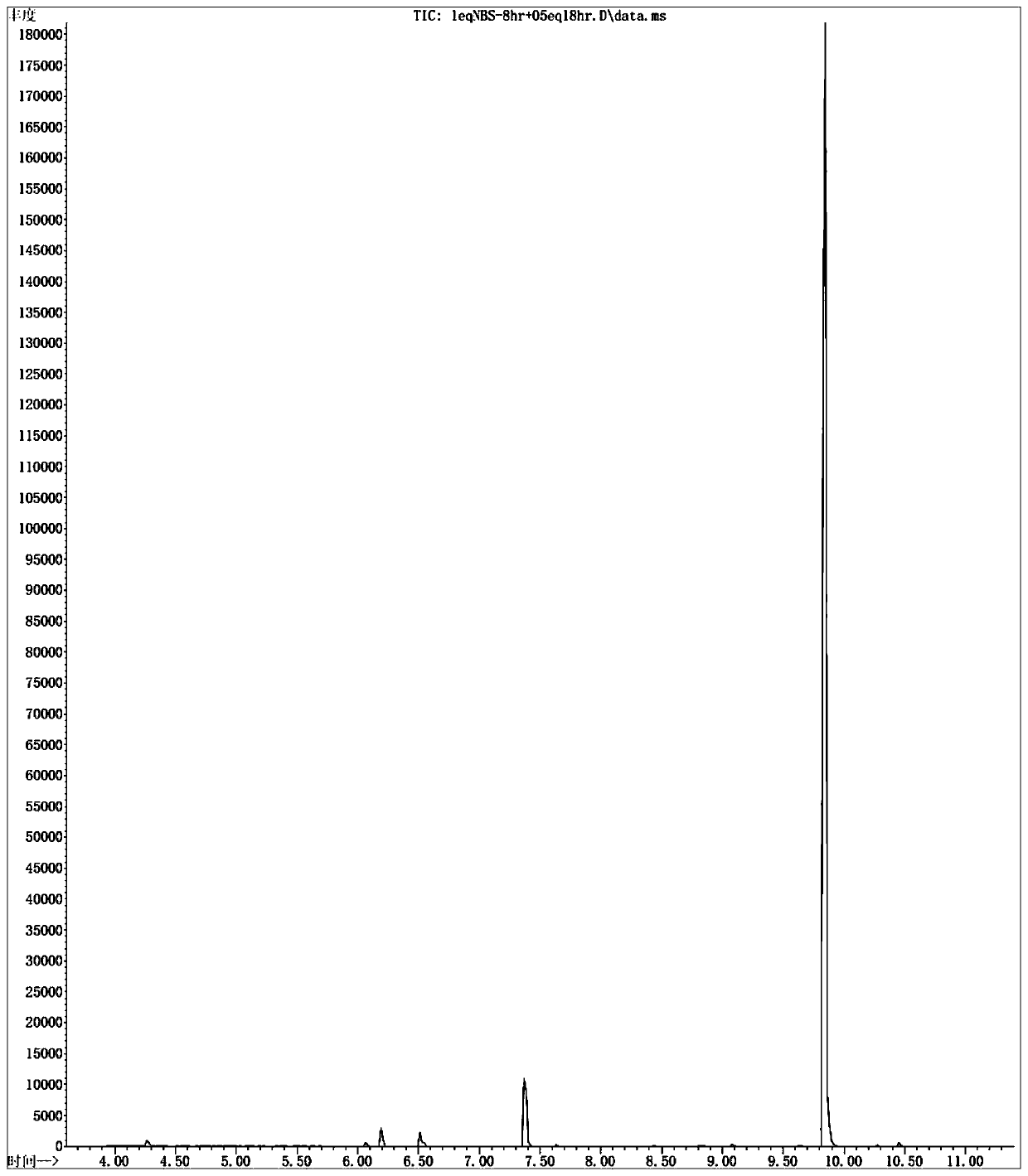
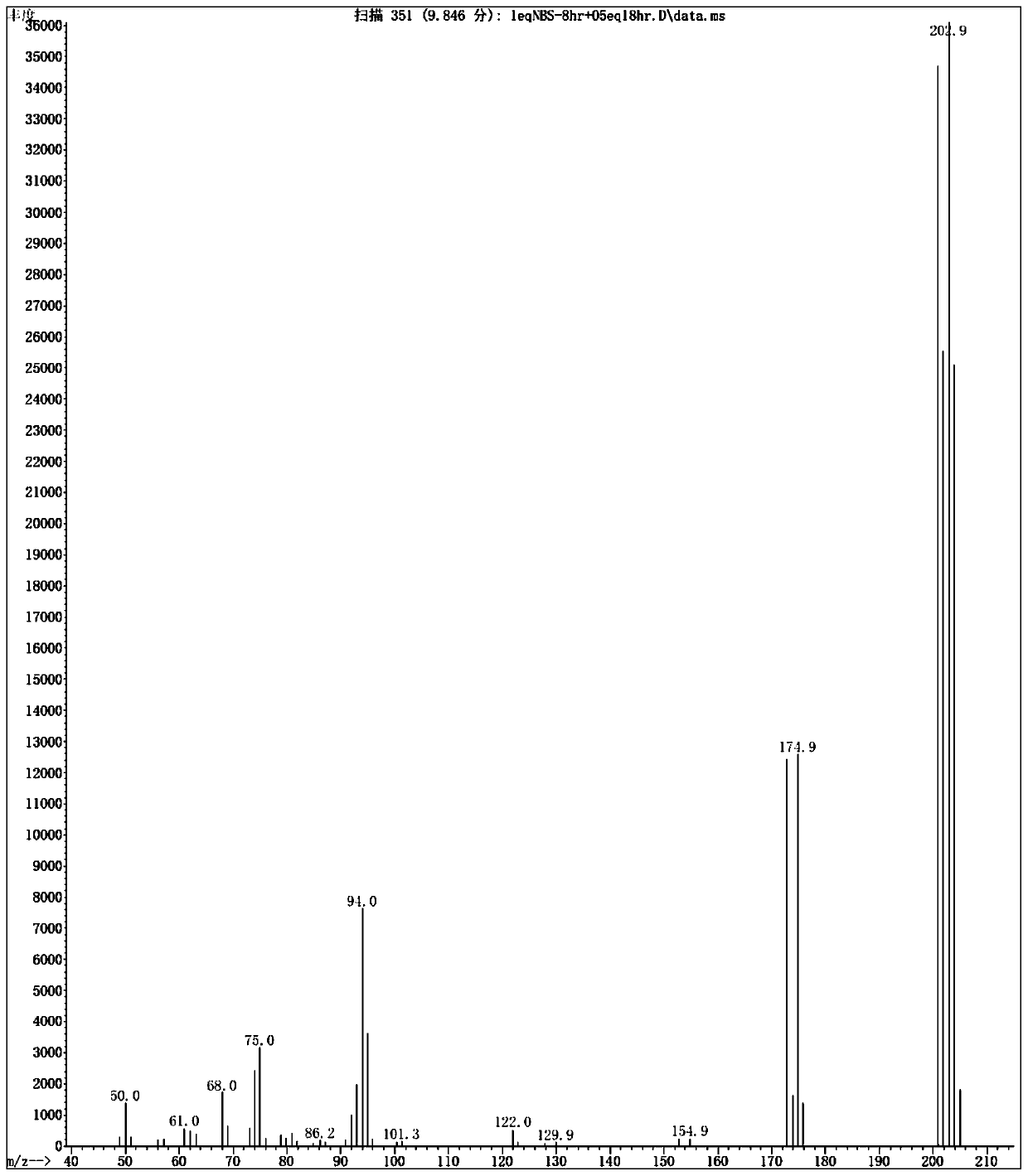
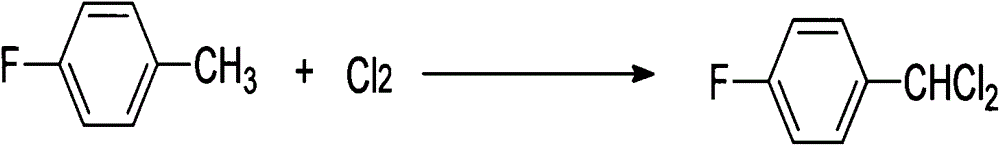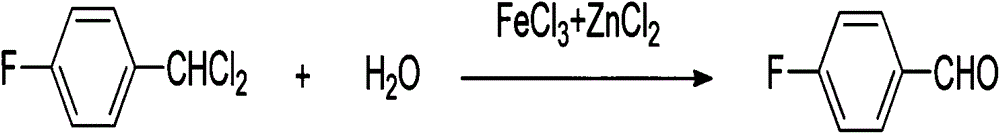Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
37 results about "4-fluorobenzaldehyde" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
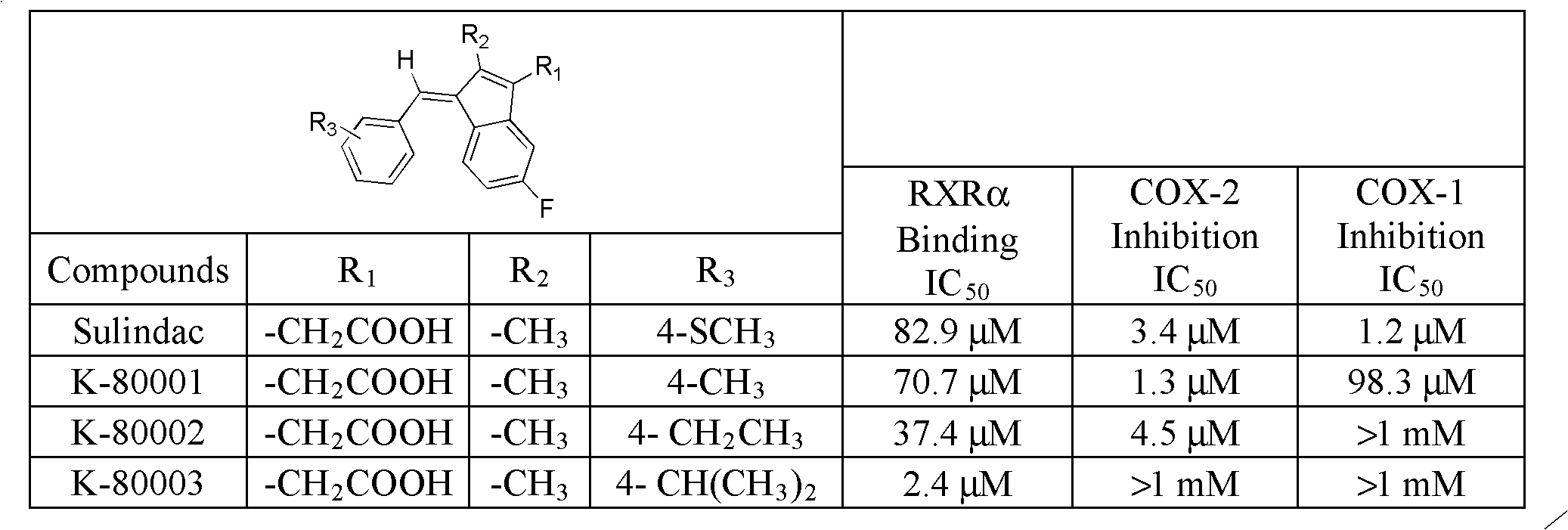
Packaging 10, 50, 250 g in glass bottle Application 4-Fluorobenzaldehyde was used in the preparation of pyrazolopyridine UR-13756.
