN UNIV
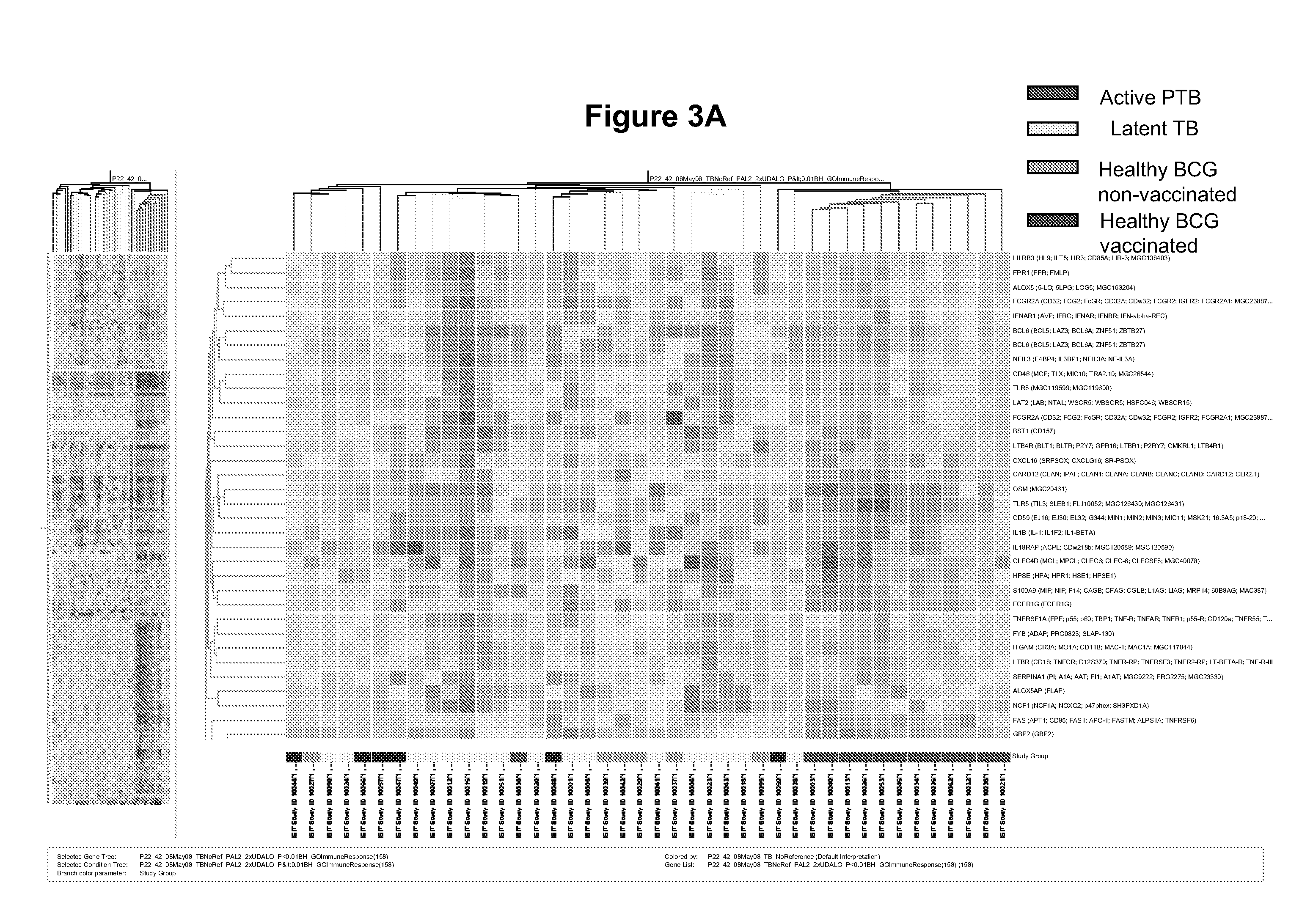
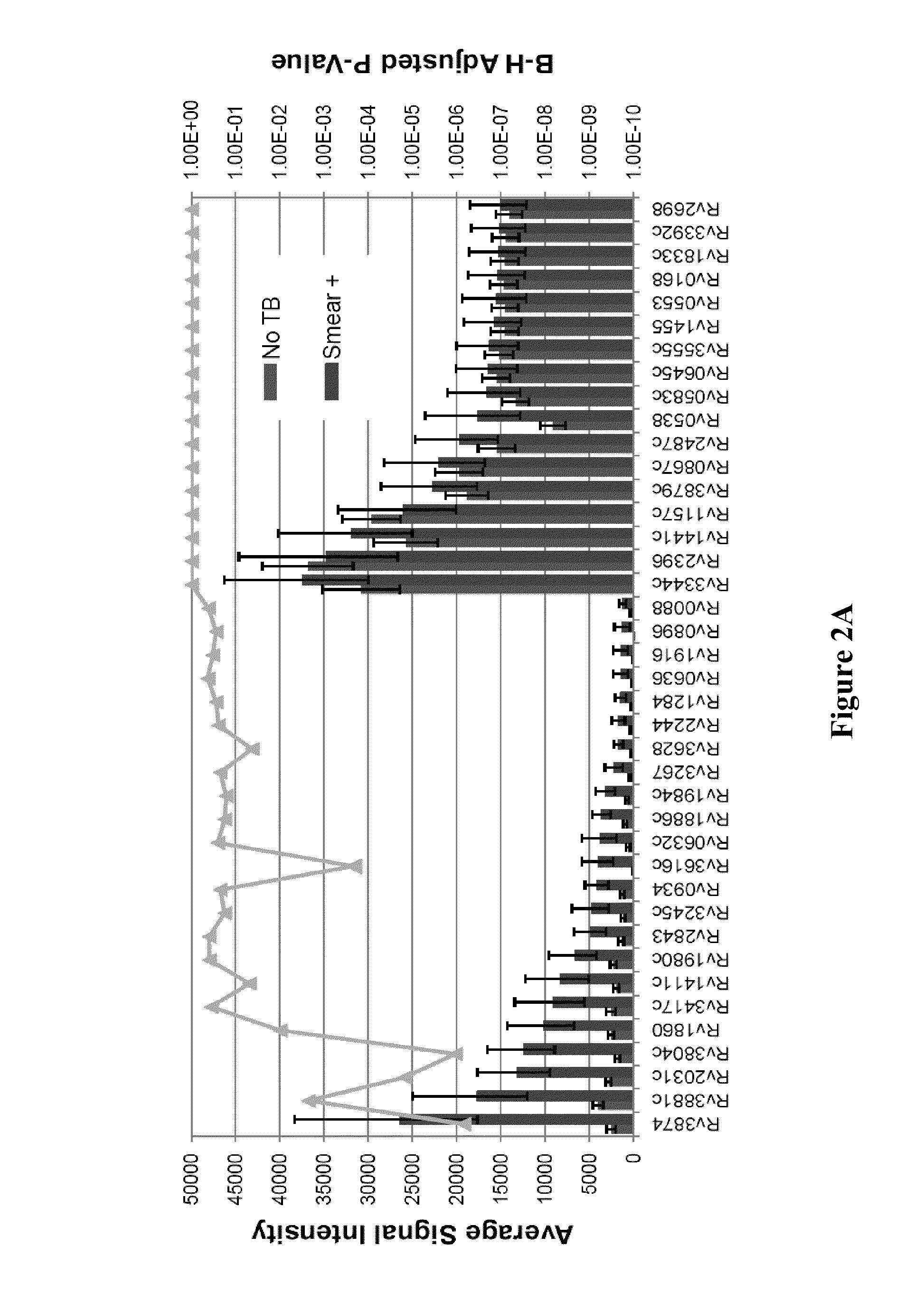
Blood transcriptional signature of mycobacterium tuberculosis infection
InactiveUS20110196614A1Microbiological testing/measurementDisease diagnosisInfected patientMycobacterium Infections
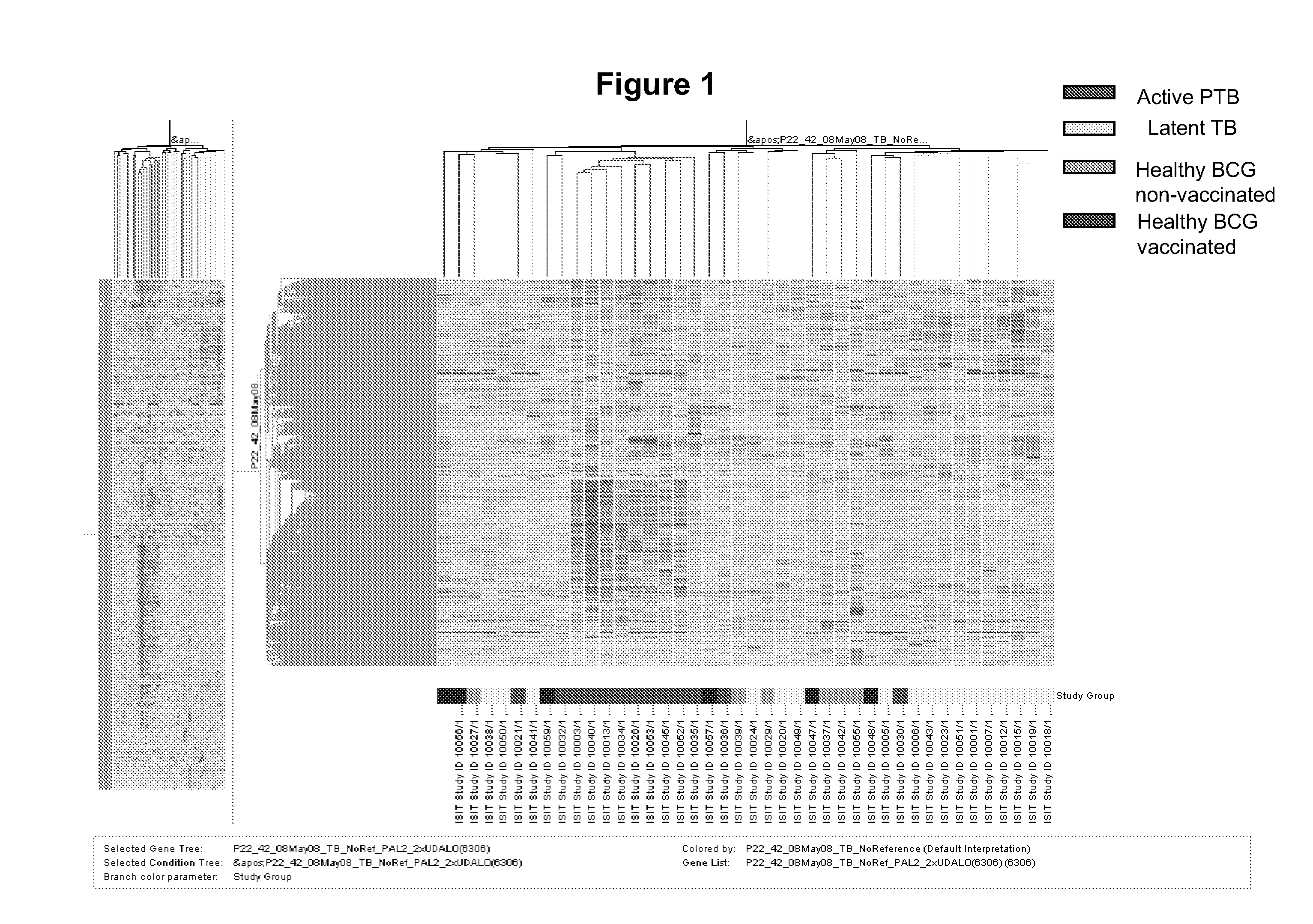
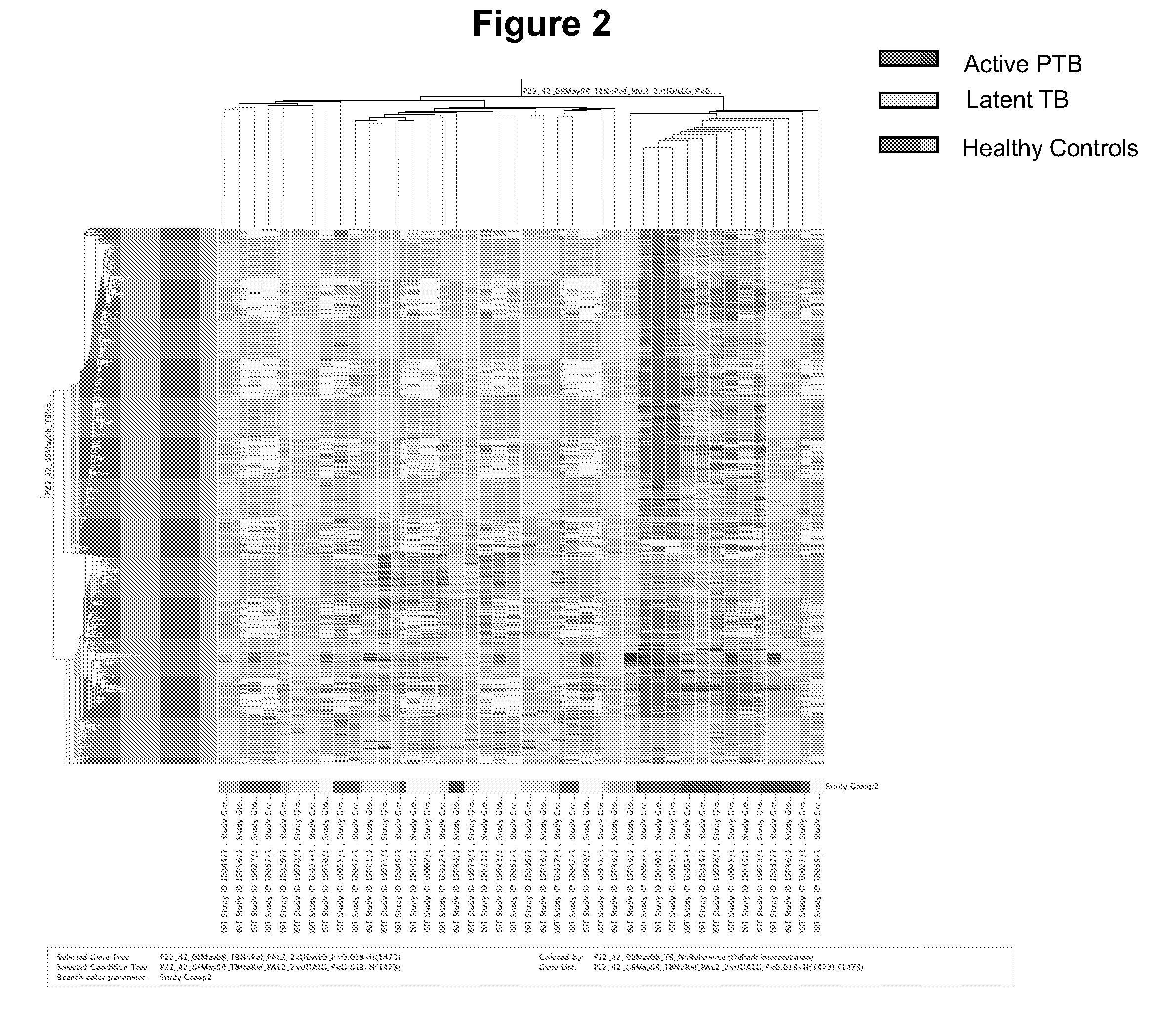
The present invention includes methods, systems and kits for distinguishing between active and latent mycobacterium tuberculosis infection in a patient suspected of being infected with mycobacterium tuberculosis, and distinguishing such patients from uninfected individuals, the method including the steps of obtaining a gene expression dataset from a whole blood obtained sample from the patient and determining the differential expression of one or more transcriptional gene expression modules that distinguish between infected and non-infected patients, wherein the dataset demonstrates an aggregate change in the levels of polynucleotides in the one or more transcriptional gene expression modules as compared to matched non-infected patients, thereby distinguishing between active and latent mycobacterium tuberculosis infection.
