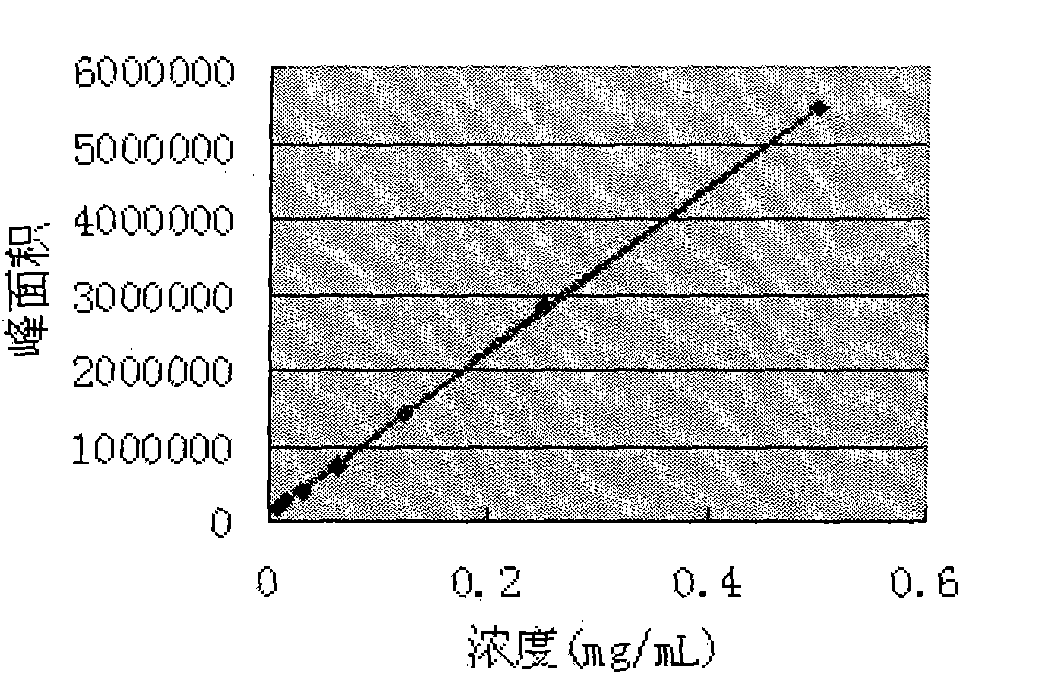
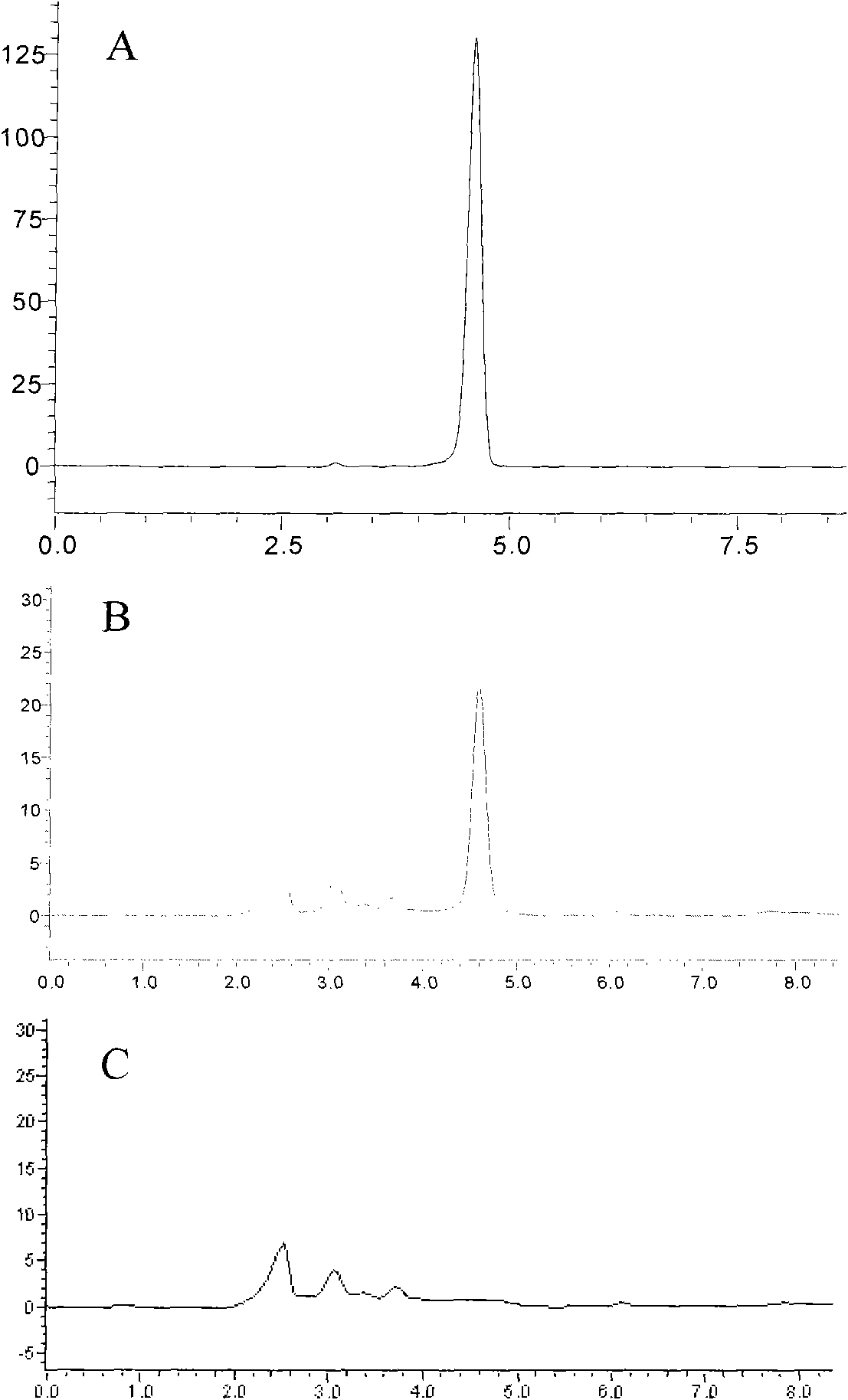
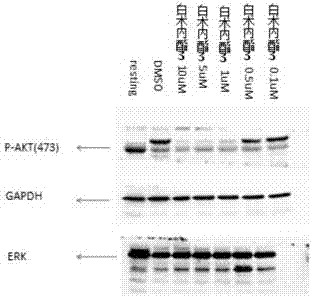
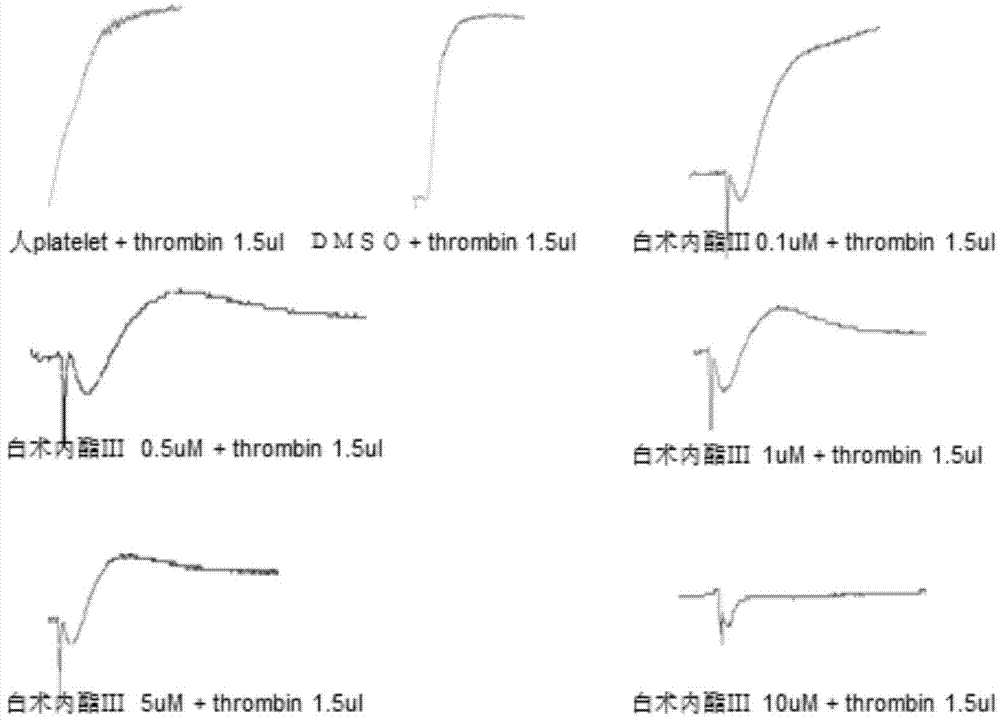
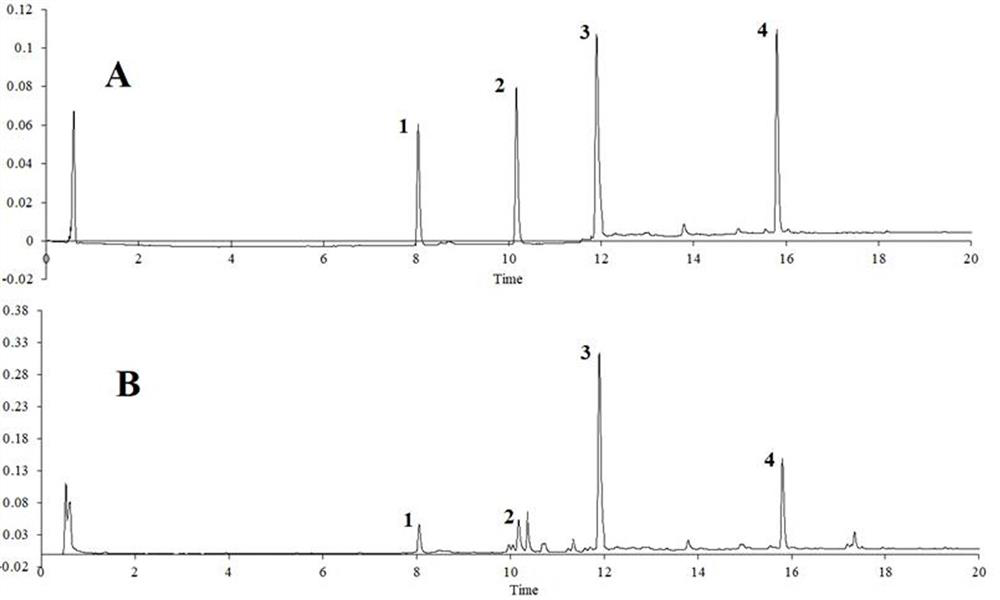
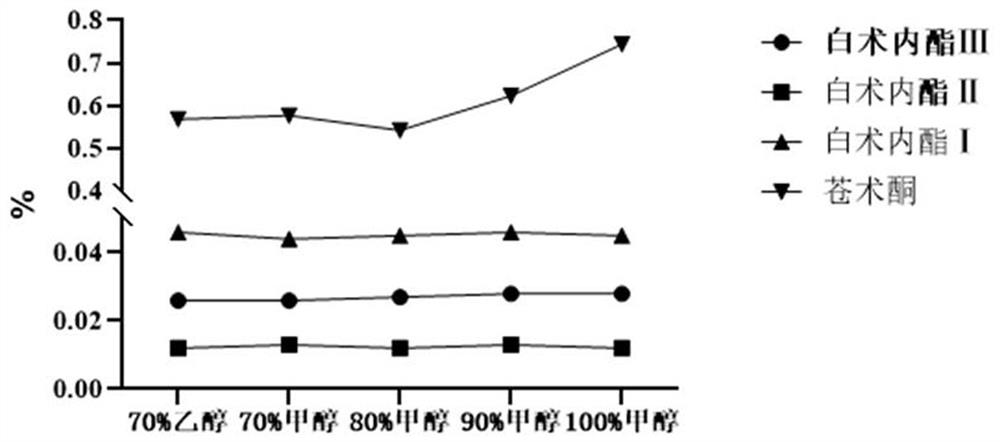
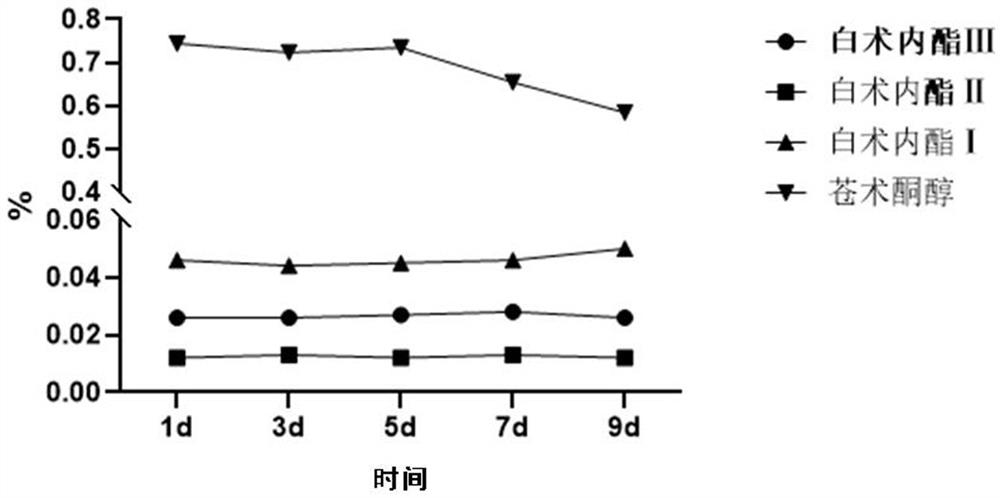
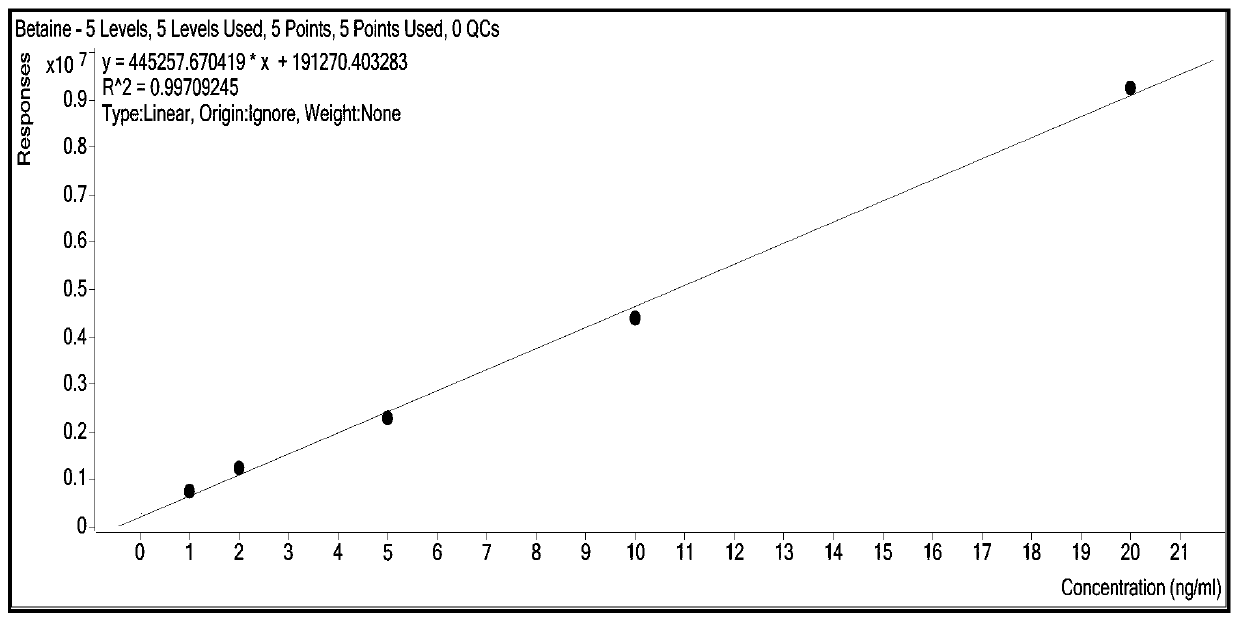
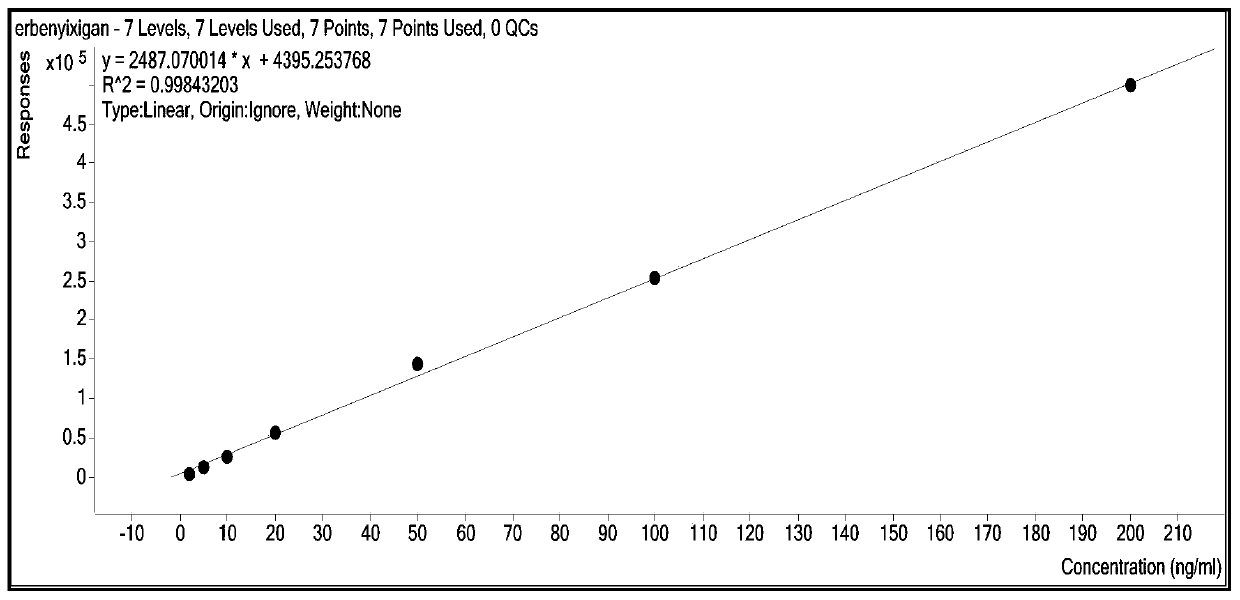
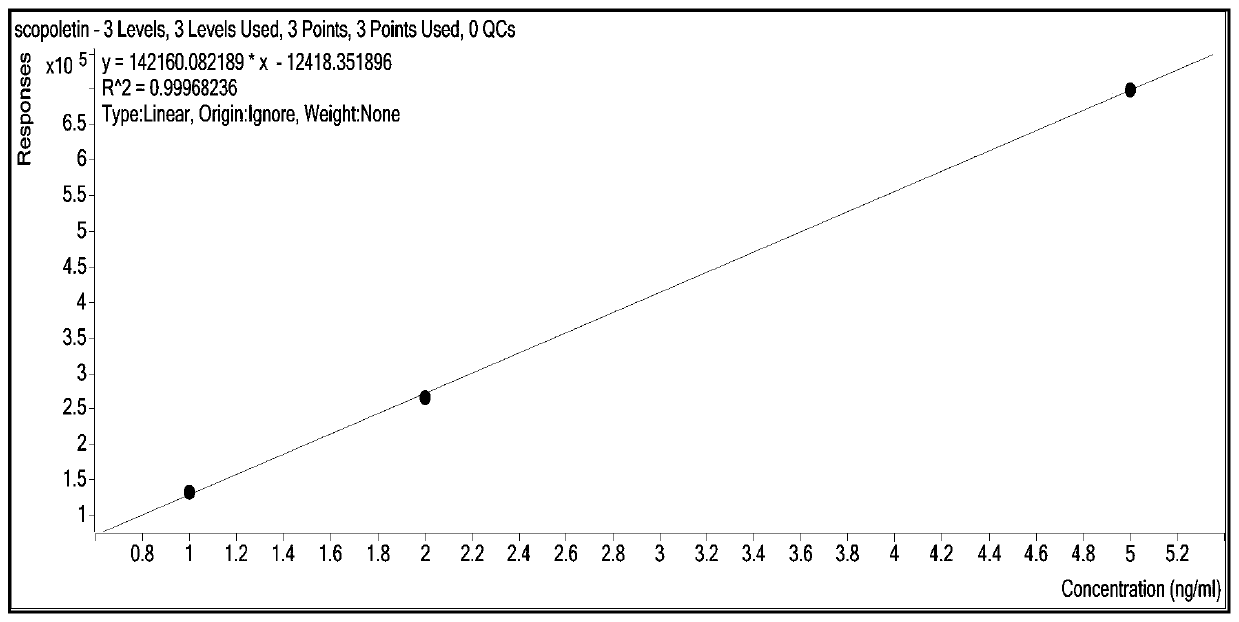
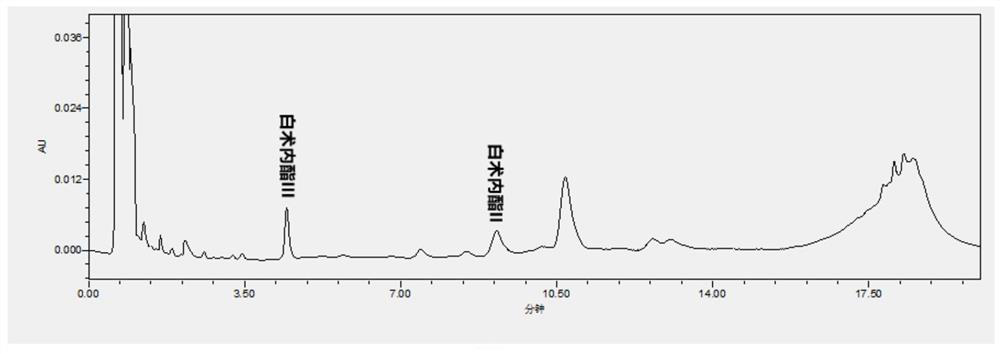
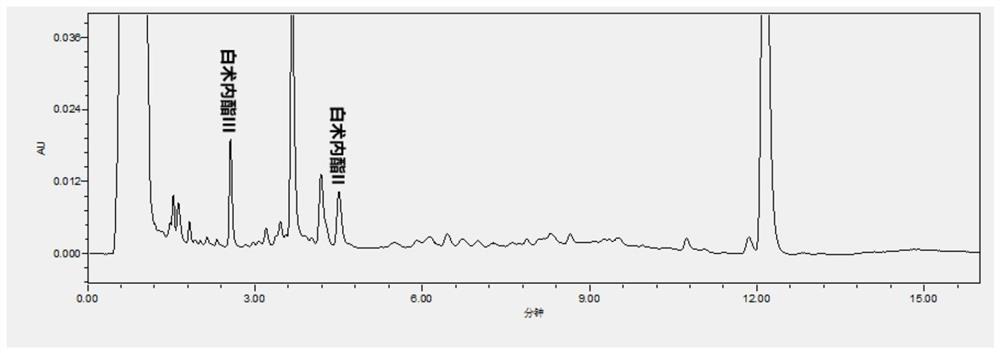
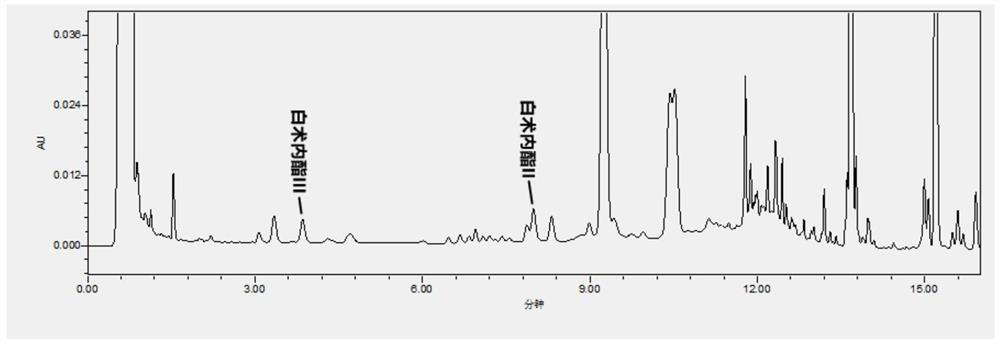
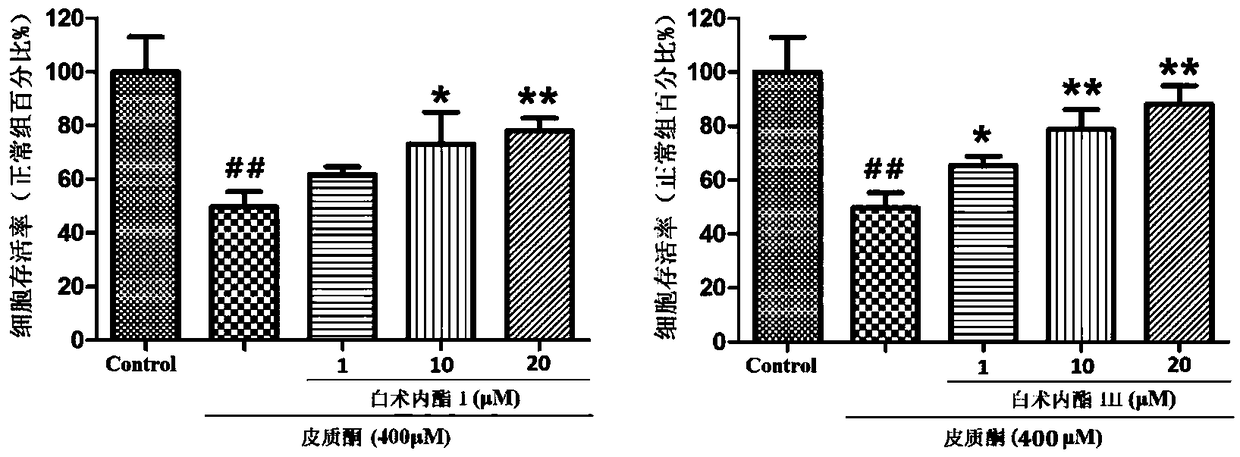
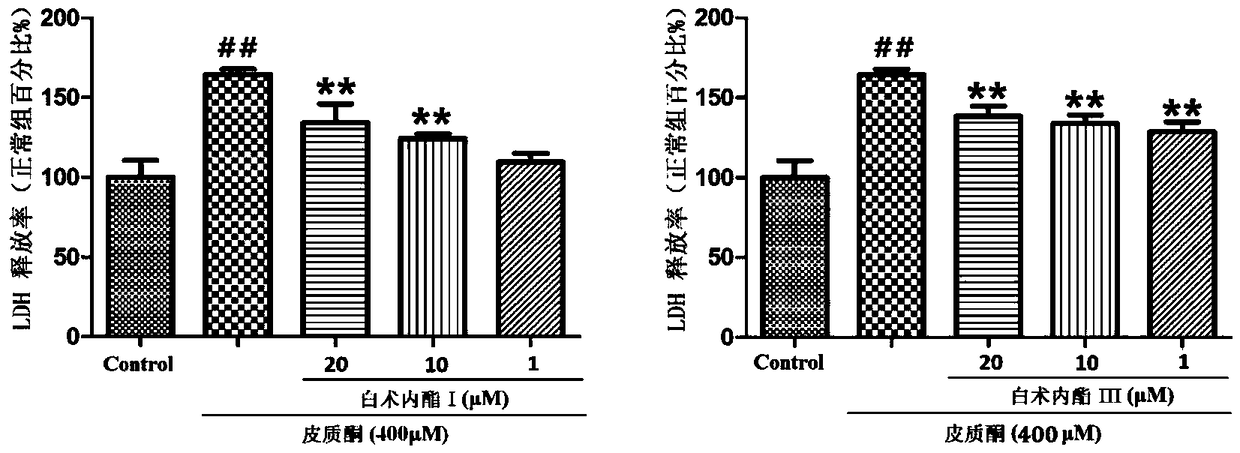
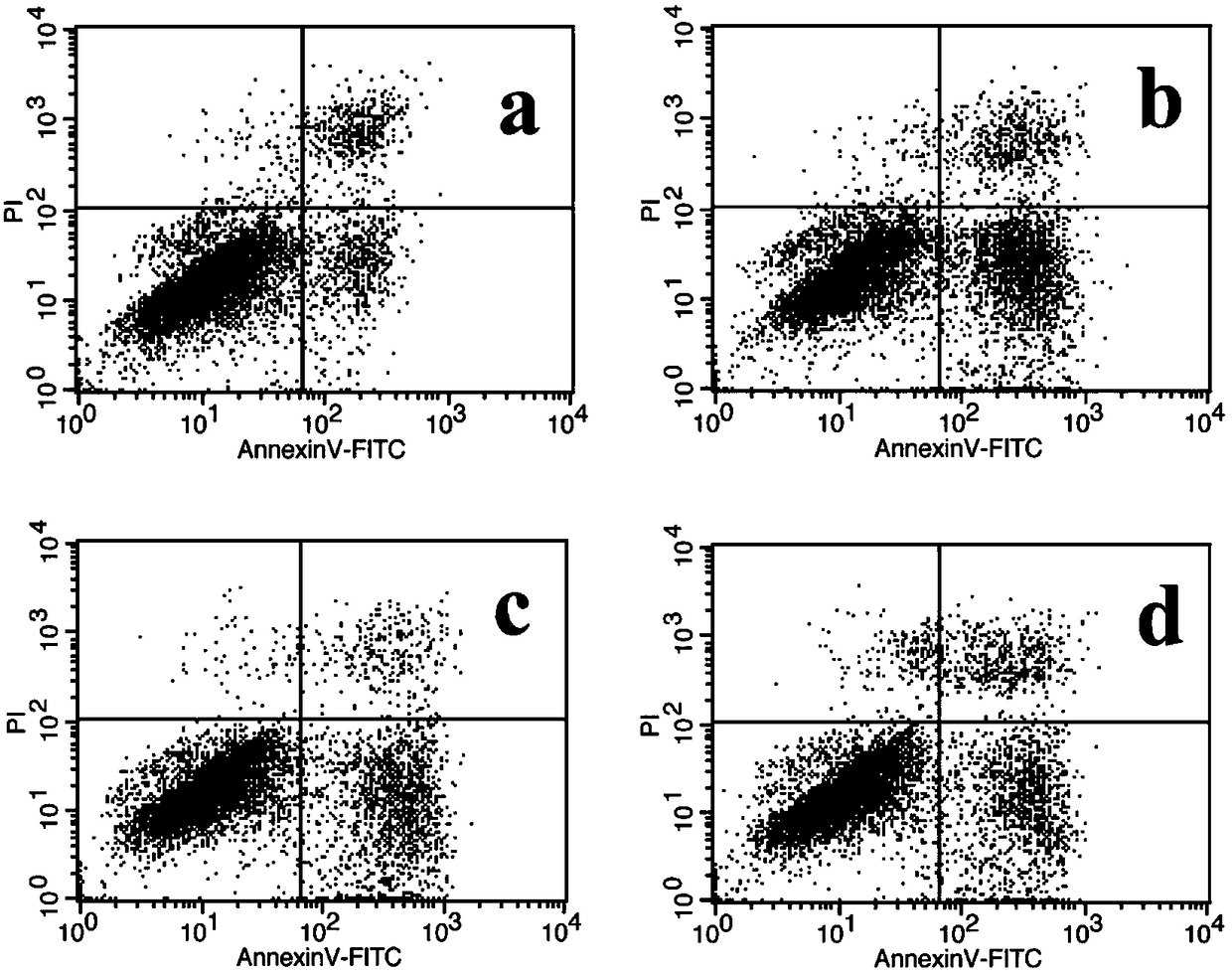
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
33 results about "Atractylenolide III" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Atractylenolide extract having antiparkinsonian effect, preparation method and application thereof
InactiveCN105267274AIncrease contentHigh extraction rateNervous disorderPlant ingredientsAntiparkinsonian drugAtractylenolide III
The invention discloses atractylenolide extract having antiparkinsonian effect, a preparation method and application thereof. Weight percentages of atractylenolide I, atractylenolide II and atractylenolide III in the atractylenolide extract are larger than 5%, 10% and 20% respectively, and purity of total lactone is larger than 35%. The application refers to that the atractylenolide extract is used to prepare antiparkinsonian drug. The preparation method includes that oxidant is added into white atractylode rhizome powder, and atractylone in white atractylode rhizome volatile oil is oxidized into atractylenolide compounds, so that content of atractylenolide is increased; zinc particles are added into an extraction solution to protect generated compounds such as atractylenolide from being further oxidized and degraded. The preparation method is simple, convenient and practical, easy to operate and more suitable for industrial production, and has remarkable progressiveness and practical value.