Preparation method of Olaparib and analogue of Olaparib
InactiveCN105085407ARaw materials are easy to getSimple processOrganic chemistryBenzoic acidIsobenzofuran
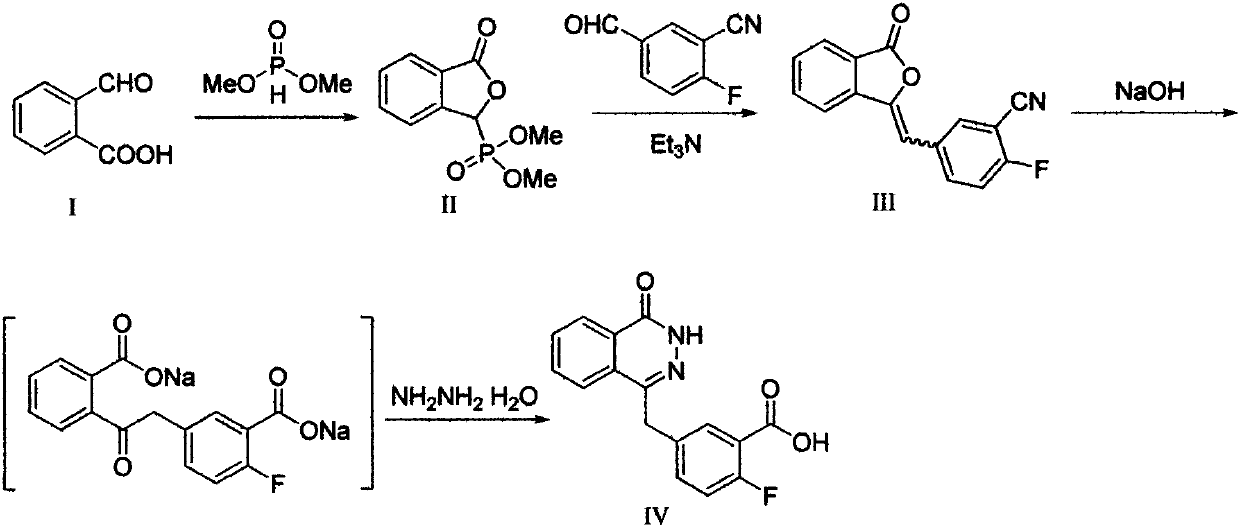
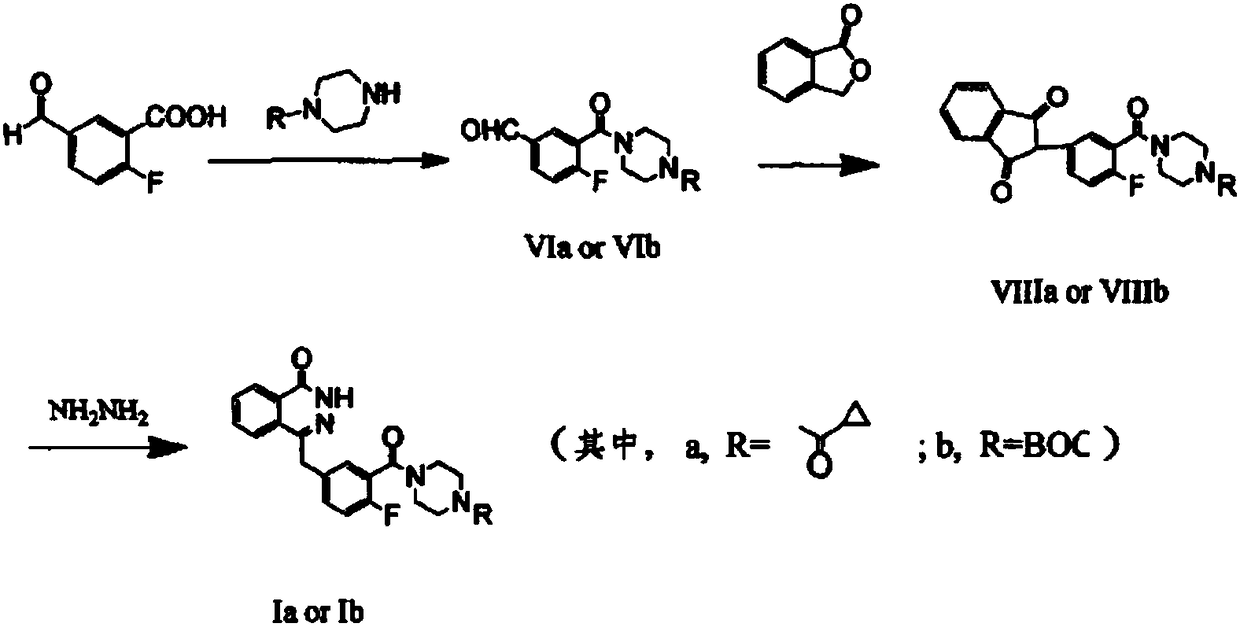
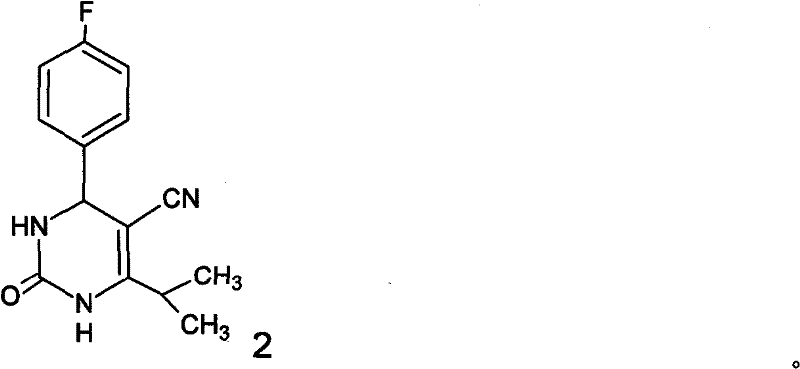
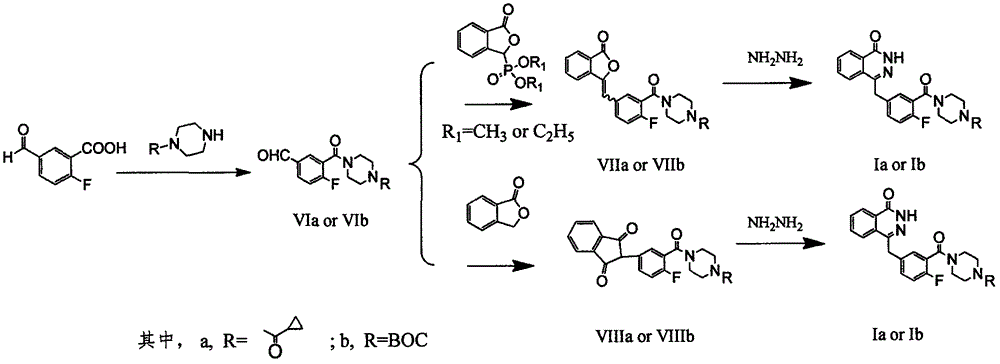
The invention discloses a preparation method of Olaparib and an analogue of the Olaparib. 2-fluoro-5-formyl benzoic acid is taken as a raw material and reacts with 1-substitutent piperazine to produce 3-(4-substitutent)piperazine-1-carbonyl)-4-fluorobenzalde which reacts with (3-oxo-1,3-dihydro-isobenzofuran-1-yl)dialkyl phosphate to produce 1-(substitutent)-4-[5-(3-oxo-3H-isobenzofuran-1-yl-methylene)-2-fluorobenzoyl]piperazine, then the 1-(substitutent)-4-[5-(3-oxo-3H-isobenzofuran-1-yl-methylene)-2-fluorobenzoyl]piperazine reacts with hydrazine hydrate to produce the Olaparib (Ia,R=cyclopropyl formyl) and the analogue (Ib,R=BOC) of the Olaparib; or 3-(4-substitutent)paperazine-1-carbonyl)-4-fluorobenzalde reacts with phthalide to produce 1-(substituent)-4-[5-(2,3-dihydro-1,3-dioxo-1H-indene-2-yl)-2-fluorobenzoyl]piperazine which reacts with the hydrazine hydrate to produce the Olaparib (Ia,R=cyclopropyl formyl) and the analogue (Ib,R=BOC) of the Olaparib.