Owner:BAYLOR RES INST +2
Fusion proteins of mycobacterium tuberculosis antigens and their uses
InactiveUS20020009459A1Improving immunogenicityPeptide/protein ingredientsAntibody mimetics/scaffoldsAntigenMycobacterial antigen
The present invention relates to fusion proteins containing at least two Mycobacterium tuberculosis antigens. In particular, it relates to bi-fusion proteins which contain two individual M. tuberculosis antigens, tri-fusion proteins which contain three M. tuberculosis antigens, tetra-fusion proteins which contain four M. tuberculosis antigens, and penta-fusion proteins which contain five M. tuberculosis antigens, and methods for their use in the diagnosis, treatment and prevention of tuberculosis infection.
Owner:CORIXA CORP
Compositions and methods for immunodominant antigens of Mycobacterium tuberculosis
Contemplated compositions, devices, and methods are drawn to various antigens from the pathogen M. tuberculosis and their use in vaccines, therapeutic agents, and various diagnostic tests. In particularly preferred aspects, the antigens are immunodominant and have quantified and known relative reactivities with respect to sera of a population infected with the pathogen, and / or have a known association with a disease parameter.
Owner:RUTGERS THE STATE UNIV +1
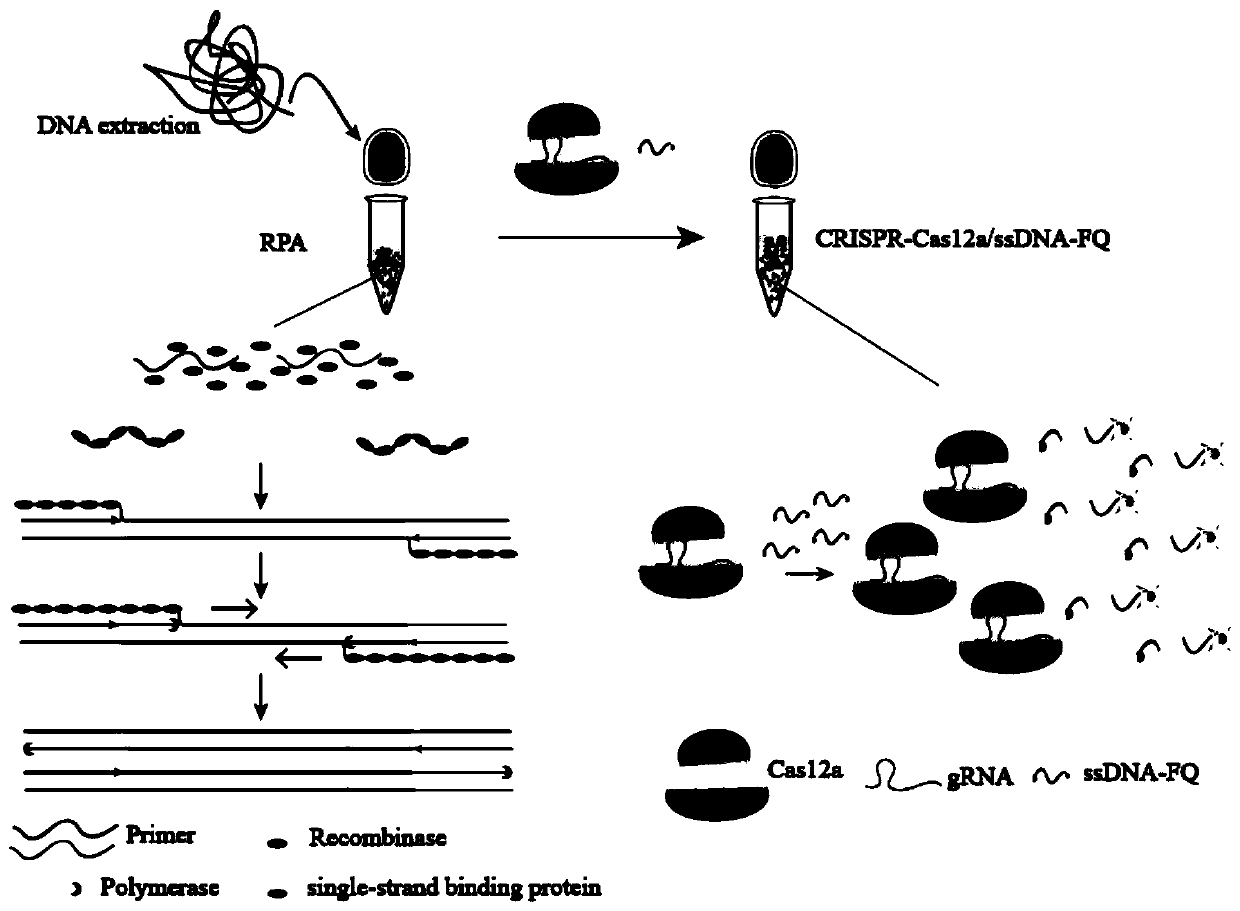
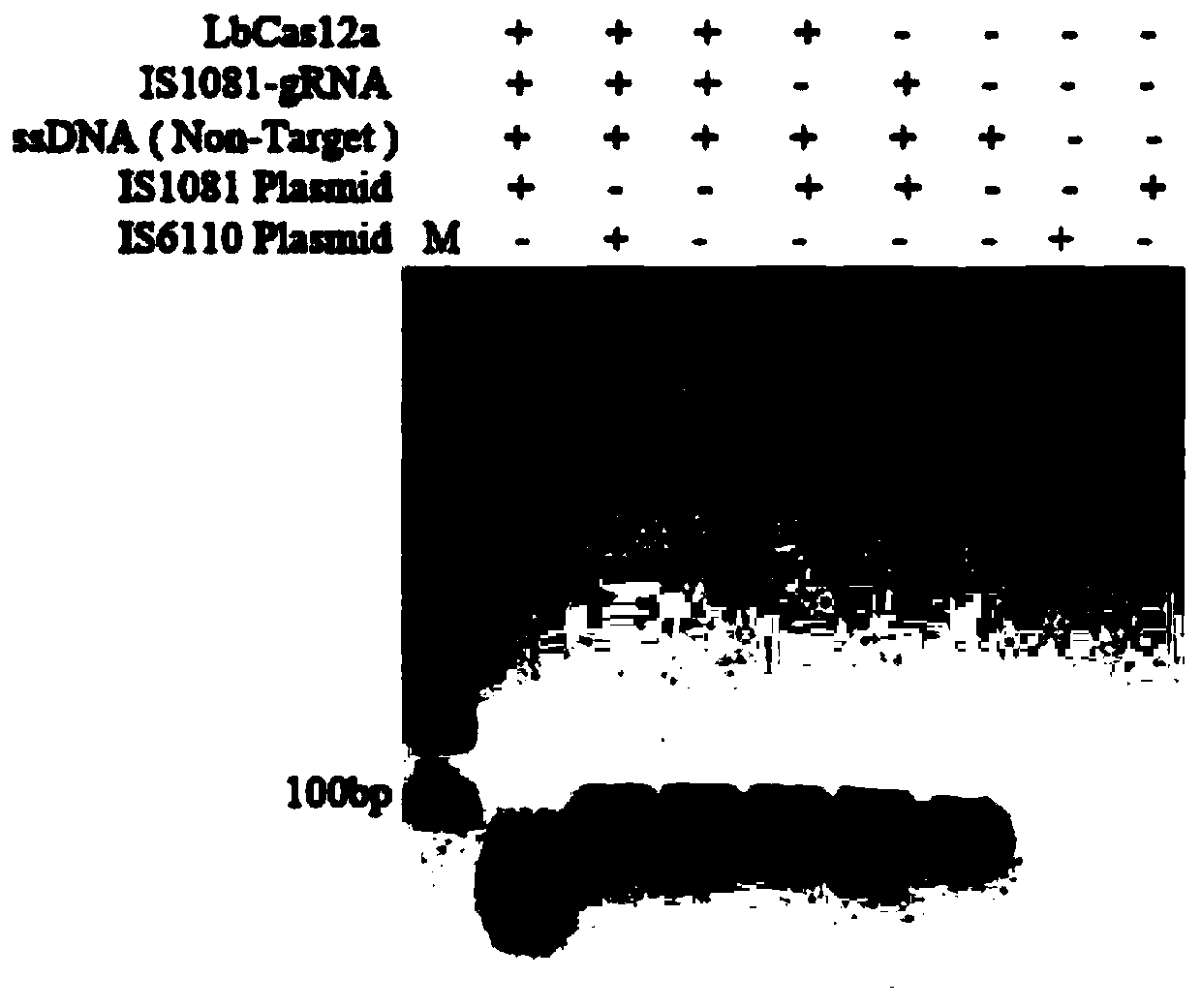
Mycobacterium tuberculosis complex detection kit based on CRISPR-Cas12a system
ActiveCN110541022AHigh detection sensitivityGet rid of dependenceMicrobiological testing/measurementNucleic acid detectionFluorescence
The invention belongs to the technical field of nucleic acid detection, and relates to a mycobacterium tuberculosis complex detection kit and method based on a CRISPR-Cas12a system. According to the kit, the detection sensitivity is improved by adopting a recombinase polymerase amplification technology, CRISPR-Cas12a is used for specifically targeting a mycobacterium tuberculosis complex target sequence and then activating the bypass cutting activity of Cas12a, and a mycobacterium tuberculosis complex can be sensitively and specifically detected from sputum. The kit and method have the advantages of being non-invasive, capable of detecting the mycobacterium tuberculosis complex frequently and repeatedly, high in detection speed and the like. Compared with an existing PCR detection method,the method based on the CRISPR-Cas12a system does not need a thermal cycler with heating and cooling functions, can detect the mycobacterium tuberculosis complex in sputum through fluorescence reading, has the advantages of being low in cost, easy and convenient to operate, high in sensitivity, good in specificity and the like and is suitable for clinical large-scale application.