Owner:LIAONING UNIV OF TRADITIONAL CHINESE MEDICINE
Content determination method for multiple components in Yupingfeng preparation
ActiveCN105021751AStrong specificityNo interference peakComponent separationFiltrationAtractylenolide II
The invention relates to a content determination method for multiple components in a Yupingfeng preparation. The method comprises the steps of: (1) taking the Yupingfeng preparation, crushing or not crushing it, then performing precise weighing, adding methanol to conduct reflux extraction for 1-3h, carrying out filtration and concentration, then adding methanol to a constant volume, thus obtaining a test solution; (2) precisely weighing the reference substance prim-o-glucosylcimifugin, calycosin-7-glucoside, cimifugin, 5-O-methylvisammioside, sec-o-glucosylhamaudol, calycosin, formononetin, atractylenolide III, atractylenolide I and atractylenolide II respectively, and adding methanol to perform dissolving to a constant volume, thus obtaining a mixed reference solution; (3) conducting determination: precisely sucking the test solution and the mixed reference solution respectively, injecting them into a high performance liquid chromatograph, carrying out gradient elution under certain mobile phase condition, and performing multi-wavelength simultaneous monitoring, thus obtaining the content. The method provided by the invention can accurately determine the content of 10 components in the Yupingeng preparation, and can objectively, comprehensively and sensitively reflect the quality condition of the Yupingfeng preparation.
Owner:GUANGZHOU XIANGXUE PHARMA CO LTD
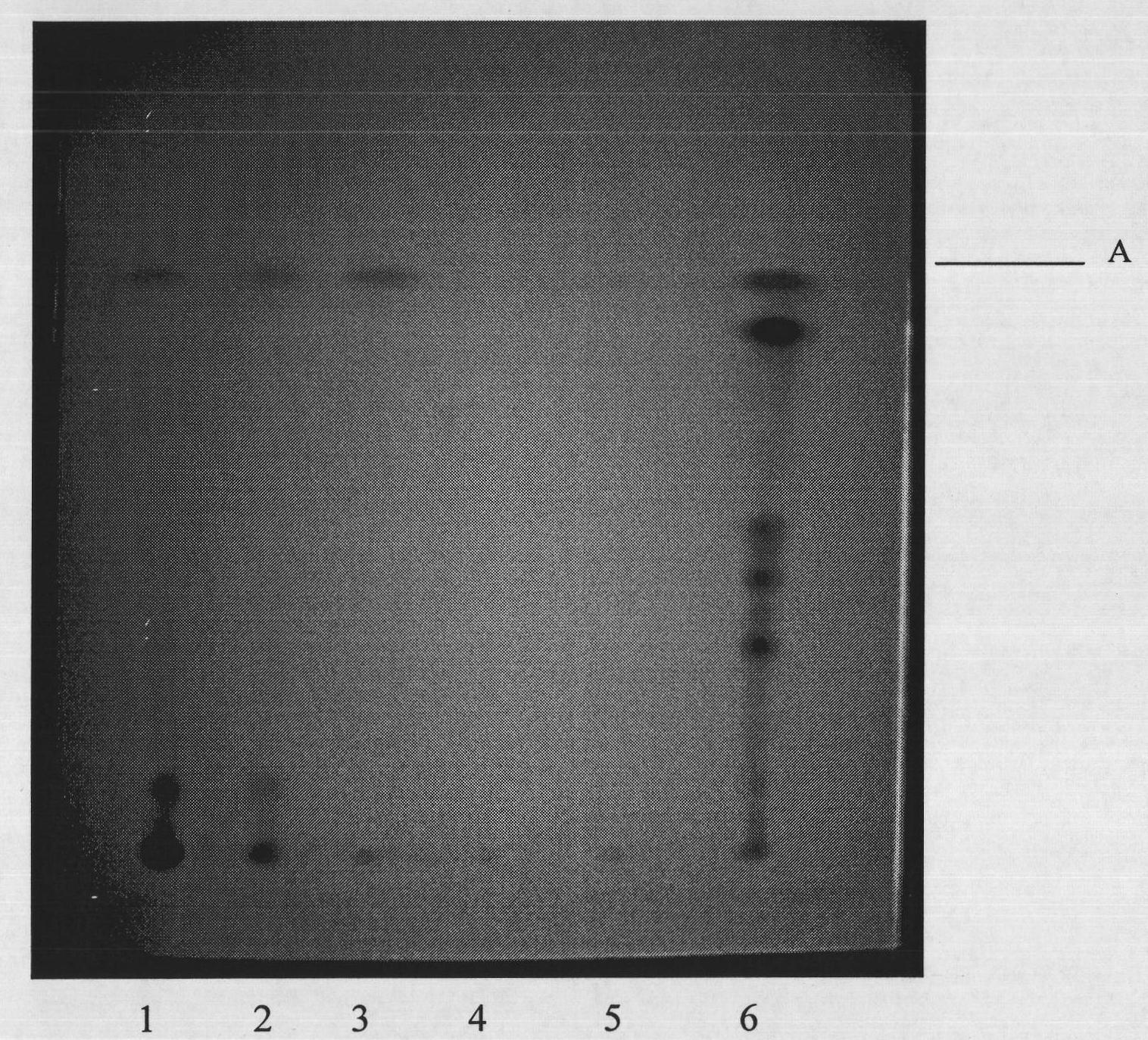
Method for identifying rhizoma atractylodis macrocephalae by adopting thin layer chromatography
InactiveCN101829176AEfficient identificationEasy to useComponent separationDigestive systemSilica gelEthyl acetate
The invention discloses a method for identifying rhizoma atractylodis macrocephalae by adopting a thin layer chromatography. The method comprises the following steps of: taking three target compounds of atractylone, atractylenolide I and atractylenolide III as comparison products, preparing a comparison product solution by using methanol and ultrasonically extracting the rhizoma atractylodis macrocephalae by using methanol to prepare a sample solution; sucking the comparison product solution and the sample solution, respectively applying samples on the same silica gel G thin-layer plate, carrying out secondary expansion by respectively taking petroleum ether-ethyl acetate with the temperature of 60-90 DEG C and cyclohexane-ethyl acetate as expanding agents, taking out and airing, carrying out identification by visually inspecting under the conditions of natural lights and the wavelength of 365nm after color development by adopting a sulfuric acid ethanol solution, and considering the sampling medicinal material which displays same color spots as the rhizoma atractylodis macrocephalae at the same Rf (Radio frequency) position of the three comparison products. The identifying method has strong specificity as well as simple and convenient operation, can be used for accurately identifying the rhizoma atractylodis macrocephalae and definitely distinguishing the rhizoma atractylodis macrocephalae from easily-confused product-rhizoma atractylodis.
Owner:ZHEJIANG ACAD OF TRADITIONAL CHINESE MEDICINE
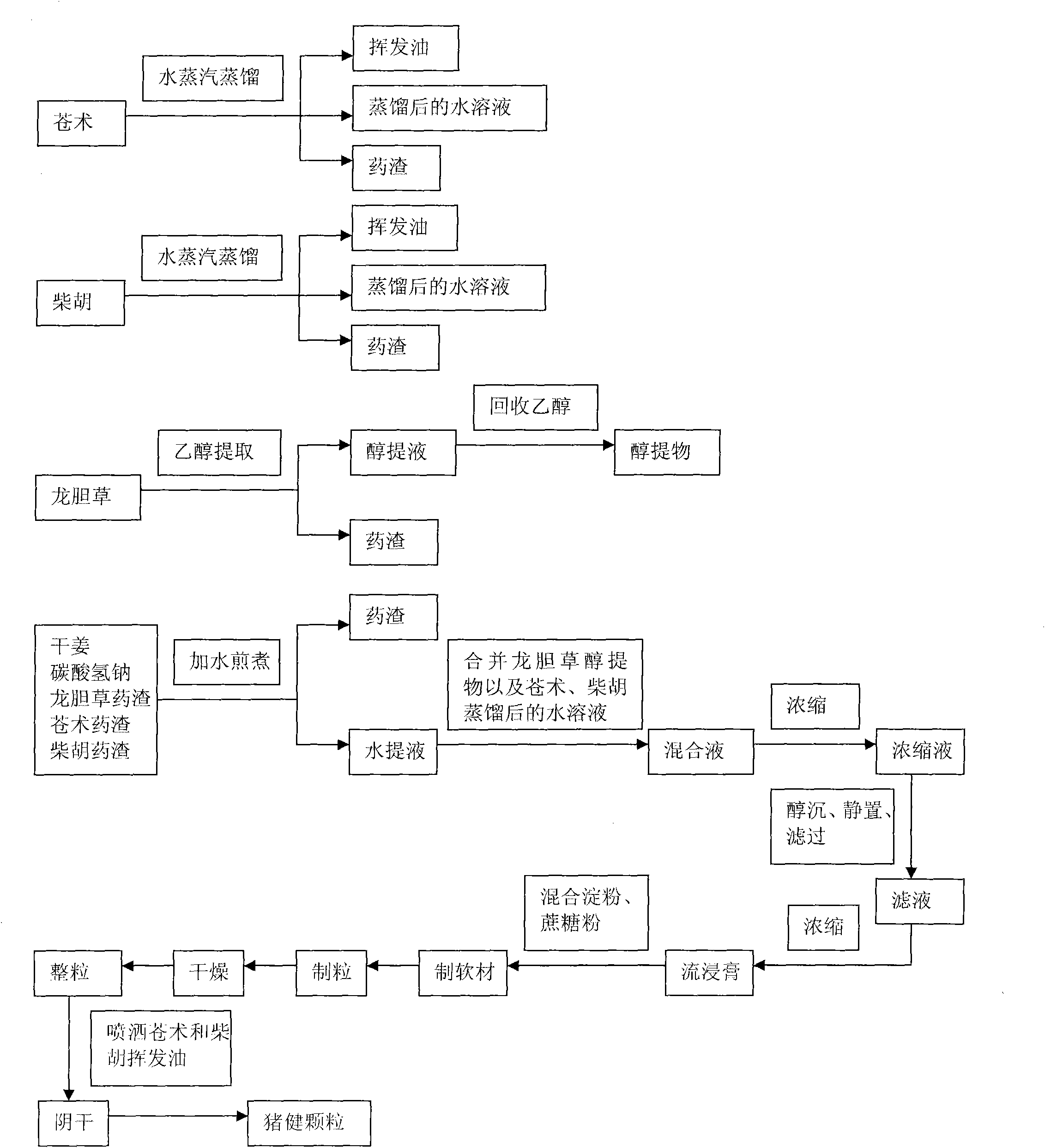
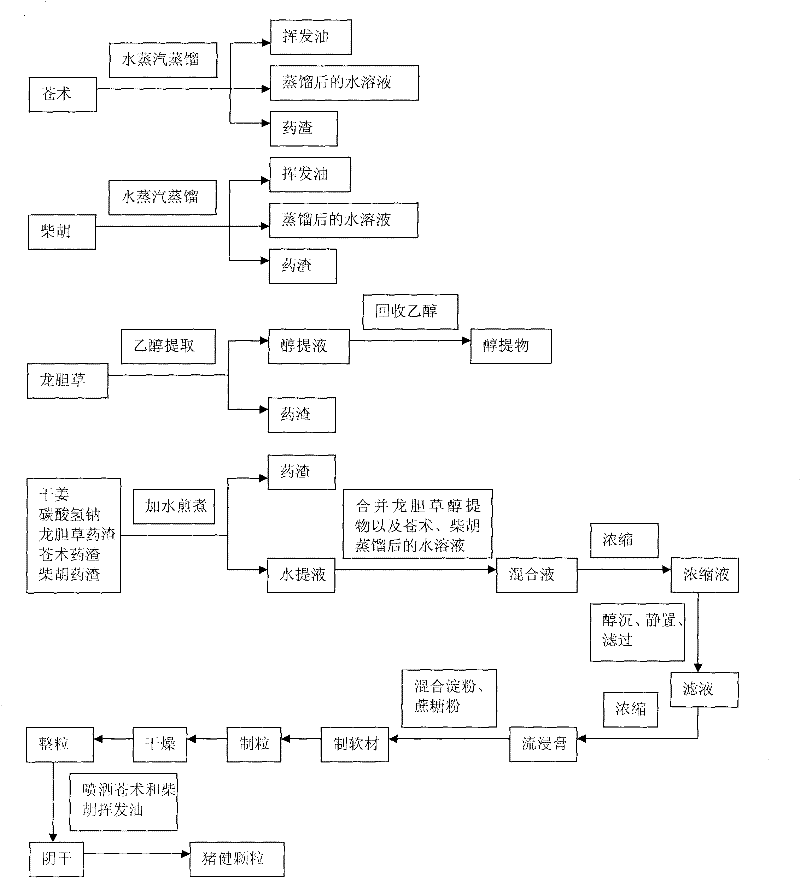
Swine health granules and preparation process thereof
ActiveCN101856489ADissolution rate is fastOrally absorbed completelyInorganic active ingredientsDigestive systemSodium bicarbonateSucrose
The invention discloses swine health granules, which are granules prepared by using gentian, atraotydin, bupleurum, dried ginger, sodium bicarbonate, starch, sucrose powder as raw materials, wherein the granules comprise no less than 2.0 percent by weight of gentiopicrin, no less than 0.05 percent by weight of atractylenolide III and no less than 11.0 percent by weight of sodium bicarbonate. A preparation process of the swine health granules comprises the following step of: extracting gentian by using ethanol; carrying out steam distillation on atraotydin and bupleurum to extract essential oil of atraotydin and bupleurum; carrying out water extraction on dried ginger, sodium bicarbonate, gentian dregs obtained by ethanol extraction and atraotydin and bupleurum dregs obtained after the essential oil is extracted; mixing water extract with ethanol extract of gentian, which is obtained after ethanol is recovered, and aqueous solution obtained after atraotydin and bupleurum are distilled; after the mixture is subjected to ethanol precipitation treatment, concentrating the obtained product into a fluid extract; uniformly mixing the fluid extract with starch and sucrose powder and preparing granules from the mixture; and finally spraying the essential oil of atraotydin and bupleurum on the surfaces of the granules to obtain the swine health granules. The swine health granules of the invention represent an improvement of the preparation form of the conventional veterinary medicament, namely the swine health powder. The effect experiment shows that the curative effect of the swine health granules is obviously superior to that of the swine health powder.