Owner:GUANGZHOU YOUMIJIAN PHARMA TECH CO LTD
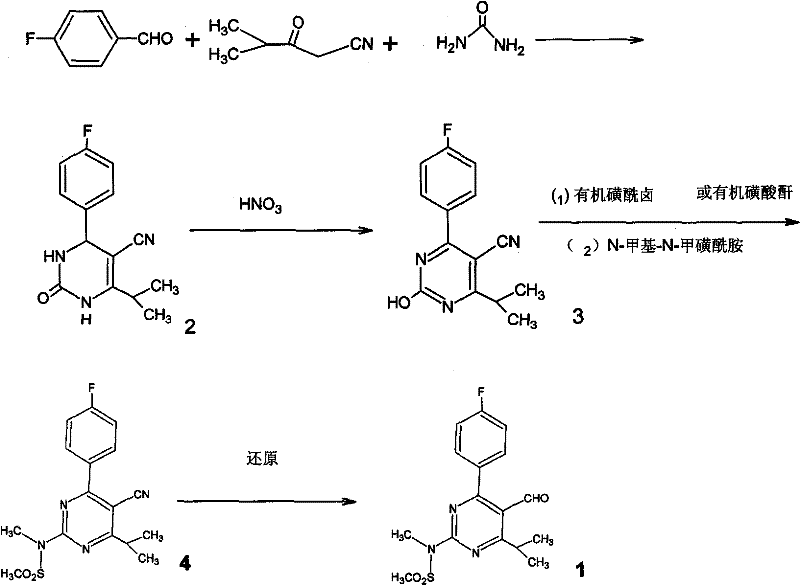
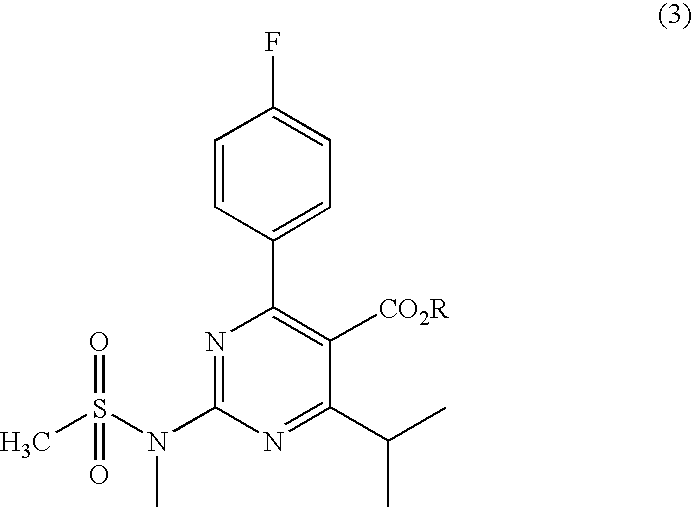
Preparation of aminopyrimidine compounds
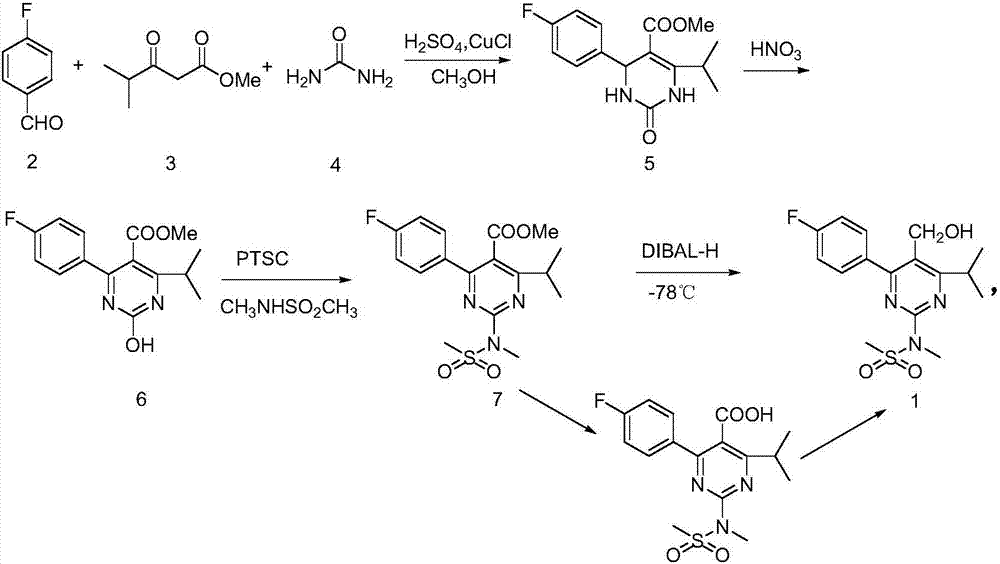
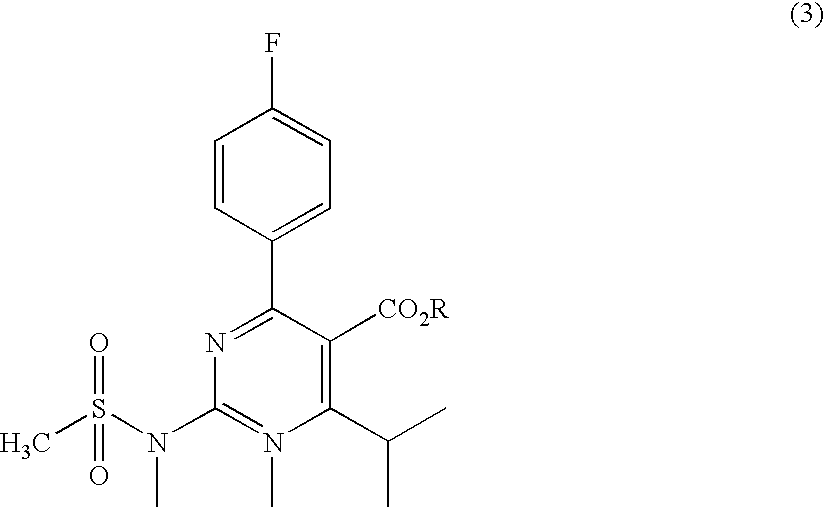
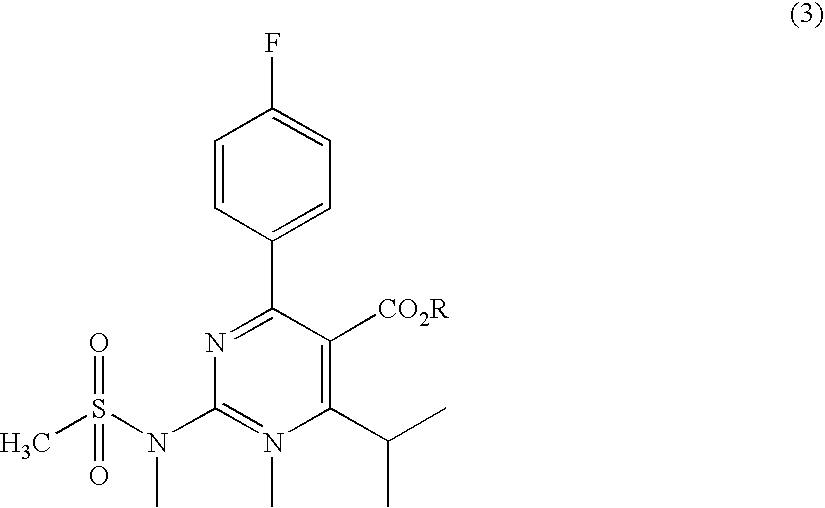
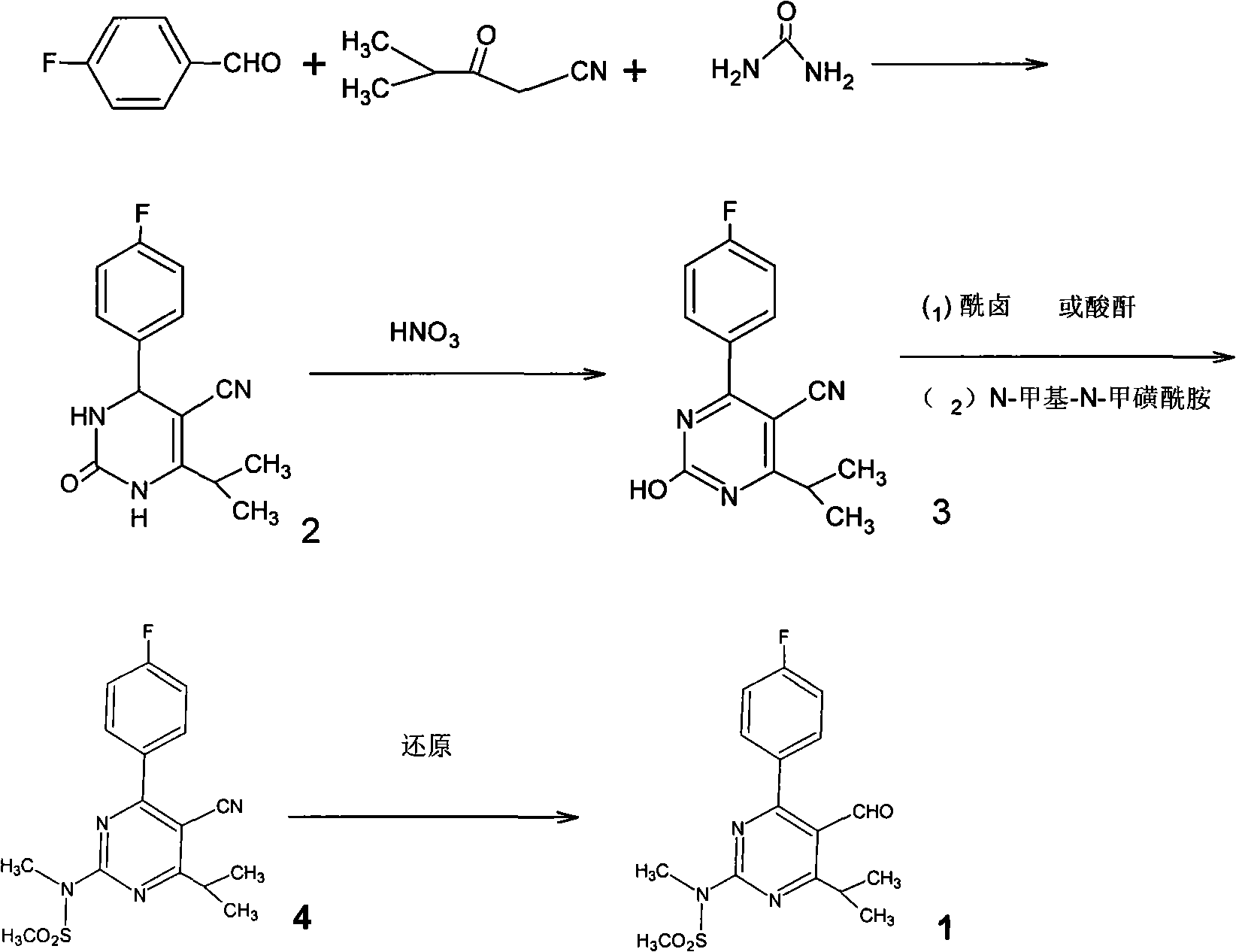
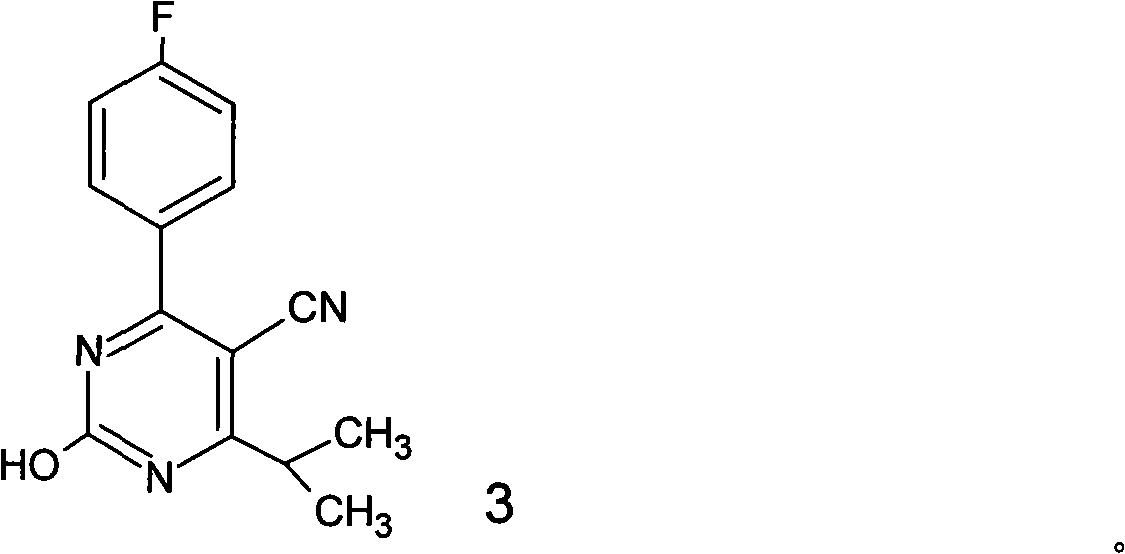
A 2-(N-methyl-N-methanesulfonylamino)pyrimidine compound of the formula (3): [R is a hydrocarbyl group], is prepared by the steps of: (I) reacting an isobutyrylacetate ester with 4-fluorobenzaldehyde and urea in the presence of a protonic compound and a metal salt; (II) oxidizing the reaction product of the step (I); (III) reacting the oxidation product of the step (II) with an organic sulfonyl halide or an organic sulfonyl anhydride; and (IV) reacting the reaction product of the step (III) with N-methyl-N-methanesulfonamide.
Owner:ASTRAZENCA UK LTD
Chiral zinc complex and copper complexes of alpha-phenylethylamine
InactiveCN102069014AGroup 4/14 element organic compoundsCarboxylic acid nitrile preparationBenzaldehyde4-chlorobenzaldehyde