Owner:MENGCHAO HEPATOBILIARY HOSPITAL OF FUJIAN MEDICAL UNIV
Kit for detecting mycobacterium tuberculosis infection and monitoring clinical treatment effect and application of kit
ActiveCN104020297AIncreased sensitivityImprove featuresDisease diagnosisBiological testingTherapeutic effectSpecific antibody
The invention discloses a kit for detecting the mycobacterium tuberculosis infection and monitoring a clinical treatment effect. The kit provided by the invention comprises a specific antibody, namely an IP-10 antibody and / or CD14 antibody, an antigen irritant and a positive contrast irritant. The kit disclosed by the invention can be used for diagnosing an active tuberculosis patient or a tuberculosis latent infection patient and is not affected by BCG (bacillus calmette-guerin) inoculation. The sensitivity and the specificity of the kit for diagnosing the active tuberculosis patient are higher than those of a commercial T-SPOT.TB kit and can be up to 100 percent. After the antituberculosis therapy is performed for 1 month, the detection rates of over 80 percent of clinical tuberculosis patients are converted to be negative, so that the kit can be used for detecting the clinical antituberculosis treatment effect.
Owner:SHANGHAI JIAOTONG UNIV SCHOOL OF MEDICINE
Mycobacterial rpoB sequences
InactiveUS7108968B2Sugar derivativesMicrobiological testing/measurementBiological bodyHybridization probe
This invention provides polynucleotide probes, sequences and methods for speciating and phenotyping organisms, for example, using probes based on the Mycobacterium tuberculosis rpoB gene. The groups or species to which an organism belongs may be determined by comparing hybridization patterns of target nucleic acid from the organism to hybridization patterns in a database.
Owner:AFFYMETRIX INC
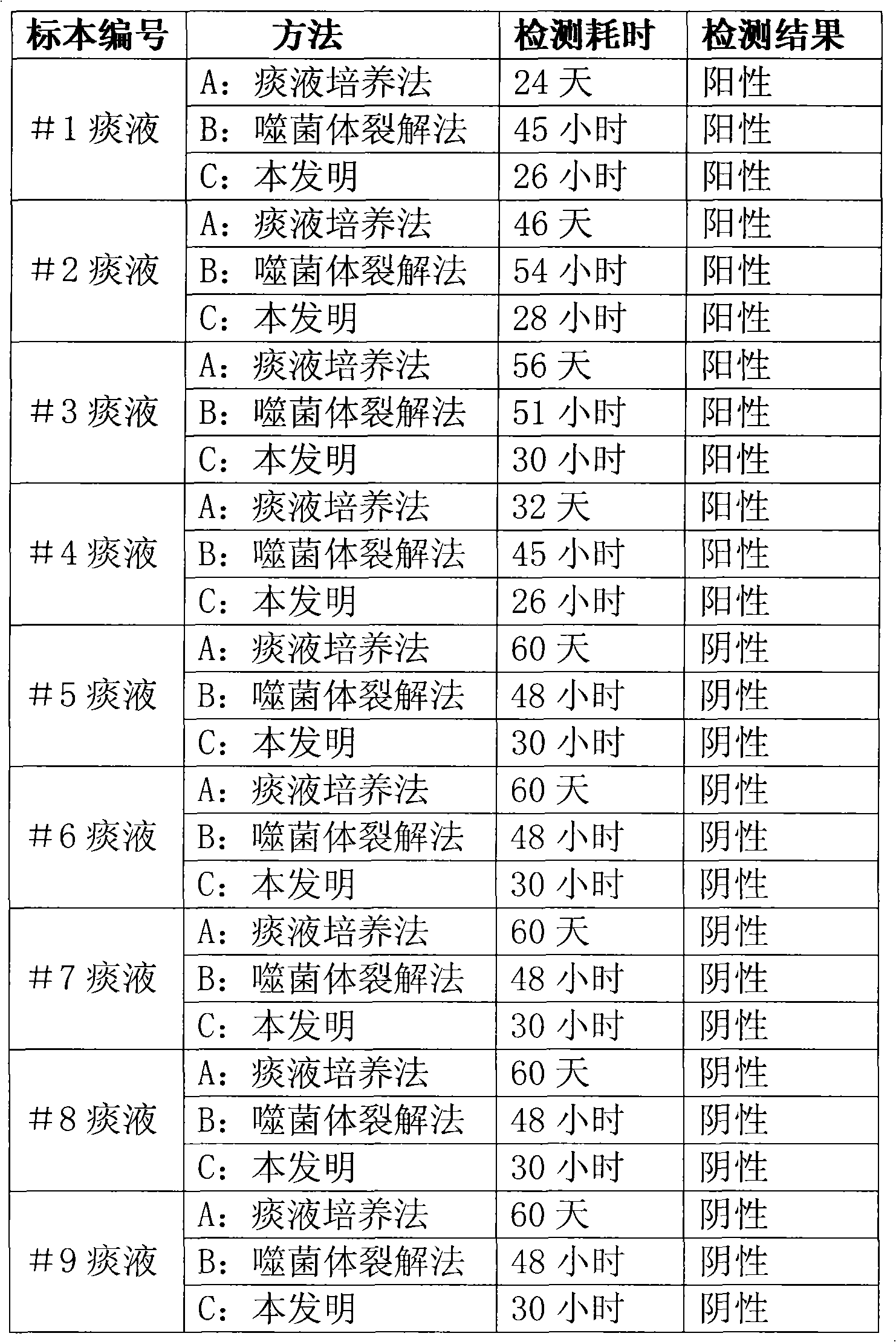
Method and kit for quickly detecting mycobacterium tuberculosis
The invention discloses a method for quickly detecting mycobacterium tuberculosis, which comprises the following steps: 1, infecting phage with the mycobacterium tuberculosis; 2, killing the phage notinfecting outside the mycobacterium tuberculosis; 3, amplifying the infecting phage; and 4, after labeling gold with antiphage antibody, adopting quick immunochromatography to detect whether the phage exists or not so as to judge whether the mycobacterium tuberculosis exists in a sample. In addition, the invention also discloses a corresponding kit, which comprises sample treating fluid, proliferous liquid, a killing agent, mycobacterium smegmatis, mycobacteriophages and a phage colloidal gold method immunity-chromatography test pen. The method and the kit can quickly and accurately detect the mycobacterium tuberculosis so as to meet the requirement of quickly diagnosing pulmonary tuberculosis and timely medicate the patient.
