Application of atractylenolide III in preparation of anti-hepatic fibrosis drugs
An anti-hepatic fibrosis, Atractylodes lactone technology, applied in the application field of Atractylodes lactone III in the preparation of anti-hepatic fibrosis drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Example 1: Observation of the effects of Atractylolide III on liver tissue damage and liver fibrosis indicators in mice.
[0019] 48 male ICR mice were purchased from Shanghai Slack Experimental Animal Co., Ltd., license number: SCXK (Shanghai) 2020-0025. Animals were given free access to food and water, and were given standard pellet feed. The experiment began after one week of adaptive feeding in a standard light cycle (12 hours of light, 12 hours of darkness), room temperature 22°C, and constant humidity. carbon tetrachloride (CCl 4 ; Analytical grade, Nanjing Chemical Reagent Co., Ltd.) and olive oil (Shandong Luhua Group Co., Ltd.) made 10% olive oil preparation at a volume ratio of 1:9. Atractylodes lactone III was purchased from Chengdu Zhibiao Pure Biotechnology Co., Ltd. (batch number: PCS0114, with a purity of over 99%, the same below), and was prepared into a solution with olive oil. Animals were randomly divided into six groups: normal group, model group, ...
Embodiment 2
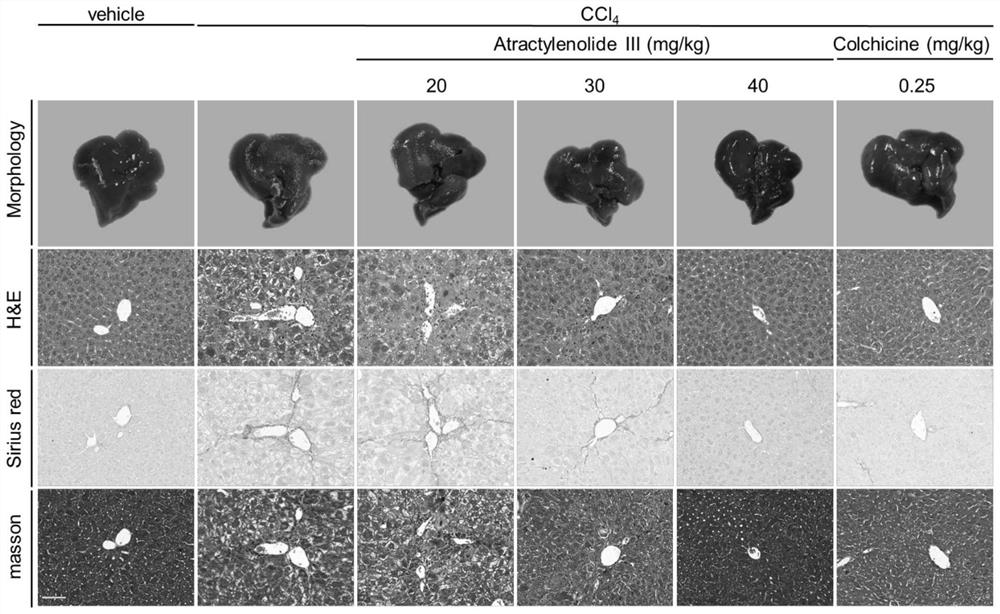
[0030] Example 2: The mouse liver tissue of Example 1, the liver morphology was photographed, the liver tissue was fixed with 4% paraformaldehyde, embedded, and sectioned for HE staining, Sirius red staining and Masson staining, to observe liver fibrosis, liver pathology and collagen deposition Impact.
[0031] HE staining of the liver sections of mice in the model group showed degeneration and necrosis of hepatocytes, showing a large area of pseudolobular tissue structure; Masson staining showed a large amount of blue collagen fiber deposition, extending outward from the portal area, and the fiber cords were thicker and more stained. Deep, indicating that there are many collagen fibers; Sirius red staining indicates that various types of collagen fibers expand outward from the portal area, and observed under a polarizing microscope, collagen deposition and expansion have formed pseudolobules, among which a large number of red type I collagen fibers, a small amount of sparse ...