The invention relates to a chiral zinc acetate complex of alpha-phenylethylamine, a chiral copper acetate complex of alpha-phenylethylamine and a chiral copper chloride complex of alpha-phenylethylamine which are used as catalyst. When the complexes are used in the nitrile silicification reactions of aromatic aldehydes such as benzaldehyde, 2-fluorobenzaldehyde, 2-methoxybenzaldehyde, 2-methylbenzaldehyde, 4-methylbenzaldehyde, 4-methoxybenzaldehyde, 4-fluorobenzaldehyde, 4-chlorobenzaldehyde and 4-bromobenzaldehyde to prepare chiral target products, the chiral catalysts have good catalytic activities and high enantioselectivity in the nitrile silicification reactions.
Owner:罗梅
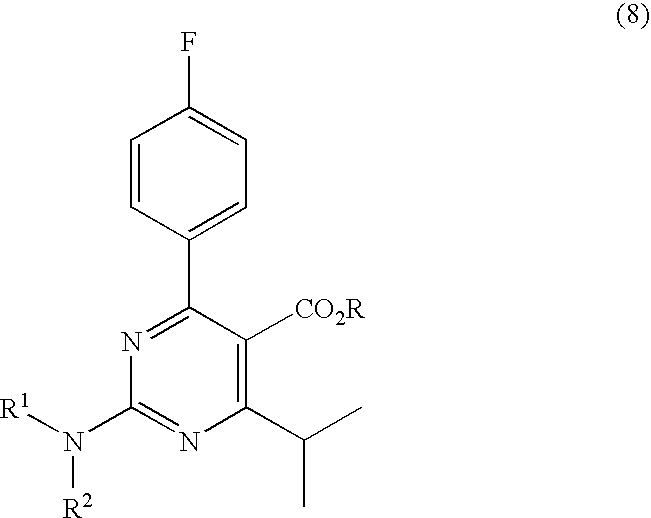
Preparation of 4-(4-fluorophenyl)-6-isopropyl-2-(N-methyl-N-methylsulfonyl amido) pyrimidine-5-formaldehyde
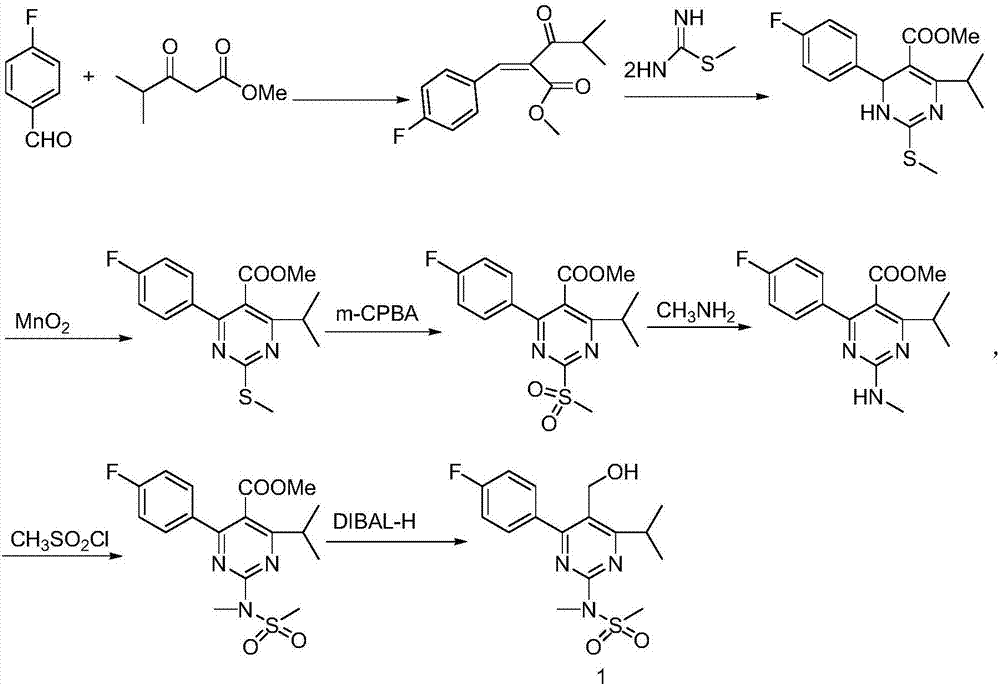
The invention provides a method for preparing a Rosuvastatin intermediate, 4-Fluorophenyl-6-isopropyl-2-(N-methyl-N-methylsulfonyl amino) pyrimidinyl-5-formyl. The method uses isobutyryl acetonitrile, 4-Fluorobenzaldehyde and carbamide as raw materials and is obtained by steps of cyclization, oxidation, substitution and reduction etc. The method of the invention dose not need expensive raw materials and has the advantages of low technological cost, simple reaction, high product yield and being applicable to industrialized production.