Owner:ABBOTT DIAGNOSTICS (SHANGHAI) CO LTD
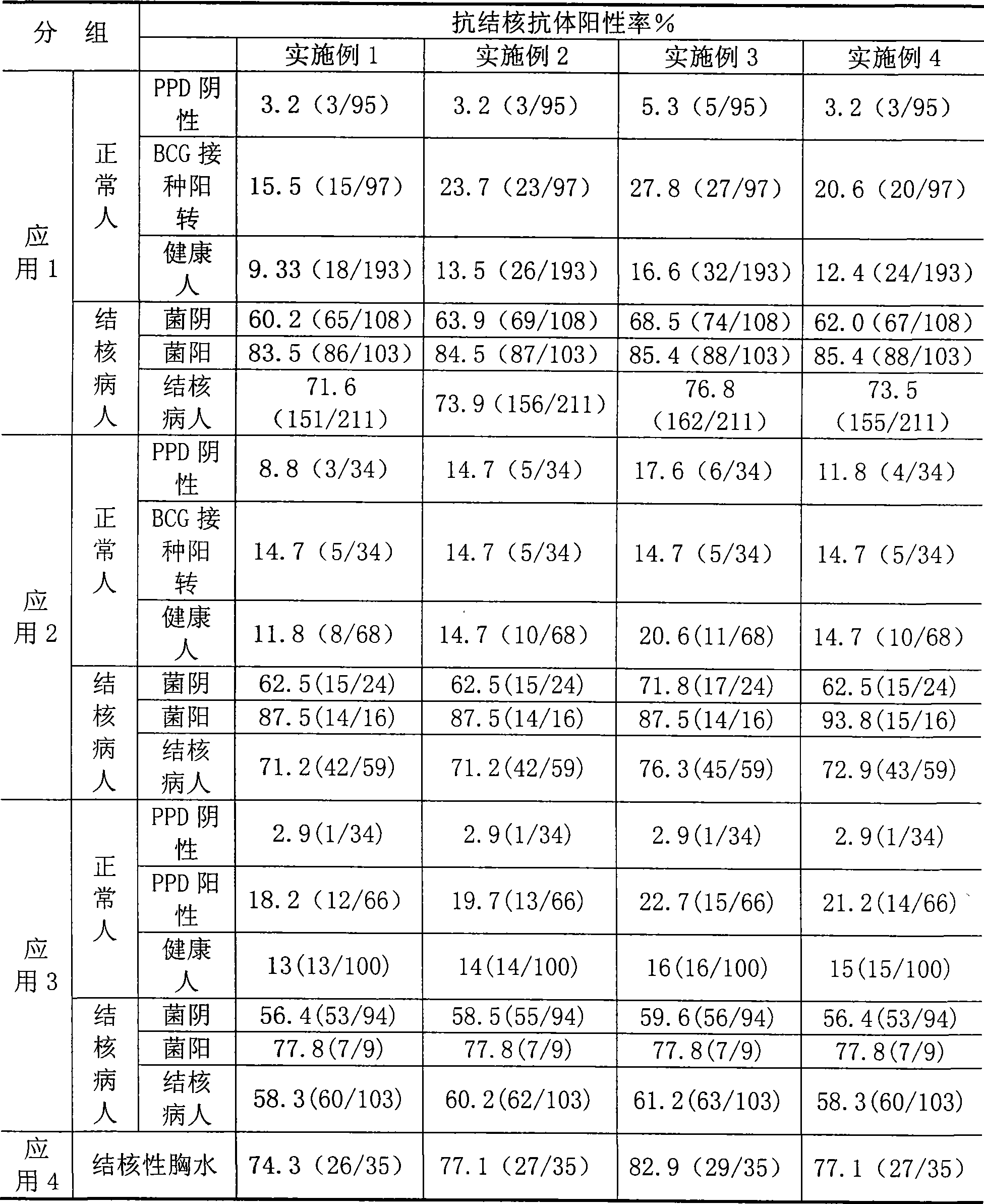
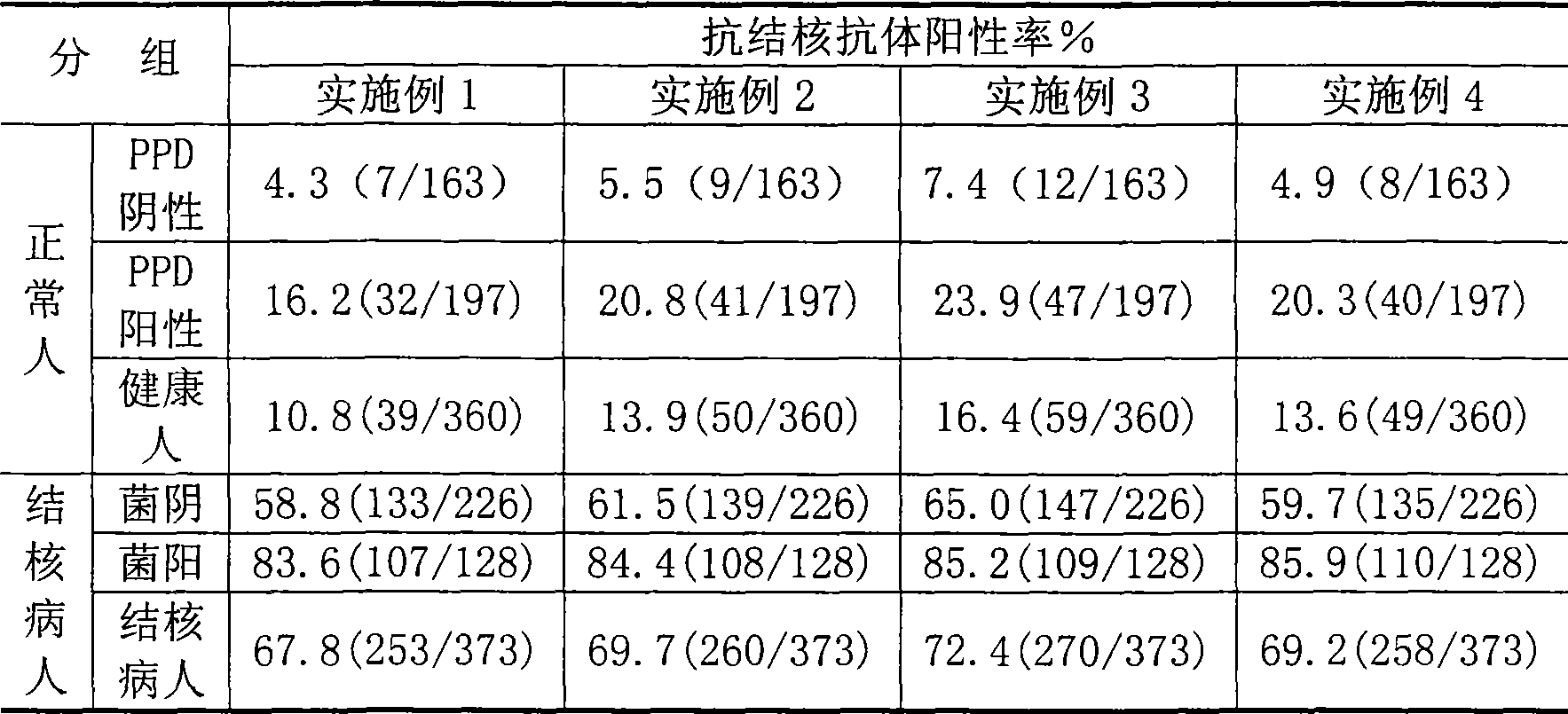
Tuberculosis antibody multi-antigen ELISA detecting kit and making method
The invention relates to a tuberculosis antibody multiple antigen ELISA detection kit and a preparation method thereof, which pertains to the field of tuberculosis medical immunology diagnostic techniques and mainly uses detection antigen, enzyme-linked antihuman IgG antibodies, substrates, positive control serum of tuberculosis patients, control serum of normal person, calf serum and polystyrene microplates to form the kit, wherein, the detection antigen adopts the mycobacterium tuberculosis complex strains of lipid Arabian mannose (LAM), 38kD and 16kD to be combined with arbitrary one or more than one mycobacterium tuberculosis recombinant proteins in the recombinant proteins of MPT63, MTB48 and CFP10-ESAT6. The mycobacterium tuberculosis has high sensitivity, strong specificity and complementarity, can be used for detecting specific antitubercular antibodies in such body fluid samples as serum, hydrothorax and the like, and assisting the diagnosis and differential diagnosis of tuberculosis.