Owner:重庆新吉亨药业有限公司
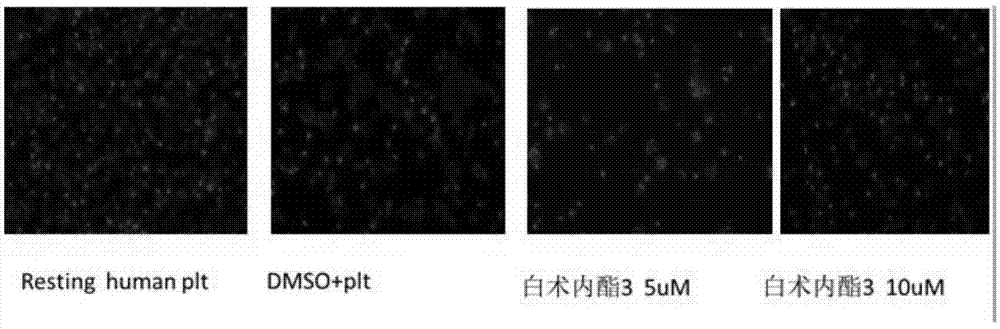
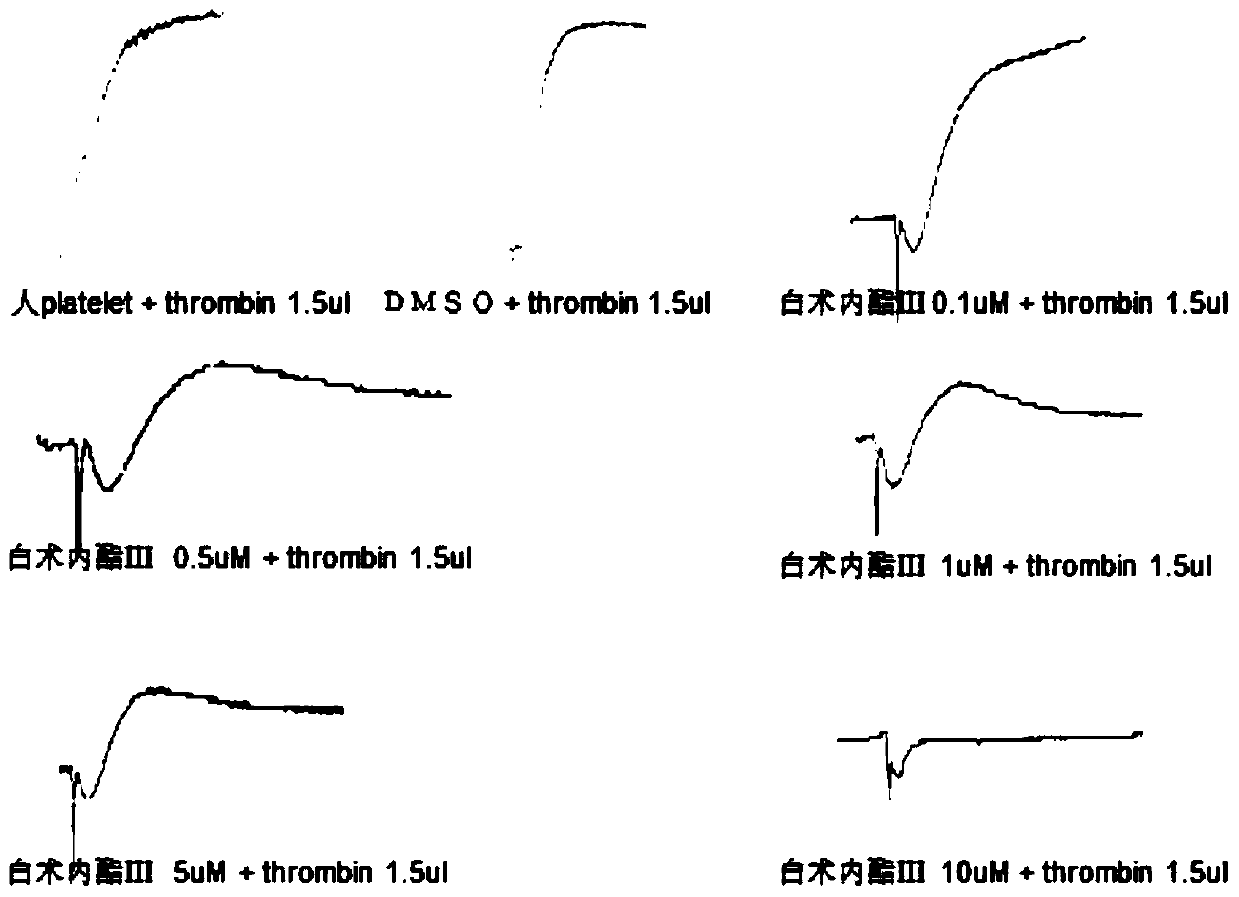
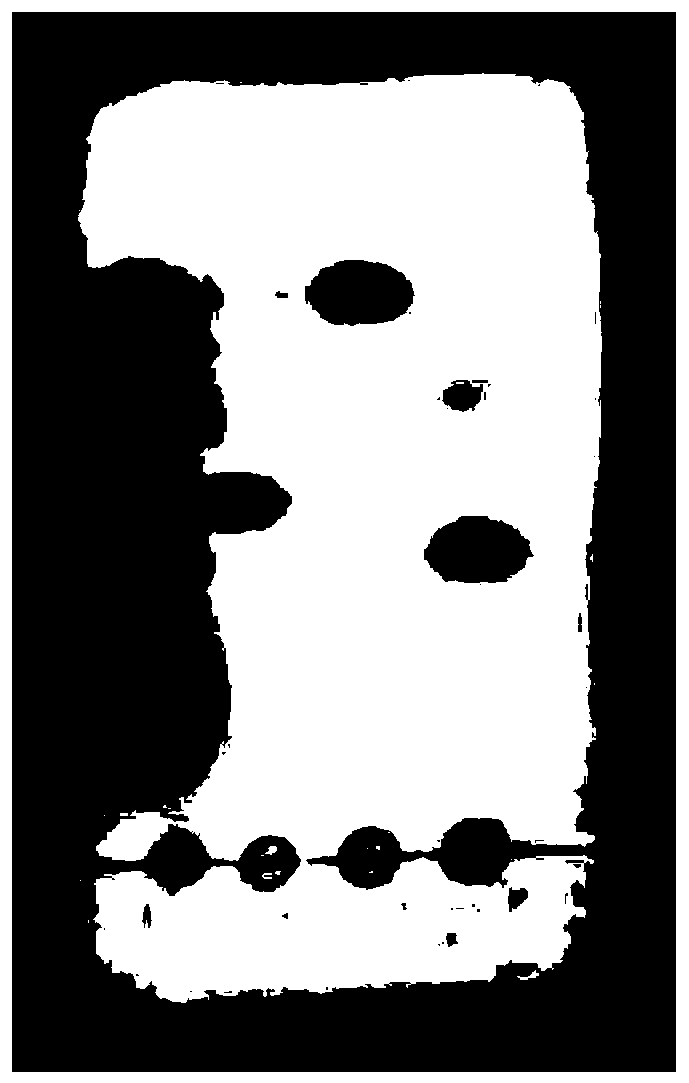
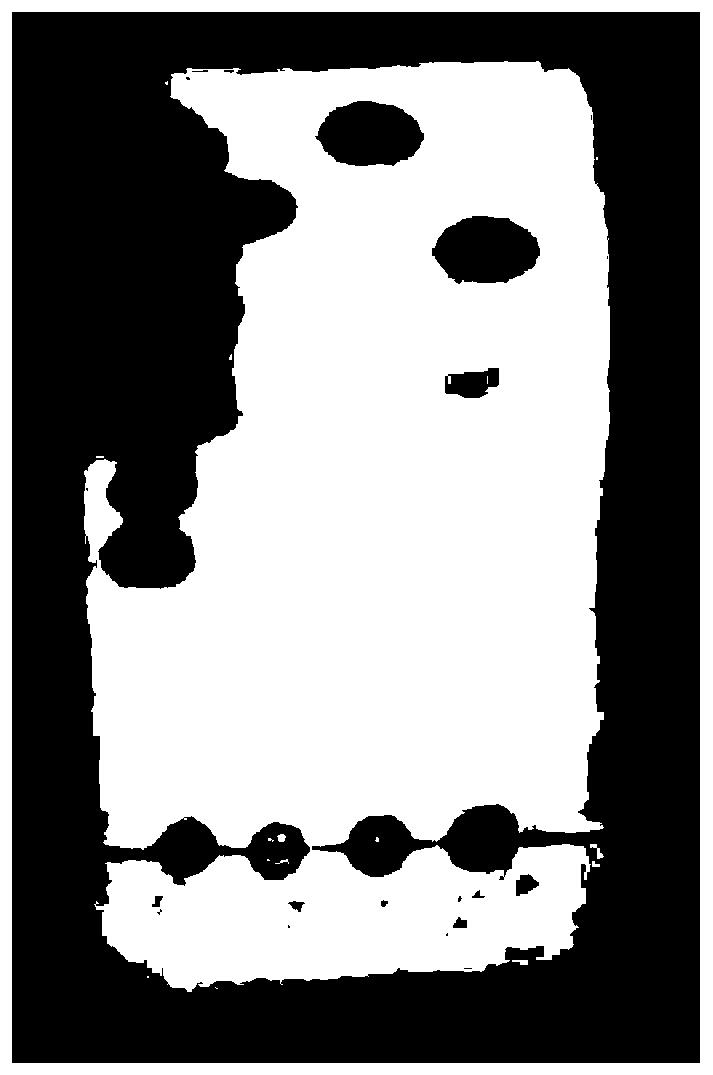
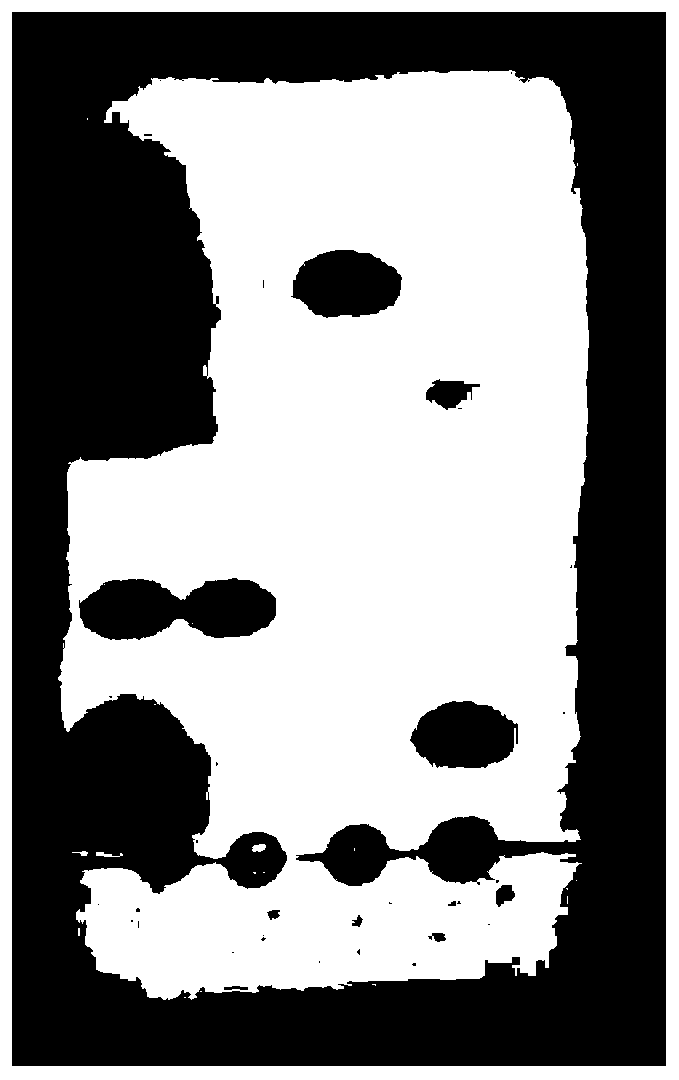
Application of atractylenolide-III derivative in preparation of platelet aggregation resisting drug and platelet aggregation resisting drug
ActiveCN104490869ASimple compositionGood curative effectOrganic active ingredientsBlood disorderAtractylenolide IIISide effect
The invention relates to a platelet aggregation resisting drug. The platelet aggregation resisting drug comprises an atractylenolide-III derivative represented by a structural formula 1 shown in specifications. The invention further relates to application of an atractylenolide-III derivative in preparation of the platelet aggregation resisting drug. The platelet aggregation resisting drug, which is simple in composition and is prepared from effective ingredients of natural medicinal raw materials or effective ingredient extracts of the medicinal raw materials, provided by the invention has a good treatment effect, is free from toxic or side effects, is not prone to the generation of tolerance, is convenient to take and is generally applicable to the problems, such as viscous blood and thrombus, caused by too-high platelet aggregation rate.
Owner:SHANGHAI NINTH PEOPLES HOSPITAL SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
Characteristic spectrum construction method and quality detection method for bighead atractylodes rhizomes
InactiveCN109580857AHigh precisionOvercoming distractionsComponent separationAtractylenolide IIAdditive ingredient
The invention relates to the field of traditional Chinese medicine detection technology, in particular to a characteristic spectrum construction method and a quality detection method for bighead atractylodes rhizomes. According to the characteristic spectrum construction method for bighead atractylodes rhizomes, a characteristic spectrum of effective ingredients, namely atractylenolide I, atractylenolide II, atractylenolide III and atractylon of bighead atractylodes rhizome medicinal materials, bighead atractylodes rhizome herbal pieces and bighead atractylodes rhizome herbal pieces stir-bakedwith bran are analyzed through efficient liquid chromatographic analysis, chromatographic peaks of the characteristic spectrum are completely displayed, uniformity is good, the characteristic chromatographic peaks are completely separated, the quantity of impurities is small, a contrast characteristic spectrum is established according to the characteristic spectrum, and meanwhile the content of atractylenolide I and the content of atractylenolide III are determined. In combination with thin-layer chromatography identification and heavy metal and harmful element determination, the comprehensive quality detection method for bighead atractylodes rhizomes with effectiveness and safety is provided, so that quality detection on bighead atractylodes rhizomes, processed products of bighead atractylodes rhizomes and relevant products is realized. Moreover, the quality detection method has the advantages of being simple, fast, stable, reliable, high in precision, good in reproducibility, easy to grasp and the like.
Owner:BEIJING ZHONGYAN TONGRENTANG CHINESE MEDICINE R & D
Method for synchronously and rapidly detecting nine functional active components in Yedao deer and turtle wine
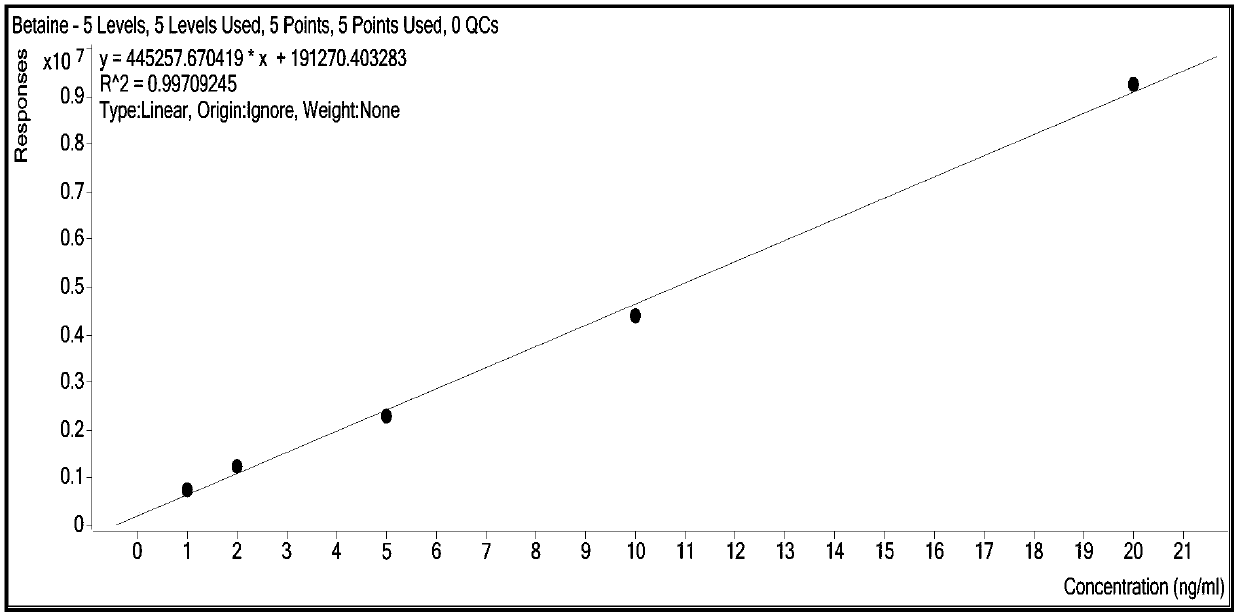
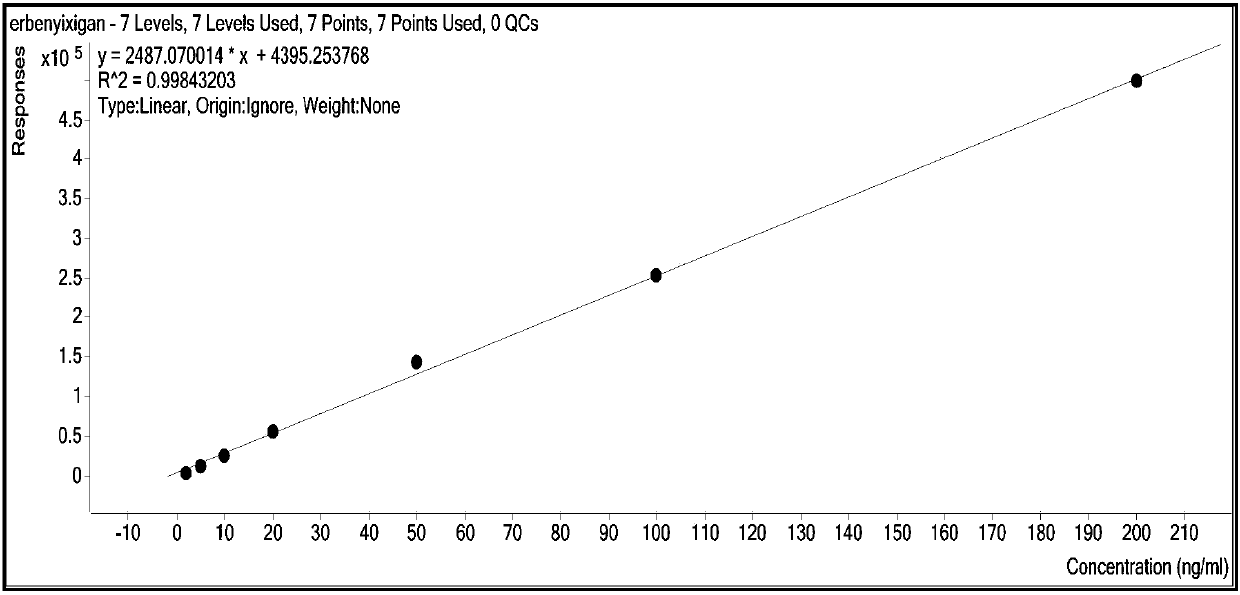
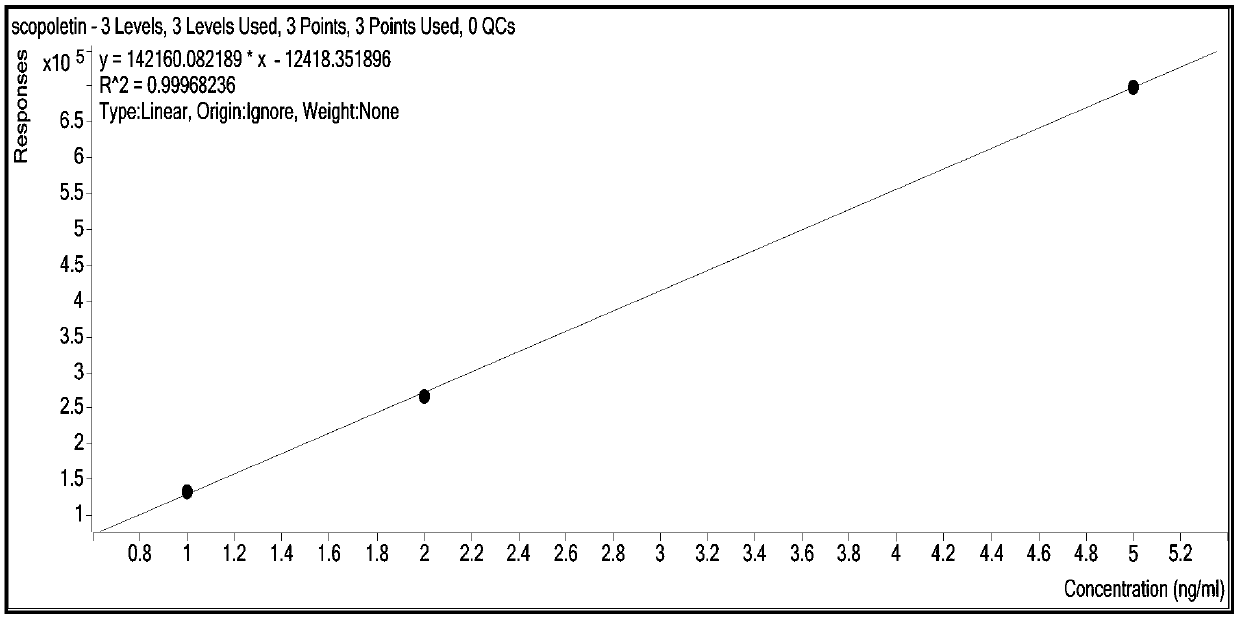
The invention relates to a method for synchronously and rapidly detecting nine functional active components in Yedao deer and turtle wine and belongs to the field of researches of functional components of health-care wine. The method comprises the following steps: carrying out qualitative analysis on the active components in the Yedao deer and turtle wine by adopting a liquid chromatography-mass spectrometry method through a positive ion scanning working mode; then carrying out quantitative analysis by adopting an external standard method. The method provided by the invention has the advantages of simple pre-treatment steps, high precision, low quantification limit and good stability and can be used for rapidly determining the content of Chinese yam sapogenin, 2,3,5,4'-tetrahydroxy stilbene-2-O-beta-D-glucoside, betaine, ligustilide, scopoletin, atractylenolide III, emodin, emodin methyl ether and codonopsis pilosula alkyne glucoside in a Yedao deer and turtle wine sample; the method can be used for synchronously and rapidly determining the components in the Yedao deer and turtle wine, a Chinese herbal medicine mixed extracting solution of a similar formula or prepared wine.