Owner:滁州市庆云医药有限公司
Process for the preparation of rosiglitazone maleate
The invention discloses a process for the preparation of a pyridine derivative namely 5-{4-2(N-methyl-N(2-pyridyl)amino ethoxy]benzyl]thiazolidine-2,4-dione maleate comprising the steps of:(a) reacting 2-chloropyridine with 2-(N-methyl amino)ethanol;(b) coupling 2-(N-methyl-N-(2-pyridyl) amino)ethanol) obtained in step (a) and 4-fluorobenzaldehyde in an aprotic polar solvent with an alkali metal hydroxide or an alkali metal alkoxide as base.(c) isolating the product of the coupling reaction viz 4-[2-(N-methyl-N-(2-pyridyl) amino) ethoxy]benzaldehyde;(d) converting said isolated benzaldehyde compound of step (c) into 5-[4-[2-N-methyl-N-(2-pyridyl) amino)ethoxy]benzyl]thiazolidine-2,4-dione in a known manner and(e) converting said thiazolidine-2,4-dione compound obtained in step (d) into a pharmaceutically acceptable maleate salt.
Owner:TORRENT PHARMA LTD
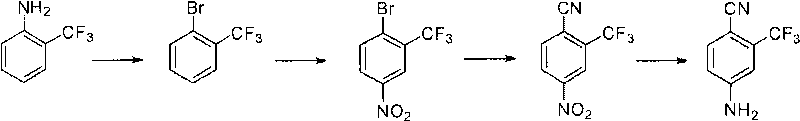
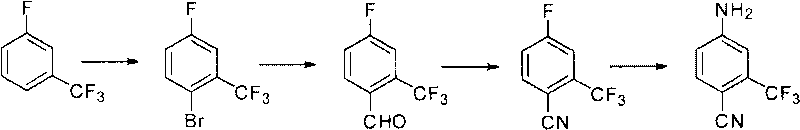
Preparation method of 2-trifluoromethyl-4-aminobenzonitrile
ActiveCN101759597ASuitable for industrializationPromote safe productionCarboxylic acid nitrile preparationOrganic compound preparationBenzeneFormylation reaction
The invention relates to a preparation method of 2-trifluoromethyl-4-aminobenzonitrile, comprising the following steps of: (a) providing m-trifluoromethyl benzene; (b) carrying out bromination reaction on the m-trifluoromethyl benzene in the presence of an acid and a bromating agent so as to obtain 2-bromine-5-fluorine-benzotrifluoride; (c) carrying out Grignard reaction on the 2-bromine-5-fluorine-benzotrifluoride to prepare a Grignard reagent, and carrying out formylation reaction in the presence of a formylation reagent so as to obtain 2-trifluoromethyl-4-fluorobenzaldehyde; (d) carrying out cyaniding reaction on the 2-trifluoromethyl-4-fluorobenzaldehyde so as to obtain 2-trifluoromethyl-4-fluorobenzonitrile; and (e) reacting the 2-trifluoromethyl-4-fluorobenzonitrile with an ammoniation reagent so as to obtain the 2-trifluoromethyl-4-aminobenzonitrile.
Owner:SHANGHAI CHEMSPEC CORP +1
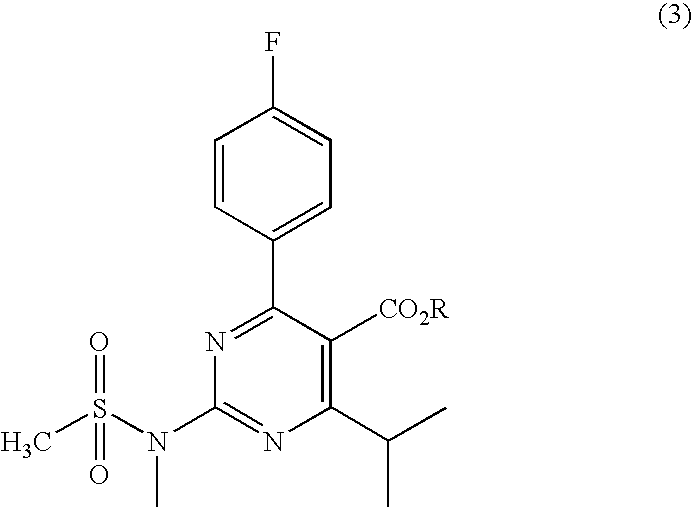
Preparation of Aminopyrimidine Compounds
A 2-(N-methyl-N-methanesulfonylamino)pyrimidine compound of the formula (3): [R is a hydrocarbyl group], is prepared by the steps of: (I) reacting an isobutyrylacetate ester with 4-fluorobenzaldehyde and urea in the presence of a protonic compound and a metal salt; (II) oxidizing the reaction product of the step (I); (III) reacting the oxidation product of the step (II) with an organic sulfonyl halide or an organic sulfonyl anhydride; and (IV) reacting the reaction product of the step (III) with N-methyl-N-methanesulfonamide.
Owner:ASTRAZENCA UK LTD
A process for the preparation of substituted phenyl ether compounds and rosiglitazone
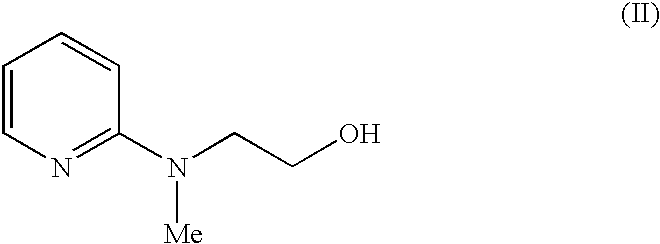
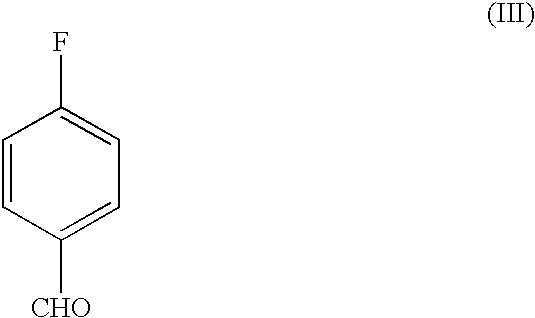
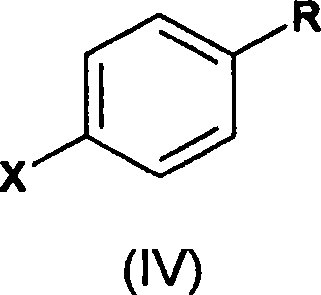
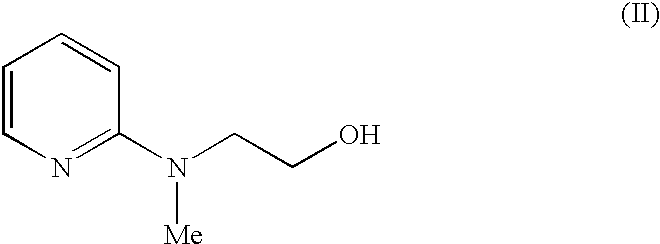
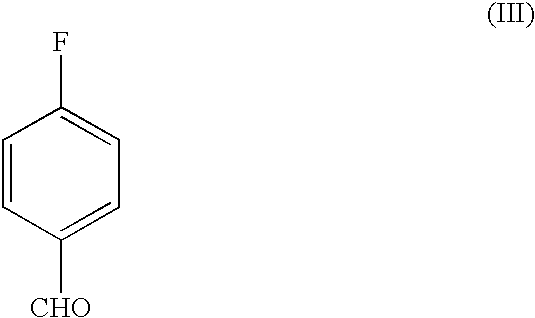
A novel process for the preparation of a compound of the formula (II), which is useful as intermediate compound for the preparation of thiazolidinedione derivatives, such as rosiglitazone, pioglitazone, troglitazone and ciglitazone, is disclosed. The novel process comprising reacting a compound of the formula (III) with a compound of the formula (IV) in a mixture of a non-polar water immiscible organic solvent and water (two phase system) with an alkali metal hydroxide or an alkali metal carbonate as a base in the presence of a phase transfer catalyst. In the first aspect of the present invention comprising reacting 2-(N-methyl-N-(2- pyridyl) ethanol with 4-fluorobenzaldehyde in the mixture of a non-polar water immiscible organic solvent, preferably toluene, and water with an alkali metal hydroxide or an alkali metal carbonate as a base, preferably potassium hydroxide, in the presence of a phase transfer catalyst, e.g. tetra n-butylammonium hydrogensulphate or benzyltriethylammonium chloride, to obtain 4-[2-(N-methyl-N-(2- pyridyl)amino)ethoxy]benzaldehyde, which is the key intermediate for preparing rosiglitazone and its salts, e.g. maleate salt or phosphate salt, useful in the treatment of Type II diabetes.