Owner:中国人民解放军总医院第二附属医院
Application of mycobacterium tuberculosis proteins in preparation of products used for diagnosis of latent tuberculosis infection
The invention provides 13 mycobacterium tuberculosis proteins in development and / or designing of products capable of discrimination, diagnosis, auxiliary diagnosis, screening and / or auxiliary screening of latent tuberculosis infection. The invention further provides protein chips prepared from 13 mycobacterium tuberculosis protein antigens. The protein chips prepared in the invention are used for detecting the levels of IgG antibodies respectively corresponding to the 13 protein antigens in serum of a patient with latent tuberculosis infection and of a normal person, and detection results of the antibodies respectively corresponding to the three protein are analyzed together so as to determine whether an examined person suffers from latent tuberculosis infection; detection results show that optimal operating points of the protein chips provided by the invention in auxiliary diagnosis of latent tuberculosis infection have specificity of 89.4% and sensitivity of 80.6%, both higher than indexes of diagnosis of latent tuberculosis infection in the prior art.
Owner:广东希格生物科技有限公司
Multiplex polymerase chain reaction (PCR) kit for identifying mycobacterium tuberculosis
InactiveCN102533959ASimple and fast operationEasy to operateMicrobiological testing/measurementSequence designAcid-fast
Owner:HUAZHONG AGRI UNIV
Method for identifying mycobacterium tuberculosis and mycobacteria other than tuberculosis, together with detecting resistance to an antituberculosis drug of mycobacteria obtained by mutation of rpob gene
The present invention provides a method for identifying Mycobacterium tuberculosis and non-tuberculosis Mycobacterium (MOTT), and for the determination of drug susceptibility of M. tuberculosis based on detection of mutations in the rpoB gene.
Owner:LEE HYEYOUNG +1
Method and kit for detecting streptomycin medicine resistant mutation of Mycobacterium tuberculosis
InactiveCN102229991AAvoid False Positive ResultsSimple and fast operationMicrobiological testing/measurementFluorescence/phosphorescenceFluorescenceNucleotide
The invention discloses a method and a kit for detecting the streptomycin medicine resistant mutation of Mycobacterium tuberculosis. The invention relates to medicine resistant mutation detection technique and provides a method for detecting streptomycin medicine resistant mutation of Mycobacterium tuberculosis, which effectively improves sensitivity and specificity, and is simple and convenient in operation and short in period. The method comprises: designing primers and probes according to the complete sequence of the Mycobacterium tuberculosis and the gene sequences of the genomes rpsL and rrs of the Mycobacterium tuberculosis; extracting the DNA of a sample of the Mycobacterium tuberculosis; constructing a polymerase chain reaction (PCR) reaction system; and performing PCR amplification and analysis on a fusion curve. In the method, experiments are performed in two tubes respectively by using the specific primers and probes, and the amplification of the nucleic acid fragment of a target nucleotide sequence and subsequent analysis on the fusion curve are realized by using heat-resistance DNA polymerase, four kinds of nucleotide monomers and other components and by using real-time PCR technique. A fluorescent PCR fusion curve method with high specificity can quickly and accurately detects common medicine-resistance mutation of Mycobacterium tuberculosis and is expected to be directly used for medicine-resistance detection of a clinic Mycobacterium tuberculosis sample.
Owner:XIAMEN UNIV +1
Method for detecting mycobacterium tuberculosis by using adaptor technique
InactiveCN1958809AImprove positive detection rateRapid diagnosisMicrobiological testing/measurementMaterial analysisMycobacterium tuberculosis cultureStructural analog
This invention relates to a method for detecting Mycobacterium tuberculosis by utilizing gamete technique. This invention is aimed to solving the problems of low discovery rate and low curative rate of tuberculosis. The method comprises: utilizing gamete technique, performing bacteria culture or bioengineering method to obtain all kinds of targets of Mycobacterium tuberculosis, obtaining highly compatible specific gametes of the targets by gamete technique screening, and converting the gametes into report gametes for rapid and precise detection of corresponding targets. The method can further combine highly compatible gametes with pathogenetic factors to defunctionalize them, or develop new drugs by finding structural analogues of highly compatible gametes, thus can provides basis for diagnosis and clinical therapy of Mycobacterium tuberculosis.
Owner:SHANGHAI PULMONARY HOSPITAL
Mycobacterium tuberculosis nucleic acid detection kit by utilizing RNA isothermal amplification
ActiveCN102181572AHigh sensitivityStrong specificityMicrobiological testing/measurementFluorescence/phosphorescencePositive controlDrug resistance
Owner:SHANGHAI RENDU BIOTECH
MTB (Mycobacterium Tuberculosis) infection diagnosis kit
ActiveCN105954521AInfection diagnosisStrong specificityBiological material analysisBiological testingBiotin-streptavidin complexInsulin-like growth factor
The invention belongs to the field of biomedicine examination, and relates to an MTB (Mycobacterium Tuberculosis) infection diagnosis kit. The MTB infection diagnosis kit provided by the invention is prepared from the following components: an antigen stimulant, a pre-coated ELISPOT (Enzyme-Linked Immunospot Assay) plate of a capture antibody, an insulin-like growth factor, a detection antibody, HRP (Horse Radish Peroxidase)-labeled streptavidin, a 3-amino-9-ethyl carbazole developing solution, antibody diluent and a positive control stimulant; the antigen stimulant is selected from one or multiple polypeptides in sequences as shown in SEQ ID NO.1 to 12. The MTB infection diagnosis kit provided by the invention is high in sensitivity and specificity and stable in property; meanwhile, when the MTB infection diagnosis kit provided by the invention is applied to in-vitro detection on MTB, the detection time can be saved, the detection steps can be simplified, and the detection efficiency can be increased.
Owner:WUHAN DANGKANG XING ZHONG BIOTECHNOLOGY CO LTD
Antigenic polypeptide pool capable of detecting mycobacterium tuberculosis infection, and application thereof
ActiveCN107011418AQuick checkSpecific detectionBiological material analysisDepsipeptidesPeripheral blood mononuclear cellMycobacterium Infections
The invention discloses an antigenic polypeptide pool capable of detecting mycobacterium tuberculosis infection. The antigenic polypeptide pool specifically stimulates mycobacterium tuberculosis infected fresh whole blood to specifically secrete IFN-gama and increase the sensitivity. The invention provides a new detection reagent for detecting mycobacterium tuberculosis infection, experiments prove that peripheral blood can be directly utilized to conduct antigenic simulation without separation of peripheral blood mononuclear cell, the experimental data shows that the antigenic polypeptide pool has high sensitivity and specificity for detecting mycobacterium tuberculosis infection, and is simple and convenient to operate and low in cost, thus having high clinical application values.