Embodiment 3
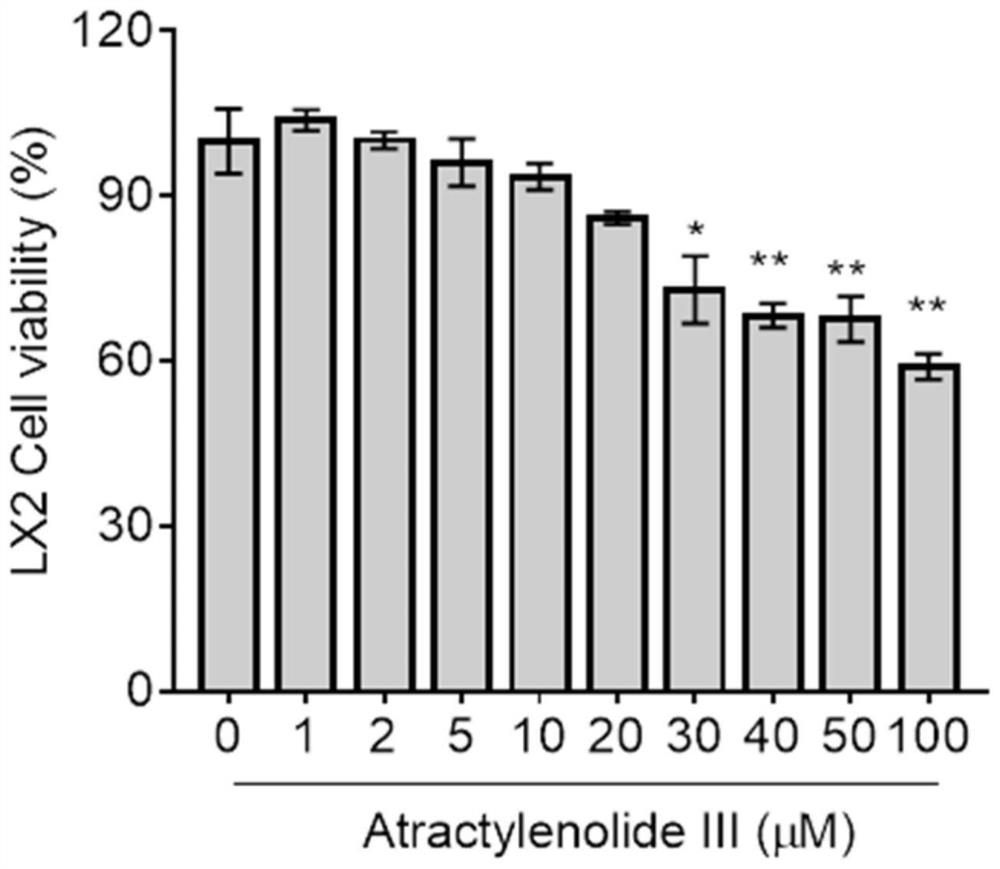
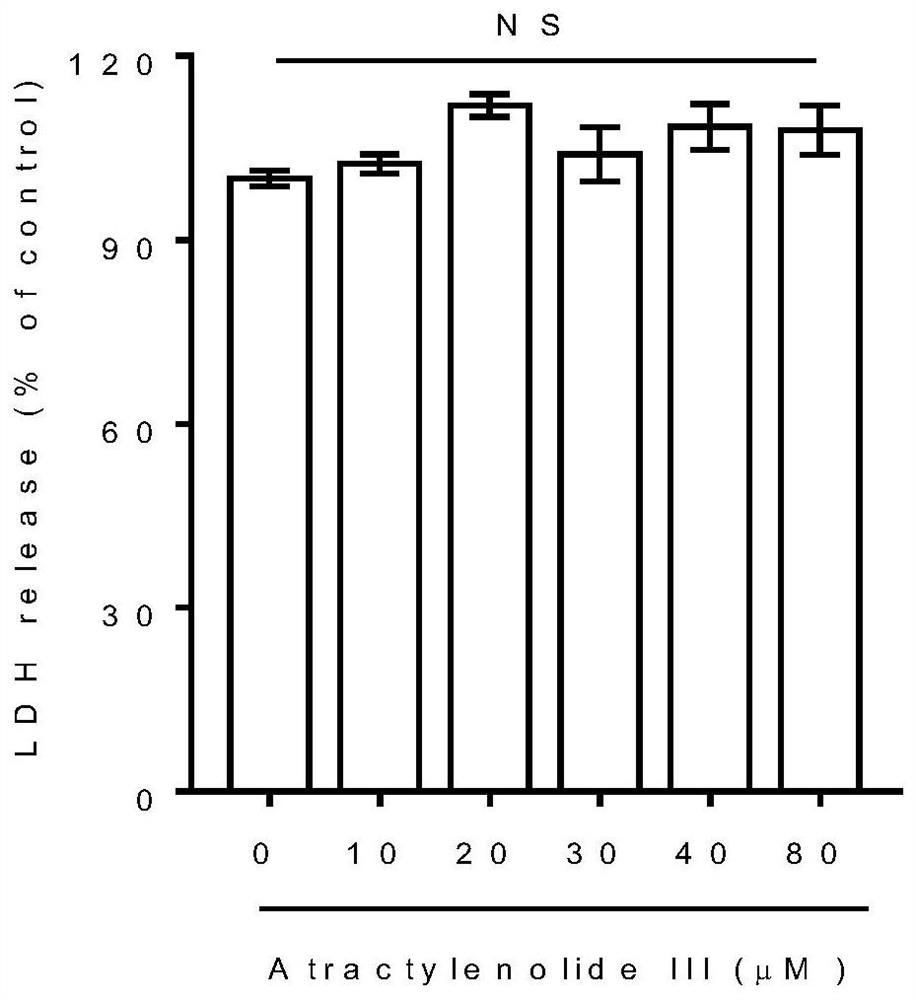
[0034] Example 3: HSCs were cultured in vitro, and the inhibitory effect of Atractylolide III on HSC proliferation was observed (detection of cell proliferation activity).
[0035] HSC-LX2 in the logarithmic growth phase was inoculated in a 96-well plate, and the concentration of the cell suspension was adjusted to 1×10 per well. 4 cells in DMEM medium containing 10% fetal bovine serum at 37°C, 5% CO 2Cultivate for 24 hours under culture conditions to make the cells adhere to the wall. The cells were divided into 10 groups. Except the normal control group, the cells in the other 9 groups were treated with atractyloid III (1, 2, 5, 10, 20, 30, 40, 50, 100 μM) for 24 hours. Each group has 6 replicate wells. After the effect was over, 20 μl of a 5% concentration of thiazolium blue (MTT) solution was added to each well, and the incubation was continued for 4 hours. Then the supernatant was sucked off, 200 μl DMSO was added to each well, and shaken on a shaker at low speed for 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com