Owner:SANDOZ AG
Process for the preparation of pyridine derivative
The invention discloses a process for the preparation of a pyridine derivative namely 5-{4-2(N-methyl-N(2-pyridyl) amino ethoxy]benzyl]thiazolidine-2,4-dione maleate comprising the steps of: (a) reacting 2-chloropyridine with 2-(N-methyl amino) ethanol; (b) coupling 2-N-methyl-N-(2-pyridyl)amino)ethanol) obtained in step (a) and 4-fluorobenzaldehyde in an aprotic polar solvent with an alkali metal hydroxide or an alkali metal alkoxide as base. (c) isolating the product of the coupling reaction viz 4-[2-(N-methyl-N-(2-pyridyl)amino)ethoxy]benzaldehyde; (d) converting said isolated benzaldehyde compound of step (c) into 5-[4-[2-N-methyl-N-(2-pyridyl) amino)ethoxy]benzyl]thiazolidine-2,4-dione in a known manner and (e) converting said thiazolidine-2,4-dione compound obtained in step (d) into a pharmaceutically acceptable maleate salt.
Owner:TORRENT PHARMA LTD
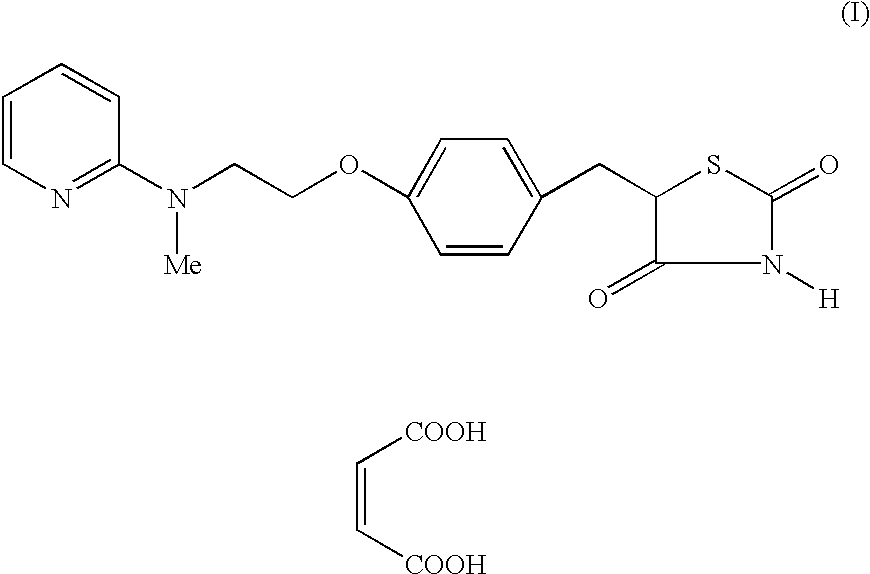
Process for the preparation of 5-[4-[2-[N-methyl-N-(2-pyridyl) amino] ethoxy] phenyl methyl] thiazolidine-2, 4-dione maleate
InactiveUS20050043539A1Cost effectiveCheaper & easily available raw-materialsOrganic chemistryN dimethylformamideBenzaldehyde
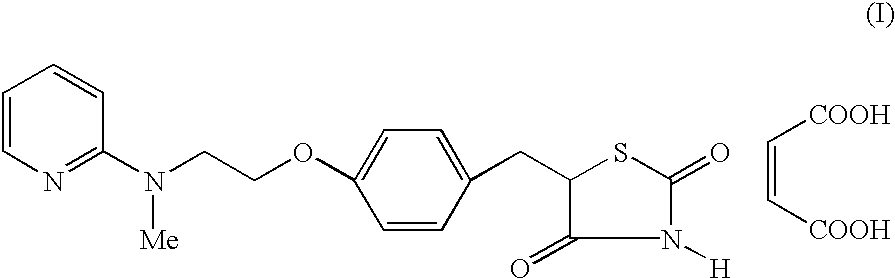
The present invention discloses a process for the preparation of 5-[4-[2-[N-methyl-N-(2-pyridyl)amino]ethoxy]phenyl methyl]thiazolidine-2,4-dione maleate (VI) comprising the steps of Coupling 2-[N-methyl-N-(2-pyridyl)amino]ethanol (I) and 4-fluorobenzaldehyde (II) in N,N-dimethylformamide, isolating the coupled product 4[2-[N-methyl-N-(2-pyridyl)amino]ethoxy]benzaldehyde (III), converting said isolated benzaldehyde compound (III) to 5-[4-[2-[N-methyl-N-(2-pyridyl)amino]ethoxy]benzylidene]thiazolidine-2,4-dione (IV) and purifying the same, reducing 5-[4-[2-[N-methyl-N-(2-pyridyl)amino]ethoxy]benzylidene]thiazolidine-2,4-dione, by a novel reduction method for making 5-[4-[2-[N-methyl-N-(2-pyridyl)amino]ethoxy]phenyl methyl]thiazolidine-2,4-dione (V). This reduction method involves reacting the compound (IV) with a novel metal legand complex and a reducing agent, purifying the product (V) obtained by a new method reported in the present invention and converting the said thiazolidine-2,4-dione compound (V) into a pharmaceutically acceptable salt.
Owner:USV LTD
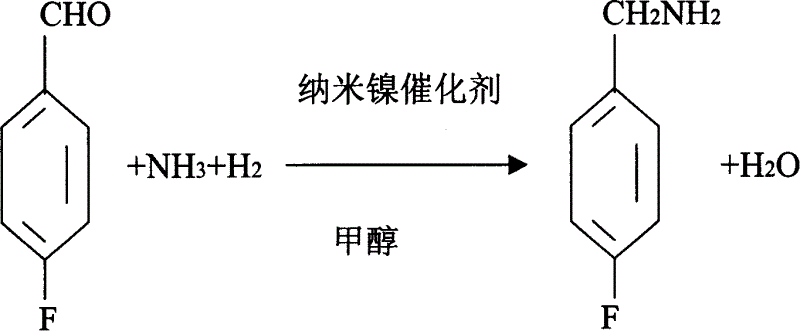
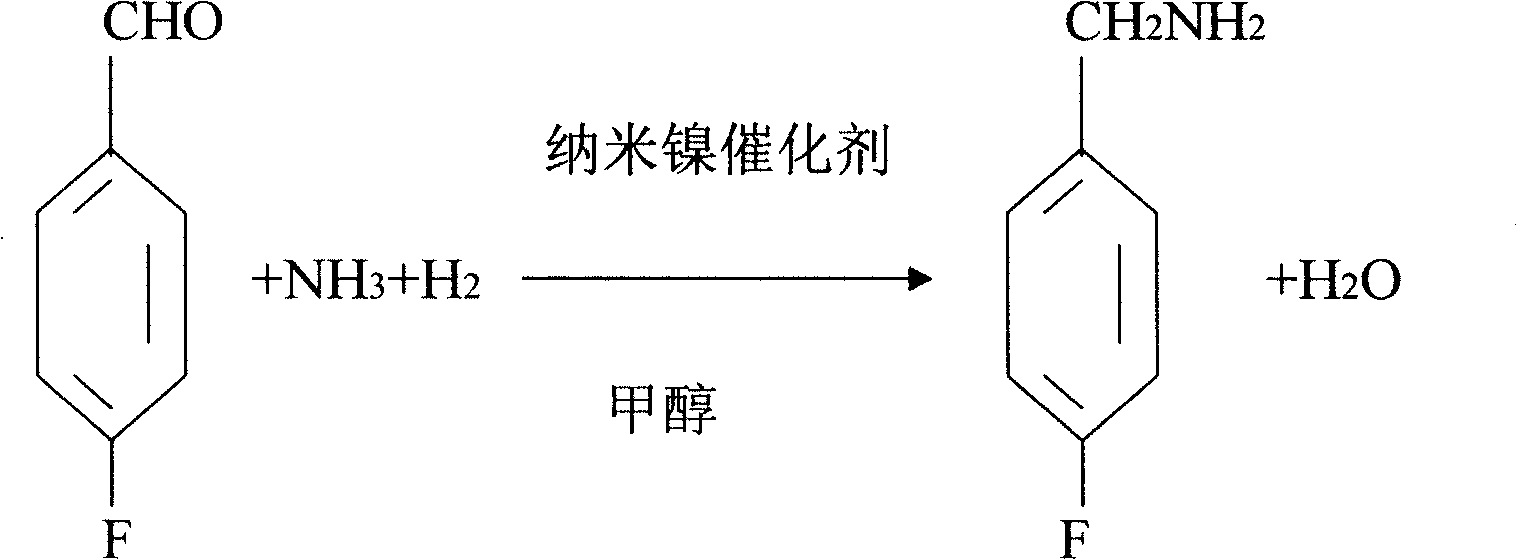
Process for preparing 4-fluorobenzylamine with nano nickel as catalyst
InactiveCN1704397AReduce dosageLow costPreparation by reductive alkylationHydrogen4-fluorobenzylamine
The invention provides a process for preparing 4-fluorobenzylamine with nano nickel as catalyst, which employs 4-fluorobenzaldehyde, hydrogen, absolute methanol, liquid ammonia as raw material. Test results show that, under the same reaction conditions, the process of the present invention can realize an average yield of above 95%, and a purity above 99%.