Owner:海南椰岛酒业发展有限公司
Application of atractylenolide in preparation of antidepressant drug
ActiveCN105582006AGood protective activityInhibit apoptosisOrganic active ingredientsNervous disorderAtractylenolide IIIAtractylenolide I
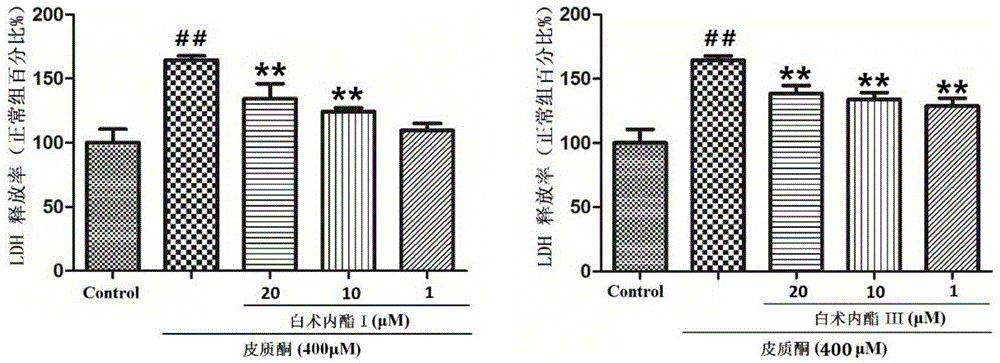
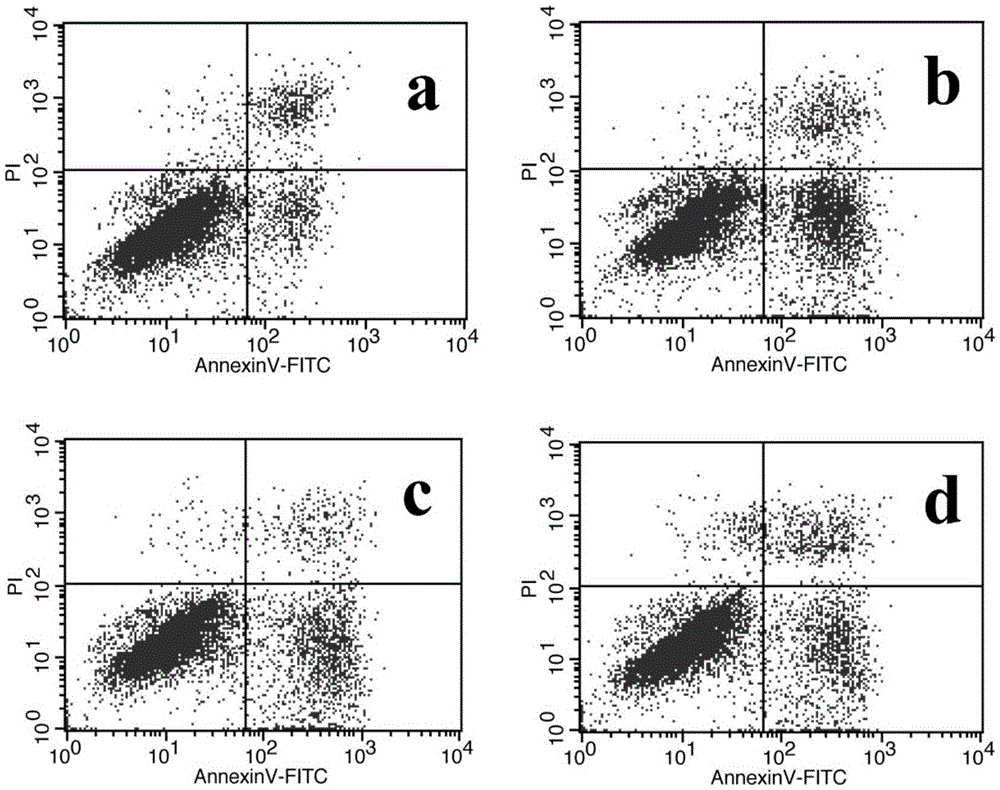
The invention provides application of atractylenolide in preparation of an antidepressant drug, and relates to the field of medicine. In-vitro activity tests prove that atractylenolide I and atractylenolide III have the better protection activity on corticosterone-damaged PC12 cells, and the mechanism is related to inhibition of neuron apoptosis; in-vivo test results show that atractylenolide I and atractylenolide III can significantly shorten the immobility time of a mouse forced swimming test and a mouse tail suspension test. Experiments prove that atractylenolide I and atractylenolide III have the significant antidepressant effect and can be used for preparing the drug for preventing and treating a depressive disorder.
Owner:SHANXI UNIV
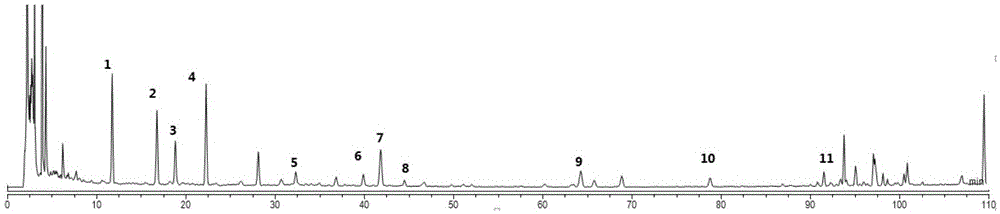
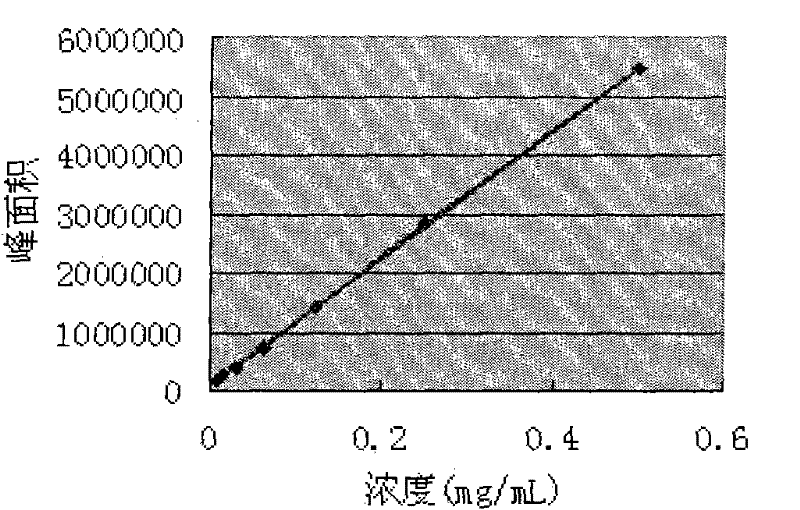
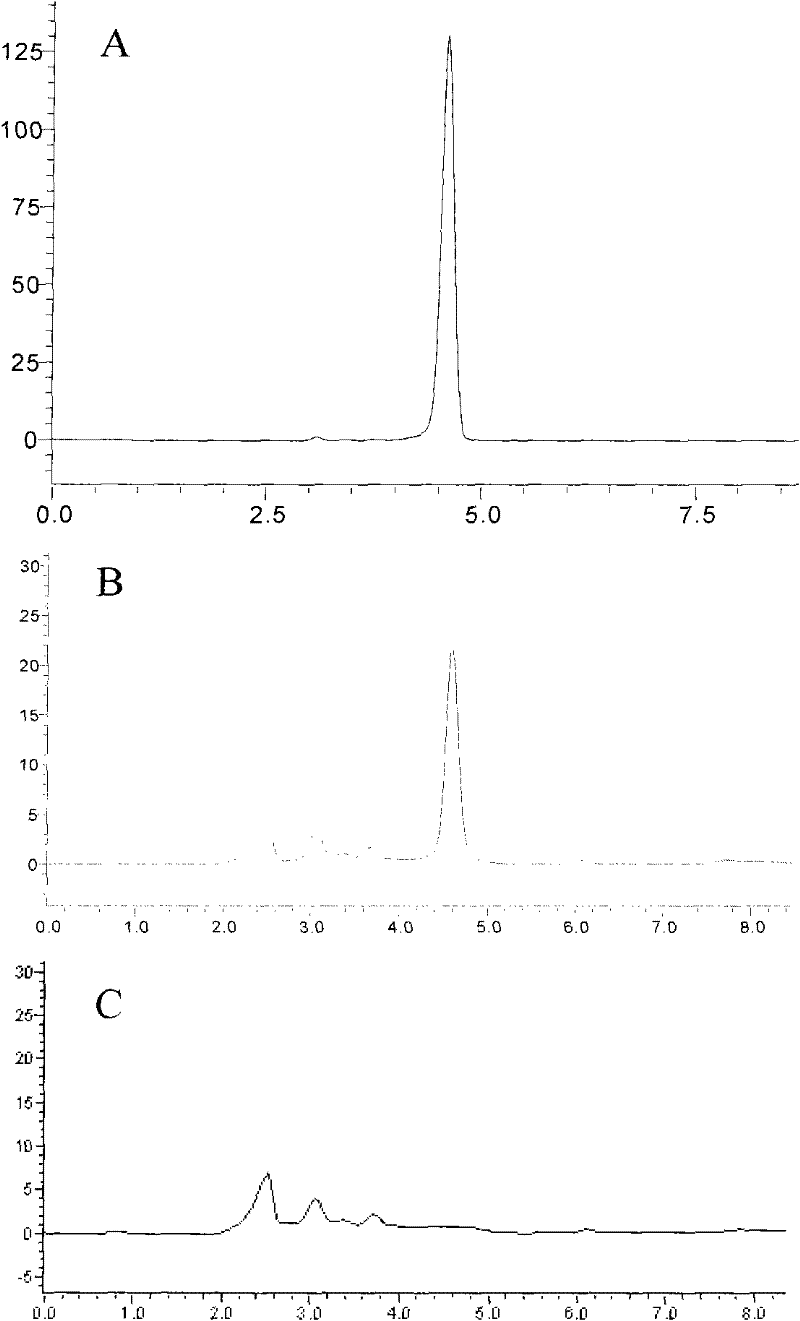
Method for disposable measuring bighead atractylodes rhizome inner ester and atractylone with HPLC microtubule method disposable
InactiveCN101113975AEasy to operateReduce mistakesComponent separationSpecial data processing applicationsAtractylenolide IIIMicrotubule
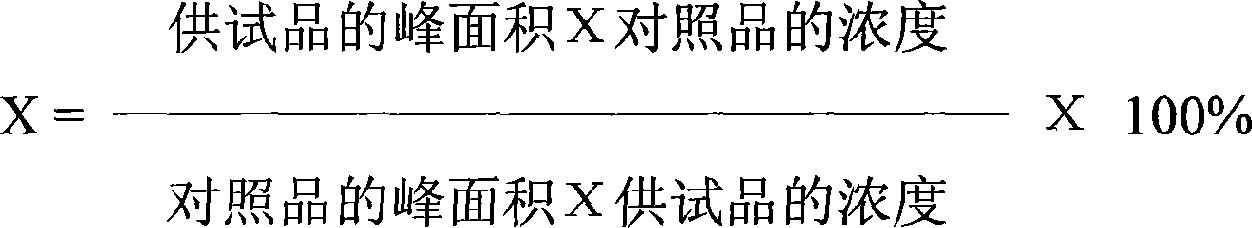
The invention discloses a method of single time measurement of atractylenolide and atractylon. The procedure includes preparing mobile phase resolution A and B made of chromatographic pure methanol, acetonitrile and redistilled water, preparing the mixed control solution made of atractylenolide I, atractylenolide III and atractylon, preparing sample solution, testing the three absorption peaks of control solution and samples at three different time respectively by chromatograph of liquid according to the setting gradient of mobile phase A and B and the time, and calculating the content of atractylenolide I, atractylenolide III and atractylon according to the formula X=(peak area of sample is multiplied by density of control then is divided by peak area of control and is multiplied by density of sample) is multiplied by 100 percent. The invention can determine the content of atractylenolide I, atractylenolide III and atractylon in atractylodes macrocephala by only one sample injection. Thus the operation is simplified, the error of multiple sample injection is reduced and analysis of the constituent ration of the three ingredients is convenient. The invention is of important academic value and meaningful in practical application.
Owner:ZHEJIANG ACAD OF TRADITIONAL CHINESE MEDICINE
UPLC method of detecting atractylenolide I and atractylenolide III in atractylodes medicine
InactiveCN110398560AMeet the requirements of quality evaluationGood precisionComponent separationUltrasound deviceAtractylenolide III
The invention provides a UPLC method of detecting atractylenolide I and atractylenolide III in atractylodes medicine. The method comprises the following steps: S1, preparation of to-be-measured atractylodes, wherein firstly, the atractylodes is comminuted, and then placed into a triangular flask, methanol is added, and then current solution is placed into an ultrasound device for ultrasound and centrifugation processing, and finally, filtration is carried out; S2, measurement and determination of a standard atractylodes sample, wherein standard atractylodes sample solution is injected into ultra performance liquid chromatography for measurement and determination to obtain a UPLC feature graph of the standard atractylodes sample; and S3, measurement and determination of a to-be-measured atractylodes sample, wherein the measurement and determination solution of the to-be-measured atractylodes sample in the step S1 is injected into the ultra performance liquid chromatography for measurement and determination to obtain a UPLC feature graph of the to-be-measured atractylodes sample, and the to-be-measured atractylodes sample obtained by preparation is quantified through the UPLC featuregraph which is of the standard atractylodes sample and is obtained in the step S2 and the UPLC feature graph of the to-be-measured atractylodes sample. The method provided by the invention can quickly complete analysis testing of the atractylodes sample, and is better in sample precision and recovery of standard addition.