Owner:武汉海吉力生物科技有限公司
Antigen stimulant and kit for detecting mycobacterium tuberculosis infection, and application of antigen stimulant
ActiveCN104597239AIncreased sensitivityImprove featuresMicrobiological testing/measurementBiological testingMycobacterium InfectionsStimulant
The invention provides an antigen stimulant for detecting mycobacterium tuberculosis infection, and a kit comprising the antigen stimulant. The invention also provides an application of the antigen stimulant in reagents for detecting mycobacterium tuberculosis infection. The antigen stimulant comprises at least one polypeptide or analogues thereof in polypeptides shown as the sequences 1-11 in a sequence table, wherein the polypeptides respectively come from tuberculosis specific antigen polypeptides ESAT-6 and tuberculosis specific antigen polypeptides CFP-10. According to the antigen stimulant provided by the invention, peripheral blood T lymphocytes of tuberculosis infection patients can be effectively stimulated to generate IFN-gamma, so that the tuberculosis infection can be diagnosed at high sensitivity and high specificity, and the influence on BCC inoculation or other underlying diseases can be avoided.
Owner:SUN YAT SEN UNIV +1
Tuberculosis special antigen rapid diagnosis kit
The invention utilizes a genetic engineering method to express in vitro the desA protein of mycobacterium tuberculosis and produces monoclonal antibodies by using the protein. A tuberculosis specific antigen rapid diagnosis reagent kit is produced by utilizing enzyme linked immunization, chemiluminescence, colloidal gold test strip method and other diagnostics methods so as to detect MTB quickly with low cost. The technique has wide application value in clinical diagnosis.
Owner:AUTOBIO DIAGNOSTICS CO LTD
Diagnosis kit for mycobacterium species identification and drug-resistance detection and mfg. method thereof
InactiveCN1444662AEfficient amplificationBioreactor/fermenter combinationsBiological substance pretreatmentsHybridization probeOligonucleotide chip
The present invention relates to diagnosis kit for Mycobacterium species identification and drug-resistance detection and manufacturing method thereof, which can discriminate a Mycobacterium Tuberculosis rpoB gene point mutation relating to the Mycobacterium species identification and drug-resistance swiftly, exactly and in large quantities using an oligonucleotide chip. The diagnosis kit for Mycobacterium species identification and drug-resistance detection in accordance with the present invention consists of an oligonucleotide chip including a Mycobacterium tuberculosis complex probe, a Mycobacterium species identification probe and a drug-resistance detection probe of a Mycobacterium tuberculosis rpoB gene, and a fluorescent material containing a biotin-binding protein so as to detect hybridization of amplified products of a specimen marked as biotine and the corresponding probe.
Owner:BIOMEDLAB CORP
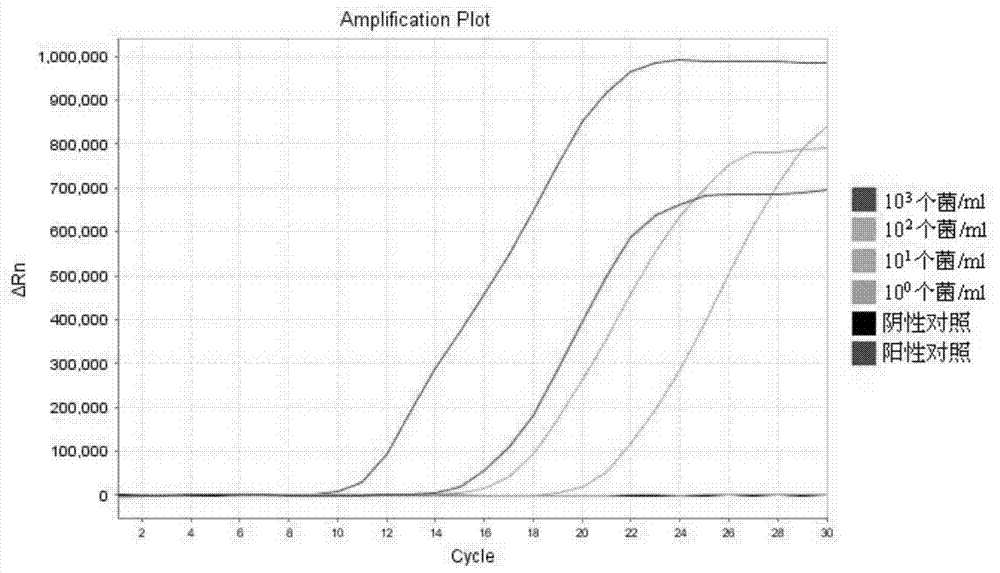
Method and detection kit for detecting mycobacterium tuberculosis complex cluster based on thermostatic technology
ActiveCN103898215AAmplifyLow backgroundMicrobiological testing/measurementMicroorganism based processesNucleotideSingle strand
The invention discloses a method and a detection kit for detecting a mycobacterium tuberculosis complex cluster based on a thermostatic technology. According to the method, 5 primers are designed aiming at a GyrB gene of mycobacterium tuberculosis, wherein TP is composed of an annealing sequence and a nucleotide sequence complementary with a target sequence; FP has a loop-stem structure, and can anneal with two complementary DNA single strands from two ends and stretch towards an opposite side; annealing sites of OP1 and OP2 are respectively located on the outer sides of the TP and the FP, anneal and stretch towards middle and simultaneously peel off double strands stretching from the TP and the FP; a single strand with the TP or FP, after being replaced, can serve as a template to continue the process, thus forming two key single-stranded intermediate products; the two intermediate products are then used for synthesizing DNA through self-guidance by taking special structures of the TP and the FP as starting points; through such circulation and repetition, a self-circulating chain displacement reaction is triggered under function of DNA polymerase, thus realizing massive amplification of the target sequence.
Owner:广州迪澳生物科技有限公司
Fusion proteins of mycobacterium tuberculosis antigens and their uses
InactiveUS20030147911A1Improving immunogenicityPeptide/protein ingredientsAntibody mimetics/scaffoldsAntigenMycobacterial antigen
The present invention relates to fusion proteins containing at least two Mycobacterium tuberculosis antigens. In particular, it relates to bi-fusion proteins which contain two individual M. tuberculosis antigens, tri-fusion proteins which contain three M. tuberculosis antigens, tetra-fusion proteins which contain four M. tuberculosis antigens, and penta-fusion proteins which contain five M. tuberculosis antigens, and methods for their use in the diagnosis, treatment and prevention of tuberculosis infection.
Owner:CORIXA CORP
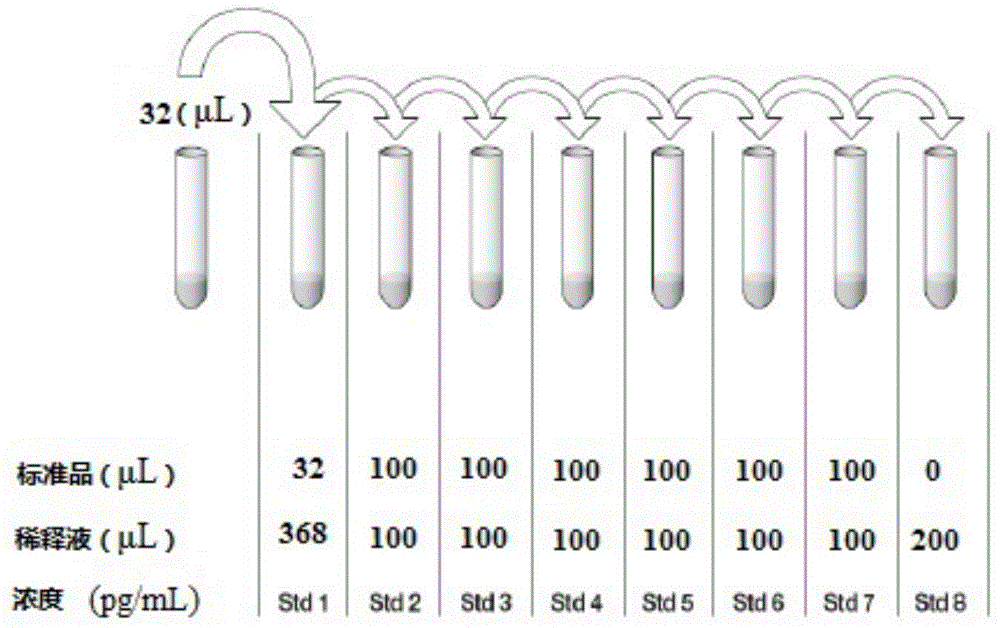
Kit for diagnosing mycobacterium tuberculosis infection based on tuberculosis specificity IL-31 detection
InactiveCN104897893AIncreased sensitivityImprove accuracyBiological material analysisAntigenMycobacterium Infections
The invention provides a kit for diagnosing mycobacterium tuberculosis infection based on tuberculosis specificity IL-31 detection. The kit comprises an IL-31 capture antibody, an IL-31 detection antibody, tuberculosis specific antigen polypeptide, a diluent, a standard substance, a developing solution, a stop solution, a buffer solution, a solid carrier and a micro-plate sealer. The kit is used for detecting the IL-31 expression quantity in plasma, can be used for tuberculosis diagnosis, and is relatively high in sensitivity and accuracy, and fast to operate, so that the diagnosing efficiency of tuberculosis can be improved.