Owner:张炳庚
Synthetic method of 4-fluorobenzaldehyde
InactiveCN101353297ALow priceShort synthetic routeOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsSulfolaneHexamethylphosphoramide
The invention relates to a synthetic method of p-flurobenzaldehyde, which belongs to the chemical and pharmaceutical field. In the synthetic method, the p-chlorobenzaldehyde and potassium fluoride react under the condition of a solvent and a catalyst at high temperature, wherein, the solvent is one of sulfolane, dimethyl sulfoxide, dimethylformamide, dimethylacetamide, hexamethyl phosphoramide, nitrobenzene and ortho-nitrotoluene; the catalyst is one of or the mixture of two or more than two of benzyl triethyl amine chloride, tetrabutylammonium bromide, hexadecyltrimethylammonium chloride, methyltrioctylammonium chloride, tetraphenylphosphonium bromide, methyltriphenylphosphonium bromide, benzyl triphenyl phosphonium bromide and polyethylene glycol dimethyl ether. Compared with the conventional preparation method, the synthetic method of the invention has the advantages of cheap raw materials, short synthetic route and little 'three wastes' discharge. As the amount of the catalyst used is reduced and the price of the solvent is low, the production cost of the p-flurobenzaldehyde is decreased.
Owner:王俊华
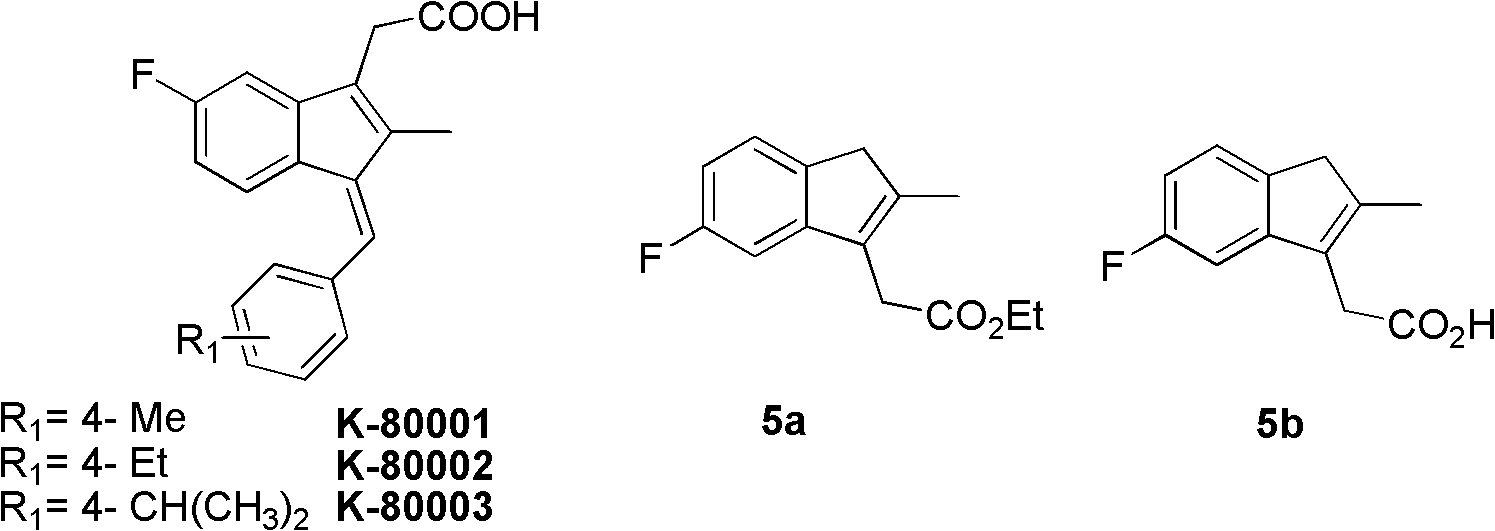
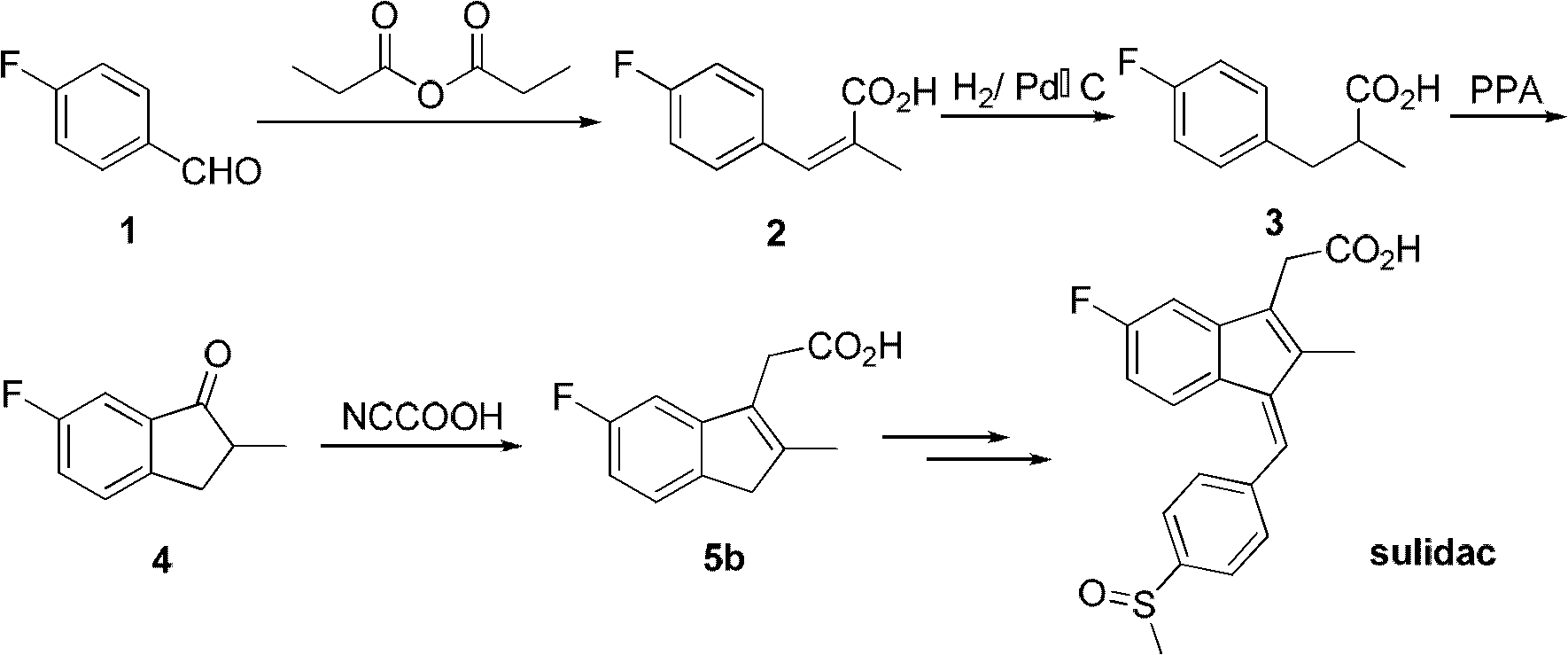
Synthesis method and application of intermediate of sulindac analogue
InactiveCN102030642AReduce dosageAvoid it happening againOrganic compound preparationCarboxylic acid esters preparationKetoneSolvent
The invention discloses a synthesis method and application of intermediates of a sulindac analogue, relating to intermediates of a sulindac analogue. The intermediates are 5-fluoro-2-metyl-3-indene ethyl acetate (5a) and 5-fluoro-2-metyl-3-indene acetic acid (5b). The synthesis method comprises the following steps of: subjecting 4-fluorobenzaldehyde as an initial raw material, propionic anhydride as a solvent and propionic anhydride to a perkin reaction to obtain 4-fluoro-2-metyl-methylcinnamic acid; catalyzing the 4-fluoro-2-metyl-methylcinnamic acid with palladium carbon with the palladium content of 5-20 percent and reducing in the hydrogen gas atmosphere to obtain 3-(4-fluorine phenyl)-2-methyl propionate; subjecting the 3-(4-fluorine phenyl)-2-methyl propionate to the intramolecilar friedel-crafts acyl browning reaction under the action of polyphosphoric acid under the heating condition to form 6-fluoro-2-methyl indene ketone; and subjecting the 6-fluoro-2-methyl indene ketone and halogenated acetate to reformatsky reaction under the action of the activated zinc powder to obtain a crude product and eliminating the crude product in an acid solution to obtain 5-fluoro-2-metyl-3-indene acetate. The intermediates can be used for preparing novel sulindac analogues with anticancer activity.