Owner:INST OF AGRI PROD QUALITY SAFETY & STANDARD JIANGXI ACAD OF AGRI SCI
Quality control method of substance reference of Linggui curcuma zedoary decoction
PendingCN112903867AComprehensive detection effectComprehensive evaluationComponent separationClinical efficacyGradient elution
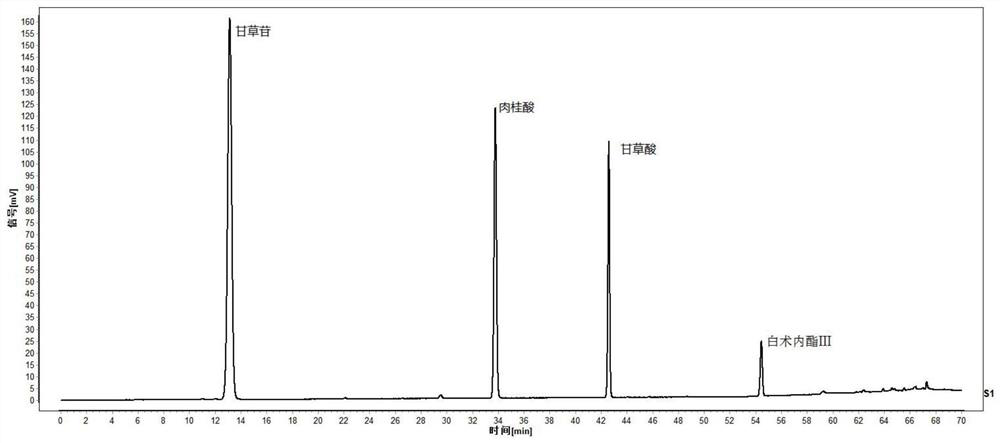
The invention relates to a quality control method of a substance reference of a Linggui curcuma zedoary decoction. The method comprises an HPLC content determination method for simultaneously determining four components of liquiritin, cinnamic acid, ammonium glycyrrhizinate and atractylenolide III of the substance reference of the Linggui curcuma zedoary decoction and an HPLC characteristic chromatogram of the substance reference of the Linggui curcuma zedoary decoction. According to the structural characteristics of the active ingredients contained in the Linggui curcuma zedoary decoction, the optimal mobile phase composition, gradient elution procedure, flow velocity, detection wavelength, chromatographic column, column temperature and other analysis conditions are screened out through a large number of experiments, the method for detecting the quality of the Linggui curcuma zedoary decoction can comprehensively, objectively and accurately detect and evaluate the quality of the Linggui curcuma zedoary decoction material reference and the preparation thereof, has important significance for ensuring the clinical curative effect of the Linggui curcuma zedoary decoction material reference, and provides a reliable quality attribute research method and evaluation system for the classical famous prescription Linggui curcuma zedoary decoction material reference and the preparation of the classical famous prescription Linggui curcuma zedoary decoction material reference.
Owner:ZHONGJING WANXI PHARMA CO LTD
Quality detection method of Shouhui bowel-relaxing capsule
ActiveCN109507356AQuality assuranceComprehensive evaluationComponent separationAtractylenolide IIIAtractylenolide I
The invention discloses a quality detection method of a Shouhui bowel-relaxing capsule and belongs to the field of analysis of traditional Chinese medicinal preparations. The quality detection methodadopts a thin-layer chromatography to identify bighead atractylodes rhizome in the capsule and simultaneously adopts an HPLC to determine the contents of atractylenolide I, atractylenolide II and atractylenolide III in the capsule. The quality detection method disclosed by the invention is stable and reliable, strong in specificity, good in reproducibility and capable of fully and effectively controlling the quality of the euphorbia pulcherrima bowel-relaxing capsule, ensuring the safety and the effectiveness of clinical medication and better meeting the needs of patients and the market.
Owner:LUNAN PHARMA GROUP CORPORATION
Quality evaluation method for Chinese herbal compound aconite lizhong decoction for treating gastric ulcer
ActiveCN109900847AQuick evaluationRapid determinationComponent separationAtractylenolide IIIChemical composition
The invention provides a quality evaluation method for Chinese herbal compound aconite lizhong decoction for treating gastric ulcer. The method defines content limits of 10 chemical components of benzoylaconitine, benzoylmesaconine, atractylenolide I, atractylenolide II, atractylenolide III, lobetyolin, liquiritin, liquiritigenin, 8-gingerol and 10-gingerol in the Chinese herbal compound aconite lizhong decoction; the content is determined through a liquid chromatography-mass spectrometry method; and meanwhile, the 10 chemical components are detected simultaneously. The method is used for evaluating the quality of the Chinese herbal compound aconite lizhong decoction for treating gastric ulcer. The content of the 10 chemical components in the Chinese herbal compound aconite lizhong decoction is detected through the liquid chromatography-mass spectrometry method, so that the 10 chemical components can be detected simultaneously and quantified through one injection; and the method is fast in determination, efficient, high in flexibility and simple and feasible in detection, and can quickly evaluate the quality of the Chinese herbal compound aconite lizhong decoction.
Owner:QIQIHAR UNIVERSITY
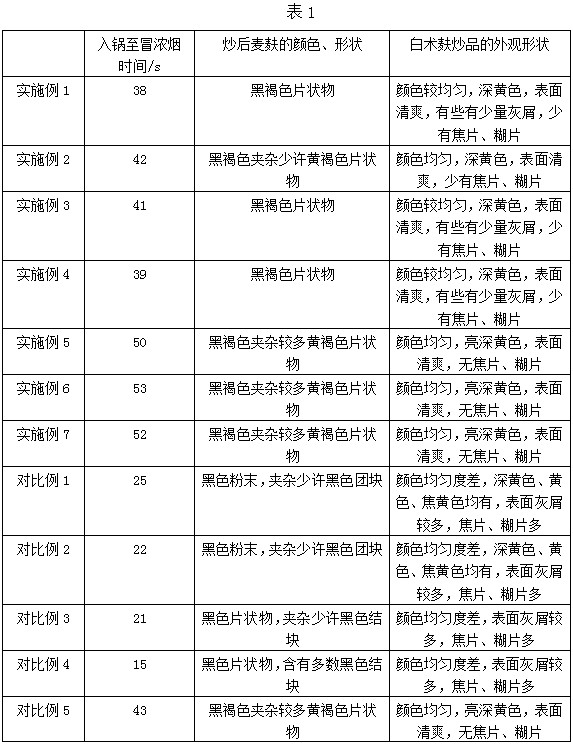
Processing technology of bran stir-fried rhizoma atractylodis macrocephalae decoction pieces
InactiveCN111700925AImprove appearance shapeUniform colorDigestive systemUnknown materialsAtractylenolide IIIAtractylenolide I
The invention discloses a processing technology of bran stir-fried rhizoma atractylodis macrocephalae decoction pieces and belongs to the technical field of preparation of traditional Chinese medicinedecoction pieces. The processing technology comprises the following steps: selection; cleaning, soaking and moistening: after cleaning mud on the surface of the rhizoma atractylodis macrocephalae, soaking the rhizoma atractylodis macrocephalae in drinking water at 30-50 DEG C, taking out and moistening the rhizoma atractylodis macrocephalae at 50-60 DEG C; cutting, drying; stir-frying: placing bran in a hot pan at 200-210 DEG C, after the bran smokes, adding the dried rhizoma atractylodis macrocephalae slices, stir-frying the rhizoma atractylodis macrocephalae slices for 25-3o min, and takingout of the rhizoma atractylodis macrocephalae slices until the surface of the rhizoma atractylodis macrocephalae slices turn yellowish brown and aroma overflows, wherein the particle size of the bran is larger than or equal to 40 meshes, the moisture conent is smaller than or equal to 10%, and the total ash of the bran is smaller than or equal to 6%; screening; and packaging. The prepared rhizoma atractylodis macrocephalae decoction pieces are uniform in color, have refreshing surfaces, have little ash and seldomly have burnt pieces and scorched pieces, have good appearance shapes and conform to the quality standard; and the content of atractylenolide I, atractylenolide II and atractylenolide III in rhizoma atractylodis macrocephalae decoction pieces is significantly increased, and the spleen and stomach strengthening functions of the rhizoma atractylodis macrocephalae decoction pieces are enhanced.
Owner:北京杏林药业有限责任公司
Petroleum ether extract of traditional Chinese medicine for preventing and treating glucose and lipid metabolic disturbance and preparation method thereof
ActiveCN102091083BHigh content of active ingredientsTo promote metabolismHydroxy compound active ingredientsMetabolism disorderSalvianolic acid BAklanonic acid
The petroleum ether extract of traditional Chinese medicine for the prevention and treatment of sugar and lipid metabolism disorders, wherein the active ingredients of the extract are composed of 1-cerotol, ß-sitosterol, n-hexacosanoic acid, atractylenolide III, oleanolic acid, berberine, jateorrhizine, salvianolic acid B, cyclotetracosane, 9,12-octadecadienoic acid, 5,7-dimethoxycoumarin, ginsenoside Rb1. The preparative method comprises extracting crude drug Radix Salviae Miltiorrhizae, Fructus Ligustri Lucidi, Rhizoma Coptidis, Herba Cirsii Japonici, Cortex Eucommiae, Rhizoma Atractylodis Macrocephalae, Radix Notoginseng and Fructus Citri Sarcodactylis with C1-3 alcohol and / or water, combining total extracts, extracting the total extracts with petroleum ether to obtain the petroleum ether extract.
Owner:QINGDAO BAILI CAIXIN MEDICAL TECH CO LTD
Application of atractylenolide III in preparation of anti-hepatic fibrosis drugs
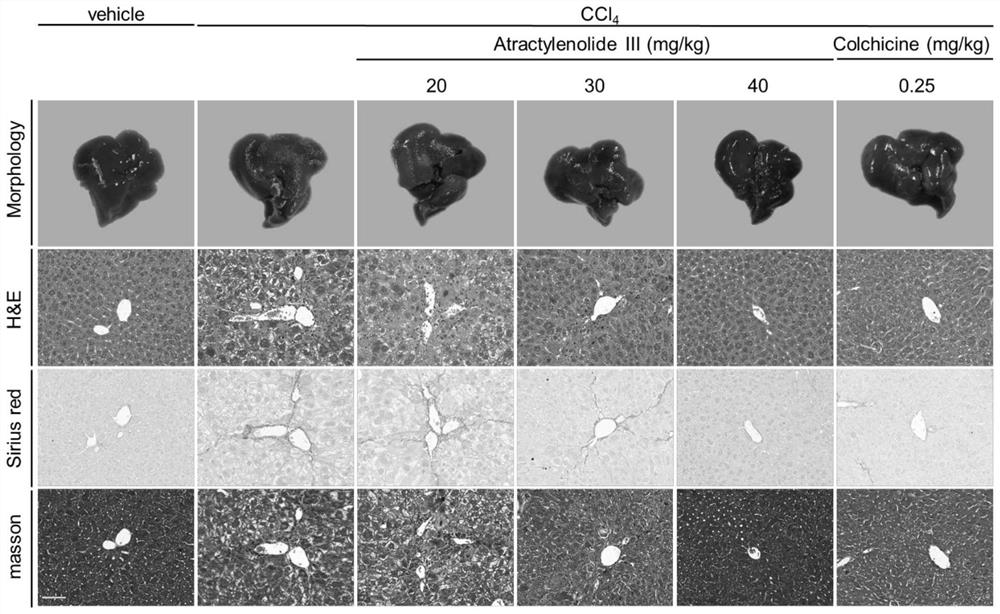
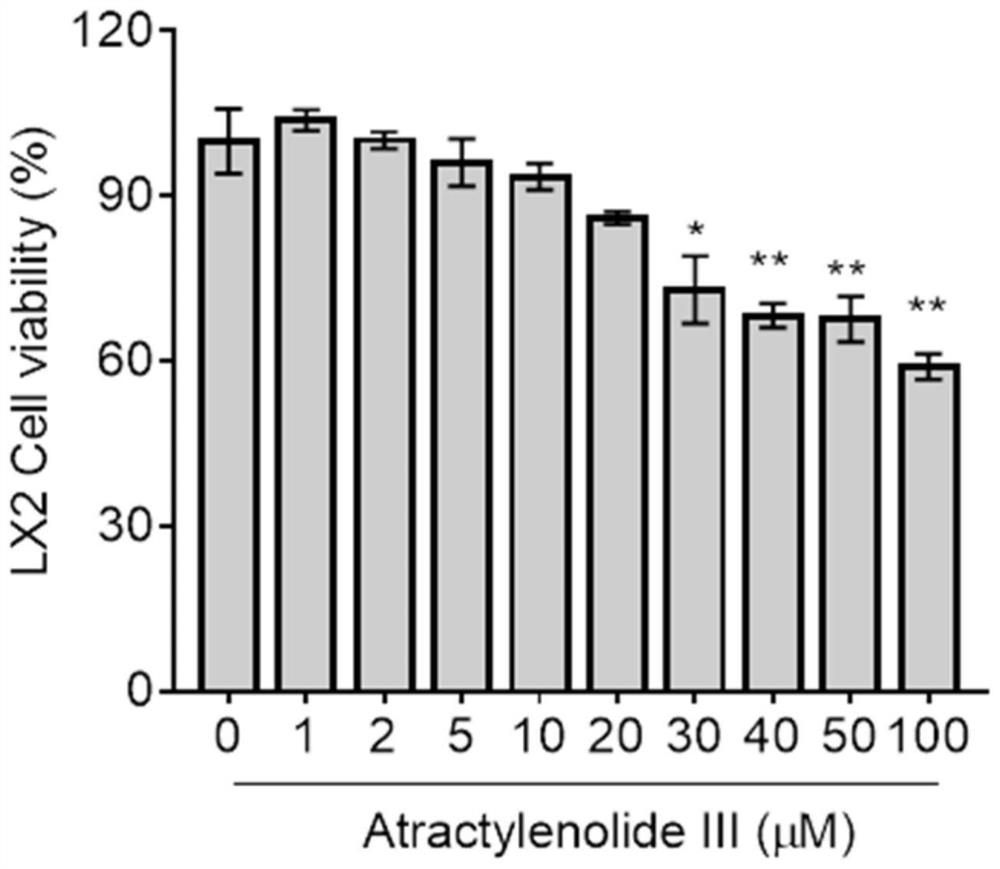
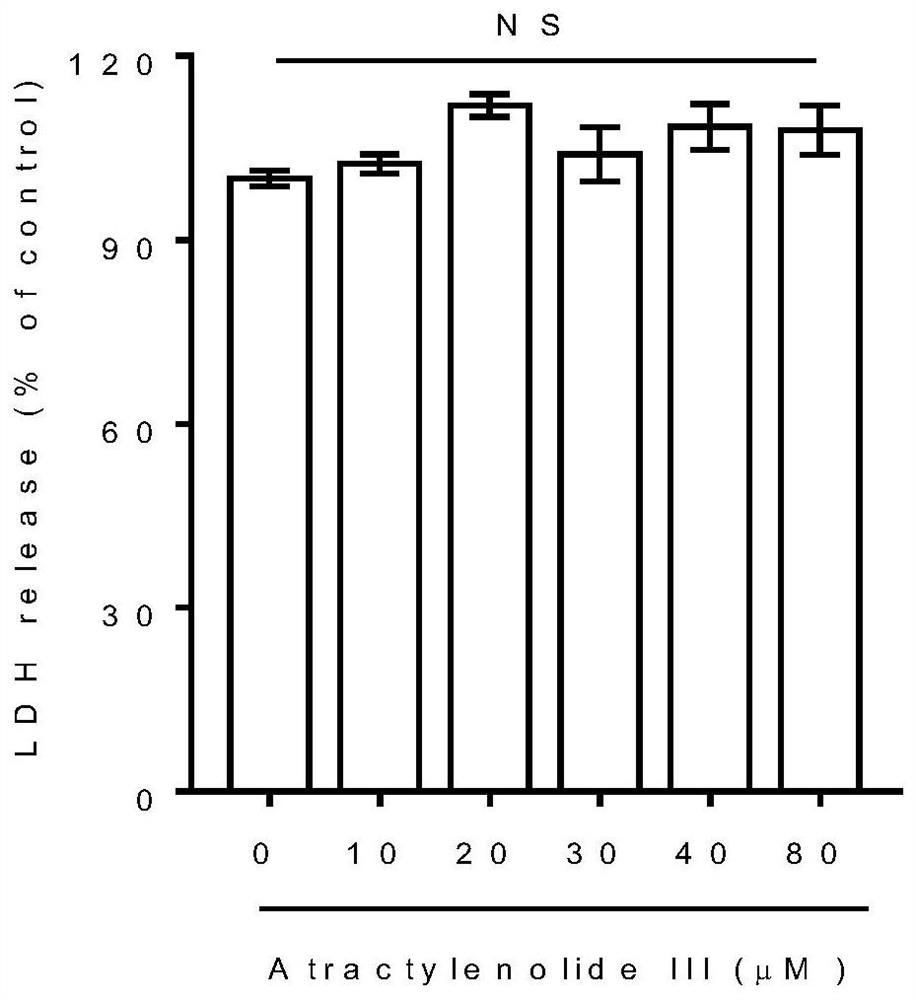
PendingCN113797194AGood anti-hepatic fibrosis activityOrganic active ingredientsDigestive systemAtractylenolide IIIAtractylenolide I
The invention particularly relates to an application of atractylenolide III in preparation of an anti-hepatic fibrosis drug. The application of the atractylenolide III in preparation of the anti-hepatic fibrosis drug researches find that the atractylenolide III reduces the contents of ALT, AST and ALP in serum of a mouse with hepatic fibrosis, reduces the contents of fibrosis indexes HA, LN and PCIII in the serum of the mouse with hepatic fibrosis, can inhibit expression of HSC activation indexes alpha-SMA and COL1 alpha 1 in a dose-dependent manner. Therefore, the atractylenolide III can be used for preparing the anti-hepatic fibrosis drug.