Owner:AFFILIATED HUSN HOSPITAL OF FUDAN UNIV
Immune colloidal gold test paper for detecting mycobacterium bovis antibody and preparation method thereof
InactiveCN101846677AStrong specificityImprove stabilityMaterial analysisEscherichia coliMycobacterium tuberculosis hominis
The invention discloses immune colloidal gold test paper for detecting mycobacterium bovis antibody and a preparation method thereof. Staphylococal Protein A (SPA) marks colloidal gold to prepare a gold colloidal pad of the immune colloidal gold test paper. The proteins of CFP10 and MPT64 are immunogenic proteins with high specificity to virulent mycobacterium tuberculosis. The genes of CFP10 andMPT64 are cloned from the genome of the mycobacterium tuberculosis, and are connected to pET-28a to construct two prokaryotic expression recombinant plasmids; the two plasmids are converted into Escherichia coli to express the proteins of CFP10 and MPT64; after the proteins are purified, the mixed proteins are taken as antigens to coat a nitrocellulose membrane to form a detection line; and the detection line is equipped with the gold colloidal pad to form the immune colloidal gold test paper. The test paper can distinguish that human bodies and animals are inoculated with BCG or subjected towild virus infection, can be used for detecting the mycobacterium tuberculosis antibody in animal serum, has the characteristics of strong specificity, high sensitivity, good stability, convenience and quickness, and has significance and actual application value for monitoring, diagnosing, purifying and controlling the mycobacterium tuberculosis.
Owner:JILIN UNIV
Biomarker for diagnosing mycobacterium tuberculosis infection and related kit
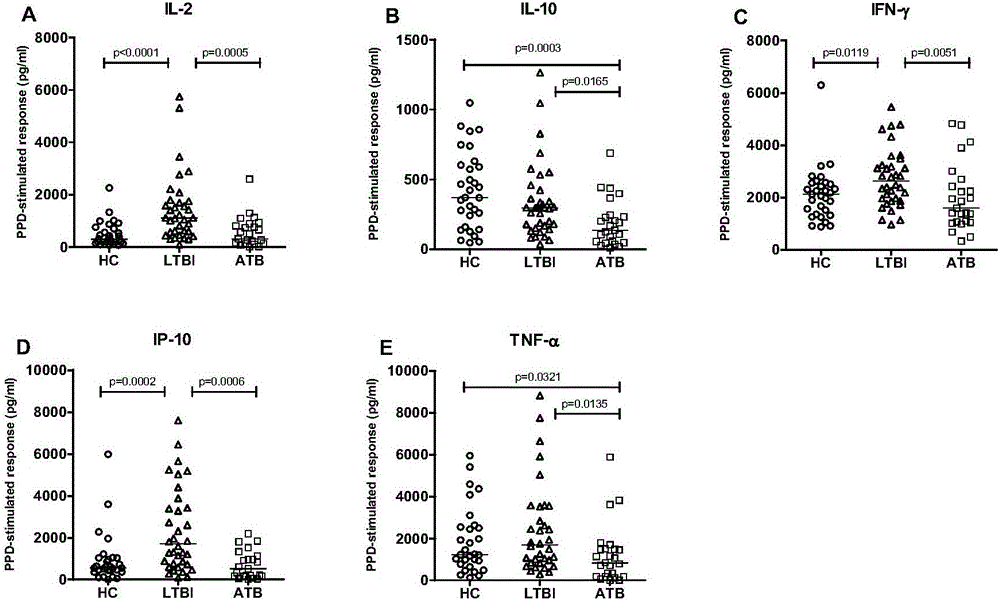
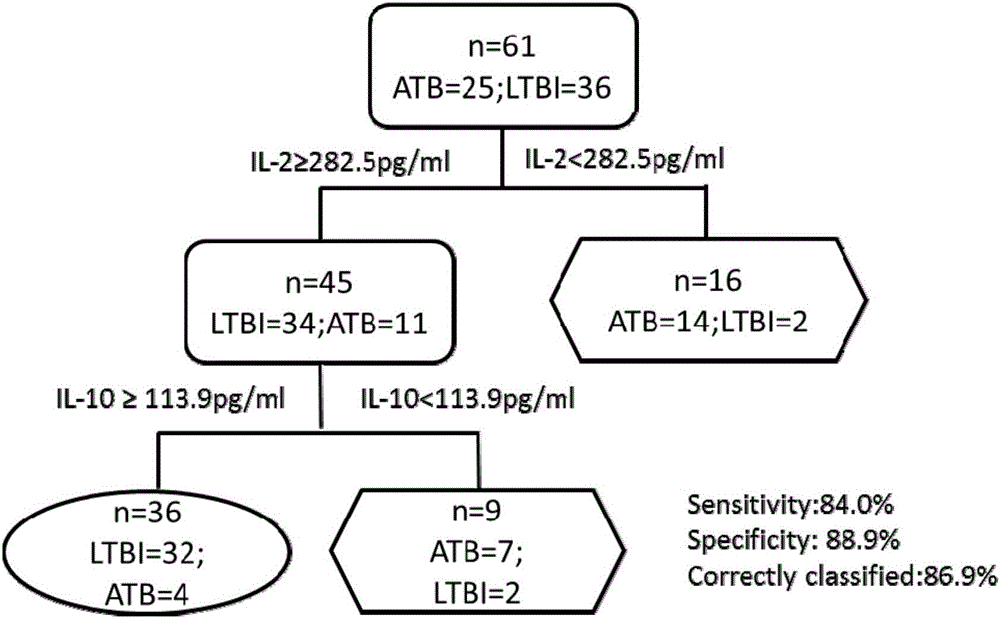
InactiveCN106501530AImprove diagnostic efficiencyEasy to operateBiological material analysisBiological testingAntigenInfection diagnosis
The invention discloses a biomarker for diagnosing mycobacterium tuberculosis infection. The biomarker comprises IL-2 and IL-10. The invention proves that cell factors IL-2 and IL-10 can serve as markers for tuberculosis infection diagnosis for the first time, the tuberculosis infection can be diagnosed by measuring the concentration of the IL-2 and the IL-10 after peripheral blood monouclear cells are stimulated by tuberculosis antigen, and active tuberculosis and latent tuberculosis infection are further distinguished. A tuberculosis infection blood detection reagent and a kit which are prepared on the basis of the two cell factors provide new technological basis for quick diagnosis of the active tuberculosis, have the advantages of high sensitivity, high specificity and the like, are quick in operation, reasonable and practical, and can obviously improve the diagnosis efficiency of the active tuberculosis.
Owner:AFFILIATED HUSN HOSPITAL OF FUDAN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com