Owner:NANJING UNIVERSITY OF TRADITIONAL CHINESE MEDICINE
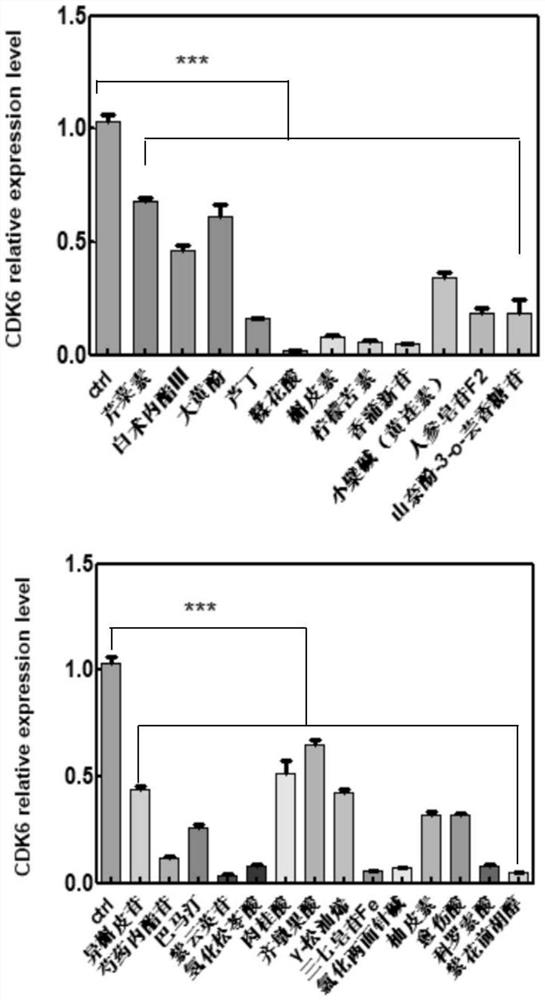
Application of composition to preparation of medicine for treating gastric cancer based on regulation and control of cancer suppression related genes
PendingCN112716935APromote apoptosisClearly targetedOrganic active ingredientsDigestive systemApigetrinOncology
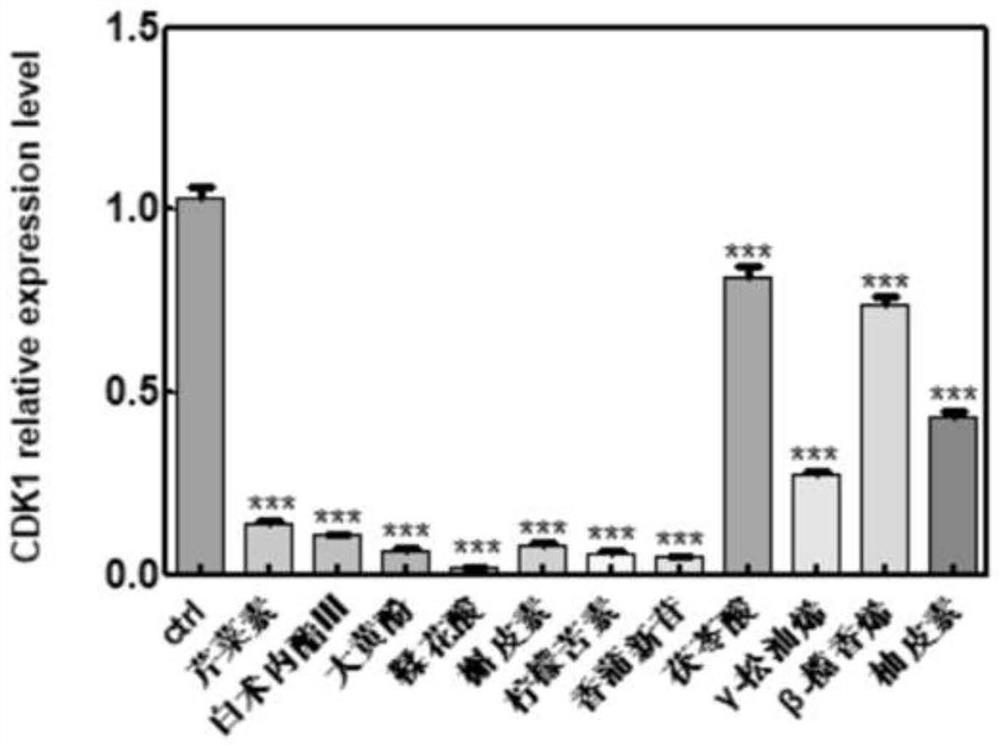
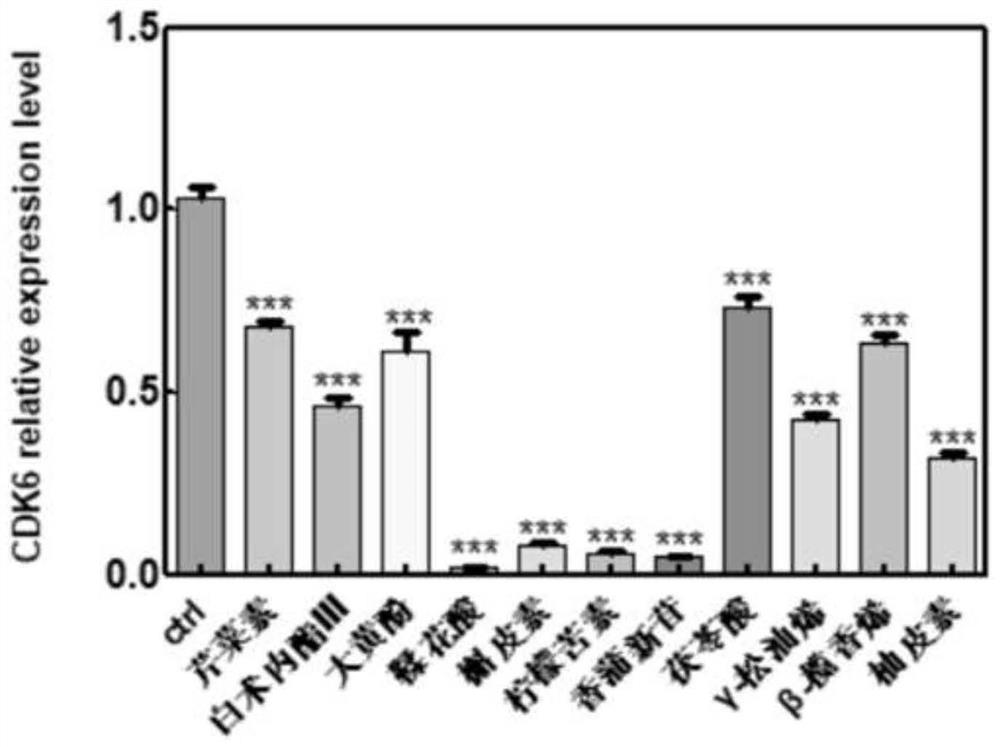
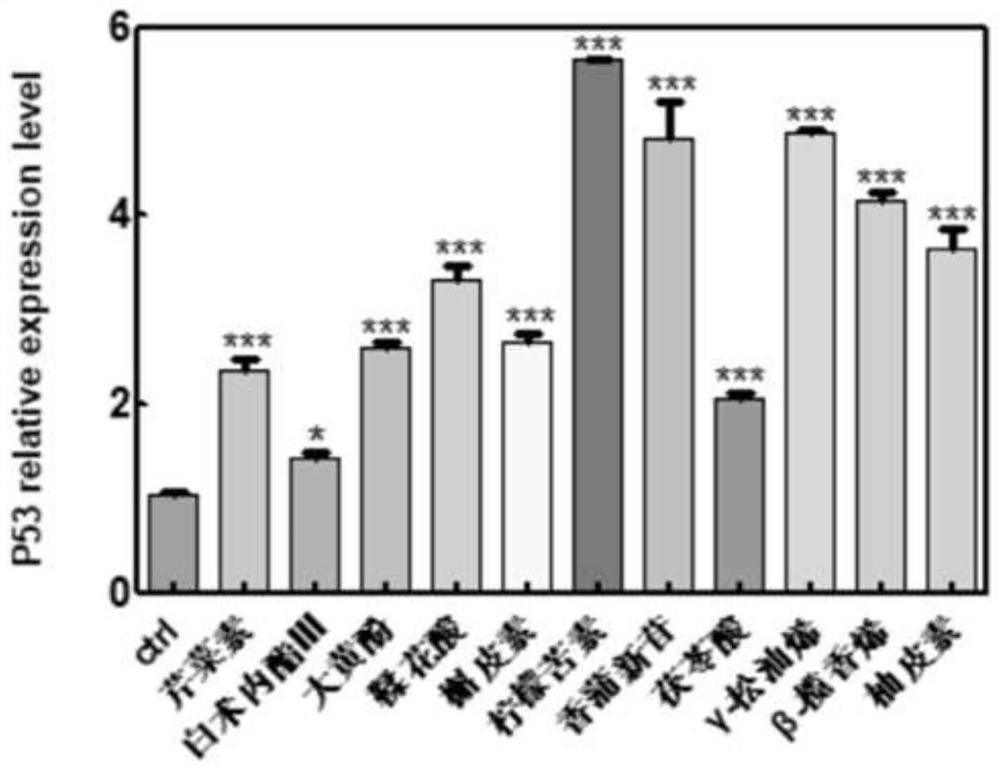
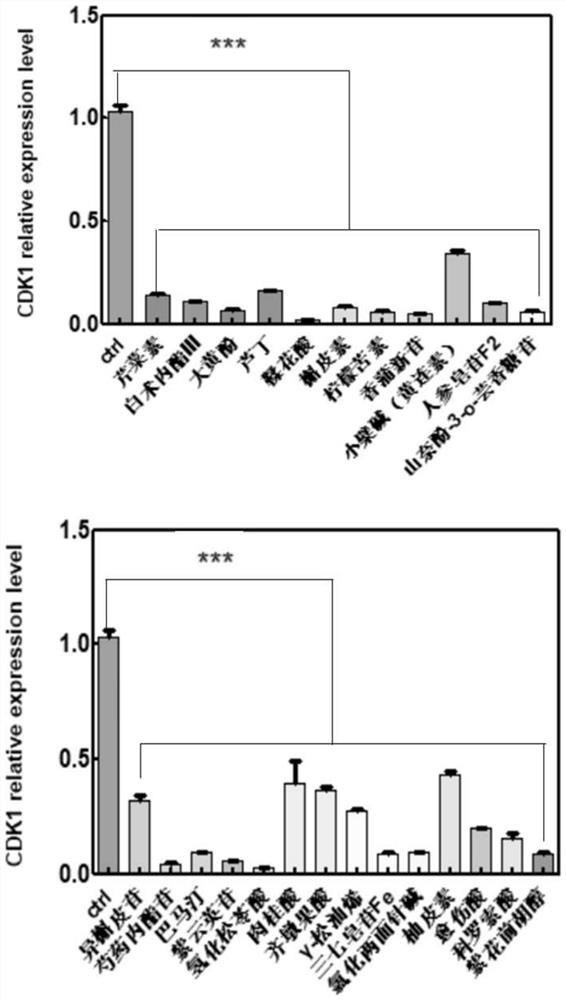
The invention belongs to the technical field of pharmaceutical preparations, particularly relates to an application of a composition to preparation of a medicine for treating gastric cancer based on regulation and control of cancer suppression related genes, and more particularly relates to application of the medicine for treating gastric cancer based on regulation and control of CDK1, CDK6, P53 and / or TGF-beta cancer suppression genes. On the basis of fingerprint spectrum analysis of a Chinese patent medicine Morhodamine, a plurality of medicine components in Morhodamine and target genes capable of being regulated and controlled by the medicine components are preliminarily determined; cancer inhibition related genes such as CDK1, CDK6, P53, TGF-beta and the like are selected as target genes for direct or indirect regulation and control of the morhodamine, and effective substances with a gastric cancer inhibition function in the morhodamine are verified. effective components including apigenin, atractylenolide III, chrysophanol, ellagic acid, quercetin, limonin, typhagoside, pachymic acid, gamma-terpinene, beta-elemene or naringenin are screened out, and theoretical support is provided for development of new drugs for treating gastric cancer.
Owner:HANDAN PHARMA
Determination method of various components in Yupingfeng preparation
ActiveCN105021751BQuality improvementImprove accuracyComponent separationAtractylenolide IIFiltration
The invention relates to a content determination method for multiple components in a Yupingfeng preparation. The method comprises the steps of: (1) taking the Yupingfeng preparation, crushing or not crushing it, then performing precise weighing, adding methanol to conduct reflux extraction for 1-3h, carrying out filtration and concentration, then adding methanol to a constant volume, thus obtaining a test solution; (2) precisely weighing the reference substance prim-o-glucosylcimifugin, calycosin-7-glucoside, cimifugin, 5-O-methylvisammioside, sec-o-glucosylhamaudol, calycosin, formononetin, atractylenolide III, atractylenolide I and atractylenolide II respectively, and adding methanol to perform dissolving to a constant volume, thus obtaining a mixed reference solution; (3) conducting determination: precisely sucking the test solution and the mixed reference solution respectively, injecting them into a high performance liquid chromatograph, carrying out gradient elution under certain mobile phase condition, and performing multi-wavelength simultaneous monitoring, thus obtaining the content. The method provided by the invention can accurately determine the content of 10 components in the Yupingeng preparation, and can objectively, comprehensively and sensitively reflect the quality condition of the Yupingfeng preparation.
Owner:GUANGZHOU XIANGXUE PHARMA CO LTD
Method for disposable measuring bighead atractylodes rhizome inner ester and atractylone with HPLC microtubule method
InactiveCN100582773CEasy to operateReduce mistakesComponent separationSpecial data processing applicationsMicrotubuleAtractylenolide III
Owner:ZHEJIANG ACAD OF TRADITIONAL CHINESE MEDICINE
Swine health granules and preparation process thereof
ActiveCN101856489BDissolution rate is fastOrally absorbed completelyInorganic active ingredientsDigestive systemSodium bicarbonateSucrose
The invention discloses swine health granules, which are granules prepared by using gentian, atraotydin, bupleurum, dried ginger, sodium bicarbonate, starch, sucrose powder as raw materials, wherein the granules comprise no less than 2.0 percent by weight of gentiopicrin, no less than 0.05 percent by weight of atractylenolide III and no less than 11.0 percent by weight of sodium bicarbonate. A preparation process of the swine health granules comprises the following step of: extracting gentian by using ethanol; carrying out steam distillation on atraotydin and bupleurum to extract essential oil of atraotydin and bupleurum; carrying out water extraction on dried ginger, sodium bicarbonate, gentian dregs obtained by ethanol extraction and atraotydin and bupleurum dregs obtained after the essential oil is extracted; mixing water extract with ethanol extract of gentian, which is obtained after ethanol is recovered, and aqueous solution obtained after atraotydin and bupleurum are distilled;after the mixture is subjected to ethanol precipitation treatment, concentrating the obtained product into a fluid extract; uniformly mixing the fluid extract with starch and sucrose powder and preparing granules from the mixture; and finally spraying the essential oil of atraotydin and bupleurum on the surfaces of the granules to obtain the swine health granules. The swine health granules of theinvention represent an improvement of the preparation form of the conventional veterinary medicament, namely the swine health powder. The effect experiment shows that the curative effect of the swinehealth granules is obviously superior to that of the swine health powder.
Owner:重庆新吉亨药业有限公司
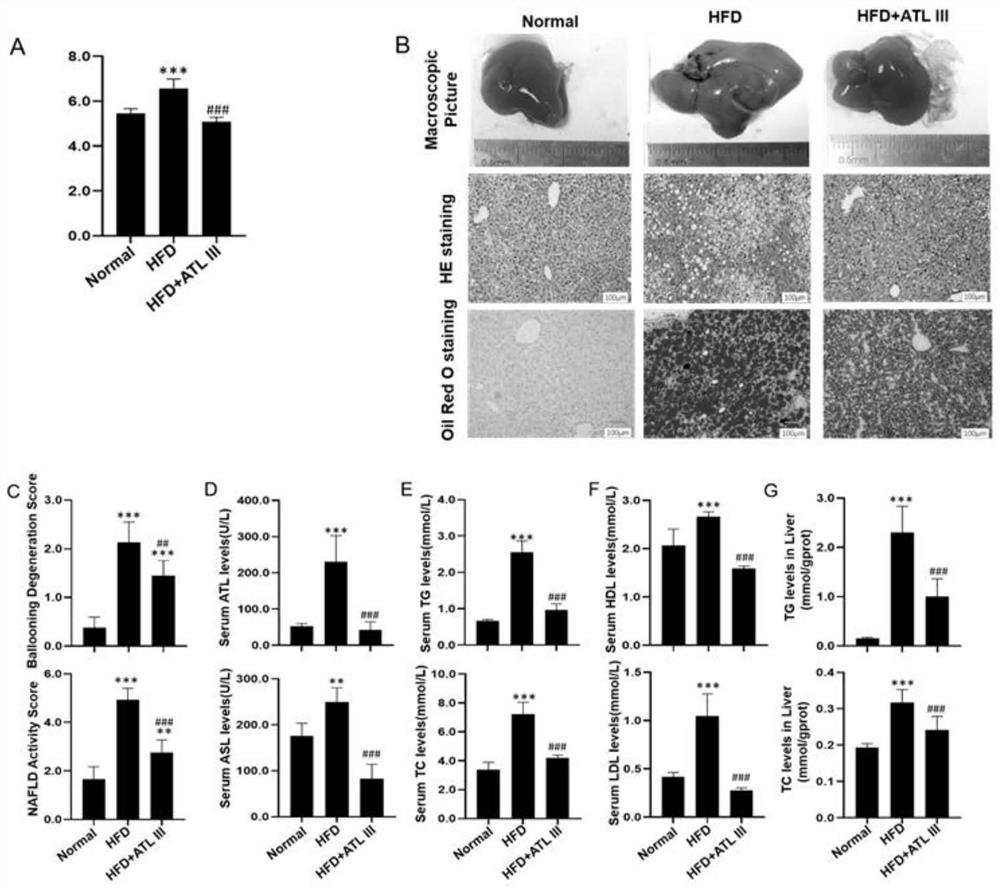
Application of atractylenolide III in preparation of medicine for treating non-alcoholic fatty liver disease
InactiveCN113577057ALose weightReduce Lipid DepositionOrganic active ingredientsCompound screeningSignalling moleculesSignal Pathways
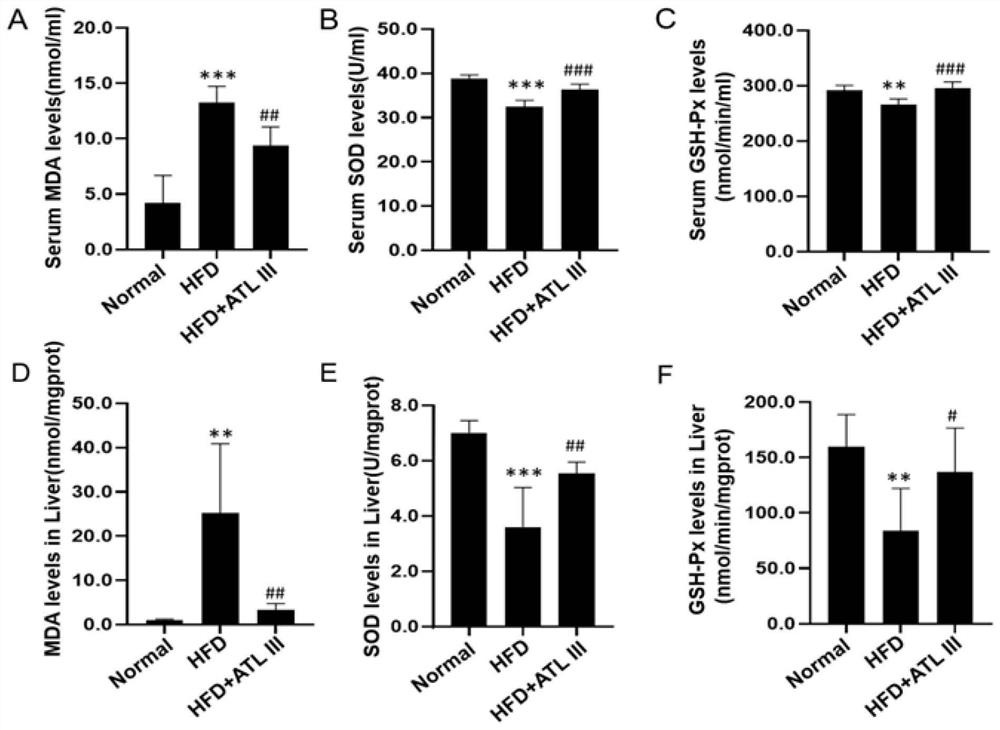
The invention relates to an application of atractylenolide III in preparation of a medicine for treating a non-alcoholic fatty liver disease. It is found that atractylenolide III can reduce liver lipid deposition, reduce liver indexes, significantly reduce serum blood fat levels and liver tissue TG and TC levels and activate related signal molecules of an AMPK / SIRT1 signal channel; the MDA level can be reduced, the SOD and GSH-Px levels are increased, and the oxidative stress is improved. The atractylenolide III can inhibit lipid deposition by activating an AMPK / SIRT1 signal channel, reduce the oxidative stress reaction of liver cells, improve the expression level of CPT1A and improve the oxidative stress of mitochondria at the same time, and play a role in treating the non-alcoholic fatty liver disease through the mechanism. The atractylenolide III is a plant-derived chemical substance, the preparation steps are simple, the environmental pollution is small, and the method is suitable for industrial production. Therefore, atractylenolide III can be used for preparing the medicine or health food for treating the non-alcoholic fatty liver disease.
Owner:SHUGUANG HOSPITAL AFFILIATED WITH SHANGHAI UNIV OF T C M
Method for preparing lobetyolin, atractylenolide III and syringin from codonopsis pilosula based on PRE-HPLC
ActiveCN113234110AHigh purityGood workmanshipSugar derivativesSugar derivatives preparationAtractylenolide IIIMedicinal herbs
The invention discloses a method for preparing lobetyolin, atractylenolide III and syringin from codonopsis pilosula based on PRE-HPLC, and the method specifically comprises the following steps: (1) single medicine extraction: taking a codonopsis pilosula medicinal material, crushing, adding 12-15 times of ethanol solution, carrying out ultrasonic extraction at 400-500W for 2-3 times, each time for 30-60min, after the extraction is finished, carrying out rough filtration, and mixing filtrate to obtain a codonopsis pilosula extracting solution; (2) concentrating and drying: performing reduced-pressure vacuum concentration and drying on the filtered extracting solution; (3) preparing and purifying: ultrasonically dissolving the dried extract, filtering through a 0.22 mu m microporous filter membrane, and preparing a liquid phase; (4) fraction collection: respectively collecting lobetyolin, atractylenolide III and syringin fractions; and (5) concentrating and drying: concentrating and drying the collected fractions under reduced pressure in vacuum. The method has the characteristics of convenience in operation, good reproducibility, short period, simultaneous preparation of three monomer components and the like, and the prepared monomer components have the characteristic of high purity.
Owner:JING BRAND
Method for evaluating quality of atractylodes macrocephala koidz by quantitative analysis of multi-components by single marker
PendingCN113899844AImprove quality controlHigh detection sensitivityComponent separationAgainst vector-borne diseasesAtractylenolide IIIClinical efficacy
The invention provides a method for evaluating quality of atractylodes macrocephala koidz by a quantitative analysis of multi-components by single marker, wherein the method comprises the steps: performing quantitative analysis of multi-components by single marker by taking atractylenolide III which is low in cost and easy to obtain as an internal reference substance, and establishing relative retention time and relative correction factors between the atractylenolide III and atractylenolide II, atractylenolide I and atractylenone; and the contents of atractylenolide III, atractylenolide II, atractylenolide I and atractylenone in the atractylodes macrocephala koidz are calculated through correction factors, and the ultra-high performance liquid chromatography is adopted for determination. According to the method, the content of index components is calculated through relative correction factors and chromatographic peak positioning, the content of four components comprising atractylenolide III, atractylenolide II, atractylenolide I and atractylenone in atractylodes macrocephala koidz can be effectively detected at the same time, the cost can be saved, the operation is simplified, the efficiency is improved, the detection sensitivity is high, the stability is good, the determination result is accurate and reliable, and the method is of great significance to quality control of a large amount of atractylodes macrocephala koidz and guarantee of clinical effects of atractylodes macrocephala koidz.
Owner:ZHEJIANG SHOUXIANGU BOTANICAL DRUG INST CO LTD +2
A simultaneous rapid detection method for nine functional active ingredients in Yedao Lugui wine
The invention relates to a method for synchronously and rapidly detecting nine functional active components in Yedao deer and turtle wine and belongs to the field of researches of functional components of health-care wine. The method comprises the following steps: carrying out qualitative analysis on the active components in the Yedao deer and turtle wine by adopting a liquid chromatography-mass spectrometry method through a positive ion scanning working mode; then carrying out quantitative analysis by adopting an external standard method. The method provided by the invention has the advantages of simple pre-treatment steps, high precision, low quantification limit and good stability and can be used for rapidly determining the content of Chinese yam sapogenin, 2,3,5,4'-tetrahydroxy stilbene-2-O-beta-D-glucoside, betaine, ligustilide, scopoletin, atractylenolide III, emodin, emodin methyl ether and codonopsis pilosula alkyne glucoside in a Yedao deer and turtle wine sample; the method can be used for synchronously and rapidly determining the components in the Yedao deer and turtle wine, a Chinese herbal medicine mixed extracting solution of a similar formula or prepared wine.
Owner:海南椰岛酒业发展有限公司
Method for determining contents of atractylenolide II and atractylenolide III in atractylodes macrocephala medicinal material
PendingCN112964805AGood peak shapeGood reproducibilityComponent separationMedicinal herbsO-Phosphoric Acid
The invention relates to a method for determining the contents of atractylenolide II and atractylenolide III in an atractylodes macrocephala medicinal material, which comprises the following steps of: taking the atractylodes macrocephala medicinal material, extracting by using 70% methanol, carrying out gradient elution by using an acetonitrile-0.1% phosphoric acid water system, and determining the contents of atractylenolide II and atractylenolide III in the atractylodes macrocephala medicinal material. According to the method, on the basis of a rhizoma alismatis decoction detection method, through optimization of a series of research on mobile phase proportion gradient, detection wavelength, an extraction solvent, an extraction mode, extraction time and a chromatographic column, the result shows that the peak time is short, the peak shape is good, the separation degree and the peak purity are qualified, the accuracy is high and the stability is good, the operation is simple, convenient and rapid, the quality detection method of the atractylodes macrocephala medicinal material is perfected, so that the quality is better controlled.
Owner:青海普兰特药业有限公司
Application of Atractylodes lactone in the preparation of antidepressants
ActiveCN105582006BGood protective activityInhibit apoptosisOrganic active ingredientsNervous disorderAtractylenolide IIIAtractylenolide I
The invention provides application of atractylenolide in preparation of an antidepressant drug, and relates to the field of medicine. In-vitro activity tests prove that atractylenolide I and atractylenolide III have the better protection activity on corticosterone-damaged PC12 cells, and the mechanism is related to inhibition of neuron apoptosis; in-vivo test results show that atractylenolide I and atractylenolide III can significantly shorten the immobility time of a mouse forced swimming test and a mouse tail suspension test. Experiments prove that atractylenolide I and atractylenolide III have the significant antidepressant effect and can be used for preparing the drug for preventing and treating a depressive disorder.
Owner:SHANXI UNIV
Pharmaceutical composition for preventing and treating cardiorenal syndrome and application thereof
InactiveCN112972470AEffective in treating cardiorenal syndromeOptimizing Compatibility DosageOrganic active ingredientsUrinary disorderSide effectCardiorenal disease
The invention provides a pharmaceutical composition for preventing and treating cardiorenal syndrome. The pharmaceutical composition is prepared from 2.2-8.8 parts of benzoylmesaconine, 2.5 to 10.0 parts of benzoylhypacoitine, 5.2 to 20.8 parts of pachymic acid A, 4.4 to 17.6 parts of atractylenolide III and 3.8 to 15.2 parts of albiflorin. The titer of the pharmaceutical composition for preventing and treating cardiorenal syndrome can reach 93.02%. According to the pharmaceutical composition disclosed by the invention, the contents of the benzoylmesaconine and the benzoylhypacoitine are limited, so that toxic and side effects caused by the benzoylmesaconine and the benzoylhypacoitine on a human body are avoided, the pharmaceutical composition can be used trustingly, and the curative effect is enhanced; and finally, a foundation is laid for further developing a novel active ingredient composition preparation.
Owner:QIANFOSHAN HOSPITAL OF SHANDONG
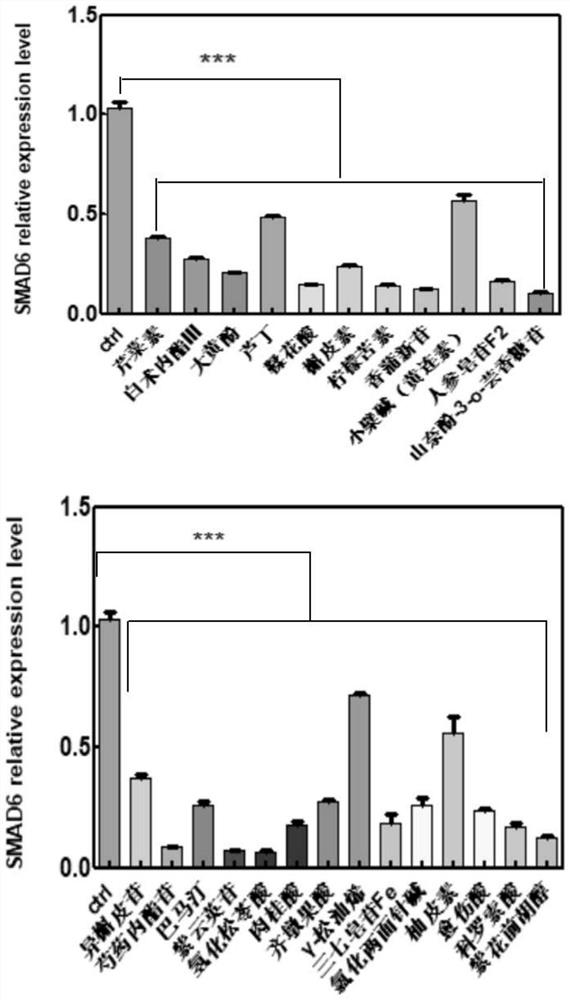
Application of composition to preparation of medicine for inhibiting gastric cancer cell proliferation based on regulation and control of CDKs and SMAD6 genes
InactiveCN112843049AClearly targetedAntineoplastic agentsHeterocyclic compound active ingredientsCorosolic acidApigetrin
The invention belongs to the technical field of pharmaceutical preparations, and particularly relates to application of a composition to preparation of drugs for inhibiting gastric cancer cell proliferation based on regulation and control of CDK1, CDK6 and SMAD6 genes. On the basis of fingerprint spectrum analysis of a Chinese patent medicine Morhodamine, CDK1, CDK6, SMAD6 and other cell proliferation and cancer inhibition related genes are selected as Morhodamine direct or indirect regulation target genes; therefore, active components, such as apigenin, atractylenolide III, chrysophanol, rutin, ellagic acid, quercetin, limonin, typhaneoside, berberine, ginsenoside F2, kaempferol-3-o-rutinoside, isoquercitrin, albiflorin, palmatine, astragalin, hydrogenated pinicolic acid, cinnamic acid, oleanolic acid, gamma-terpinene, notoginsenoside Fe, nitidine chloride, naringenin, traumatic acid, corosolic acid or decursinol, can be screened out. The medicine provides theoretical support for developing novel medicines for treating gastric cancer.
Owner:HANDAN PHARMA
Application of Atractylolide Ⅲ Derivatives in Preparation of Anti-platelet Aggregation Drugs and Anti-Platelet Aggregation Drugs
ActiveCN104490869BSimple compositionGood curative effectOrganic active ingredientsBlood disorderAtractylenolide IIISide effect
Owner:SHANGHAI NINTH PEOPLES HOSPITAL SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
Quality testing method of Shouhui Tongbian Capsules
ActiveCN109507356BQuality assuranceComprehensive evaluationComponent separationAtractylenolide IIIAtractylenolide II
The invention discloses a quality detection method of a Shouhui bowel-relaxing capsule and belongs to the field of analysis of traditional Chinese medicinal preparations. The quality detection methodadopts a thin-layer chromatography to identify bighead atractylodes rhizome in the capsule and simultaneously adopts an HPLC to determine the contents of atractylenolide I, atractylenolide II and atractylenolide III in the capsule. The quality detection method disclosed by the invention is stable and reliable, strong in specificity, good in reproducibility and capable of fully and effectively controlling the quality of the euphorbia pulcherrima bowel-relaxing capsule, ensuring the safety and the effectiveness of clinical medication and better meeting the needs of patients and the market.
Owner:LUNAN PHARMA GROUP CORPORATION
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com