Owner:XIAMEN UNIV
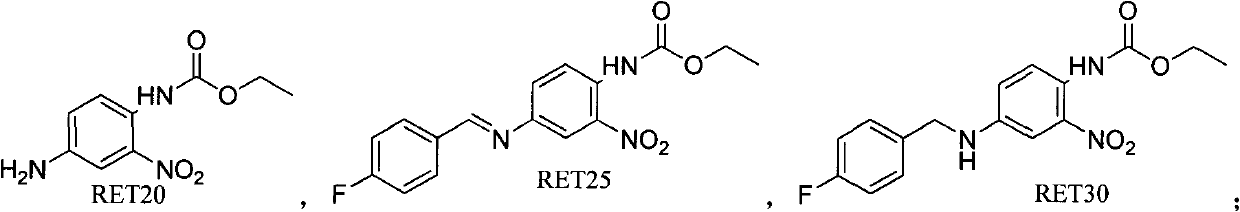
Preparation method of retigabine and intermediate thereof
ActiveCN103373941ACarbamic acid derivatives preparationOrganic compound preparationToluene4-fluorobenzaldehyde
The invention relates to the medicine synthesis field and in particular relates to a preparation method of retigabine and an intermediate thereof. The method specifically comprises the following steps of: (1) preparing a compound with a structure of formula (RET25) by the compound with the structure of formula (RET20) and 4-fluorobenzaldehyde under the action of p-toluene sulfonic acid; (2) preparing the compound with the structure of formula (RET30) by the compound with the structure of formula (RET25) through sodium borohydride reduction; and (3) preparing the retigabine by the compound with the structure of the formula (RET30) through raney nickel hydrogenation reduction. The total impurity content of the retigabine obtained by the preparation method disclosed by the invention is less than 0.16% in terms of area percentage unit of HPLC (High Performance Liquid Chromatography).
Owner:SUZHOU NOVARTIS PHARMA TECHONOLOGY CO LTD
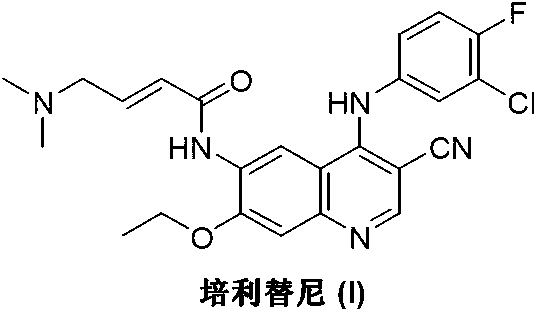
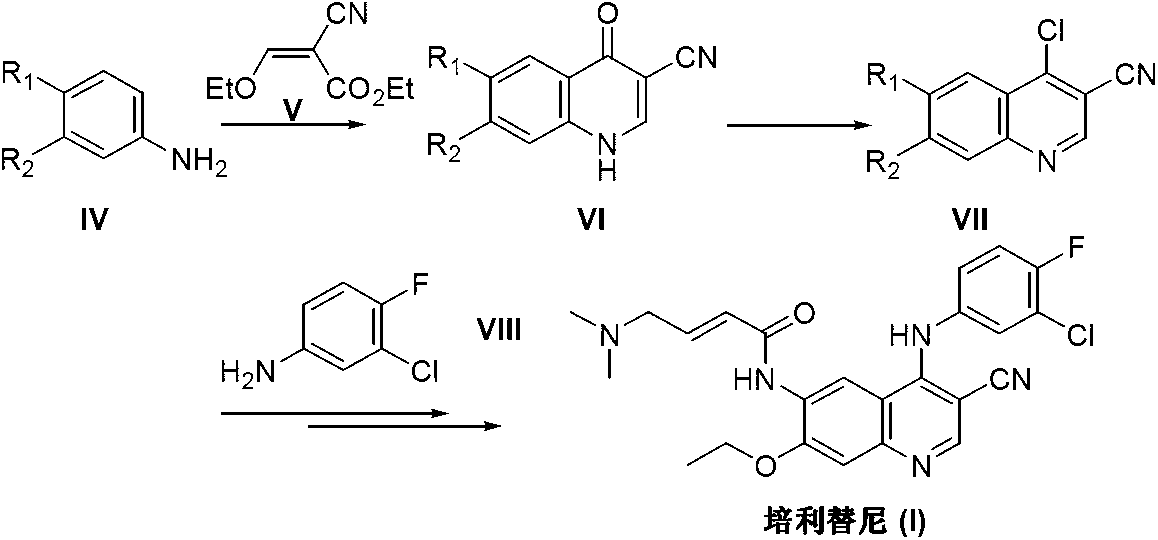
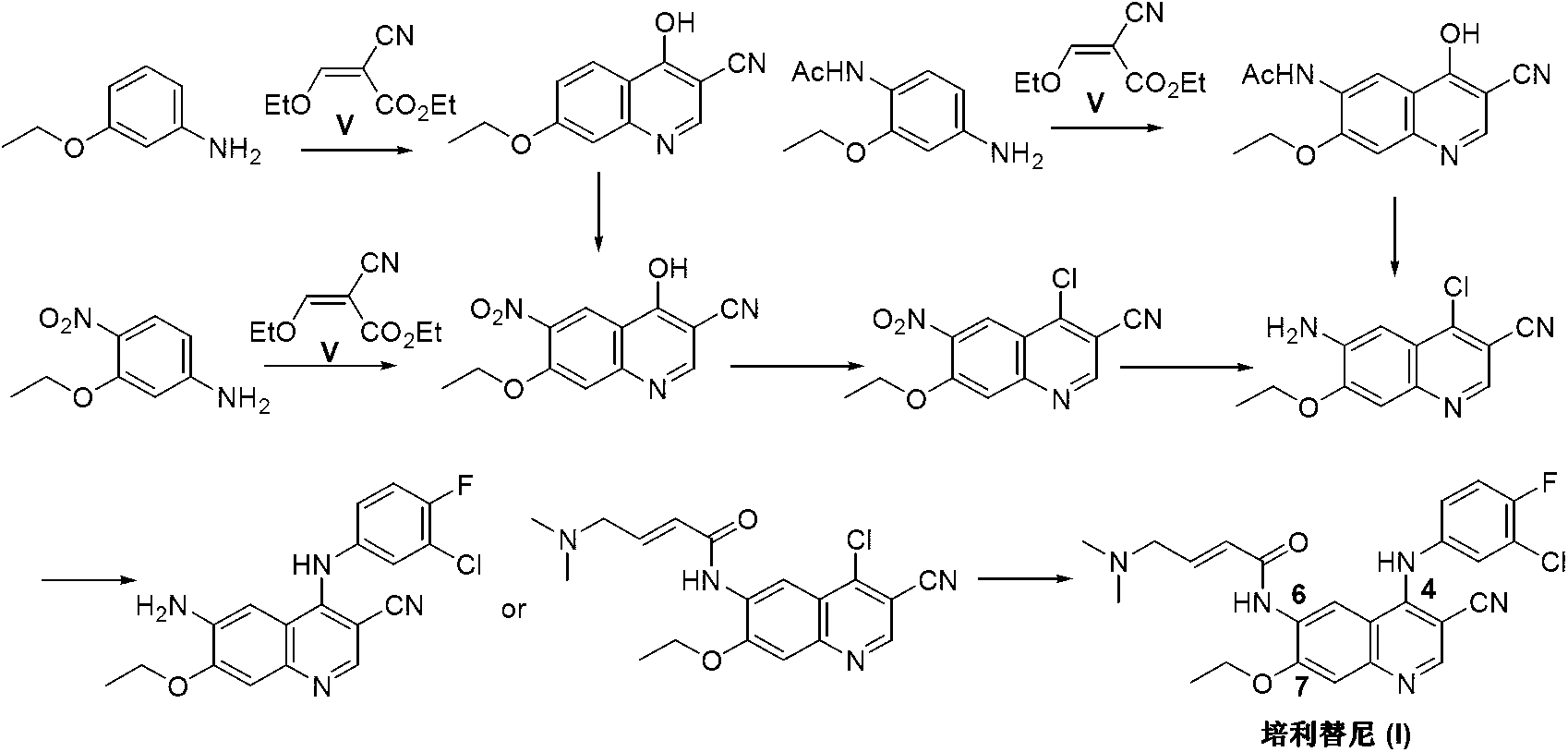
Preparation method of pelitinib
The invention discloses a preparation method of pelitinib (I). The preparation method includes steps as follows: 6-[(E)-4-(dimethylamino)-2-butene acylamino]-7-ethoxy-4-amino-3-quinoline formonitrile (II) and 3-chlorine-4-fluorobenzaldehyde (III) perform condensation and reduction reaction, and the pelitinib (I) is obtained. According to the preparation method, raw materials are easy to obtain, the process is simple and concise, and the method is economical, environment-friendly and suitable for industrial production.
Owner:SUZHOU MIRACPHARMA TECH +1
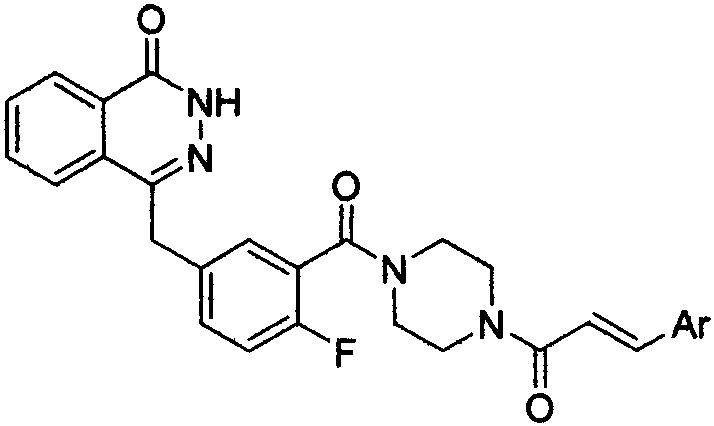
Pyridazinone derivative, and preparation method and medical application thereof
InactiveCN110272412AHigh activityEnhanced inhibitory effectOrganic chemistryAntineoplastic agentsBenzoic acidBenzaldehyde
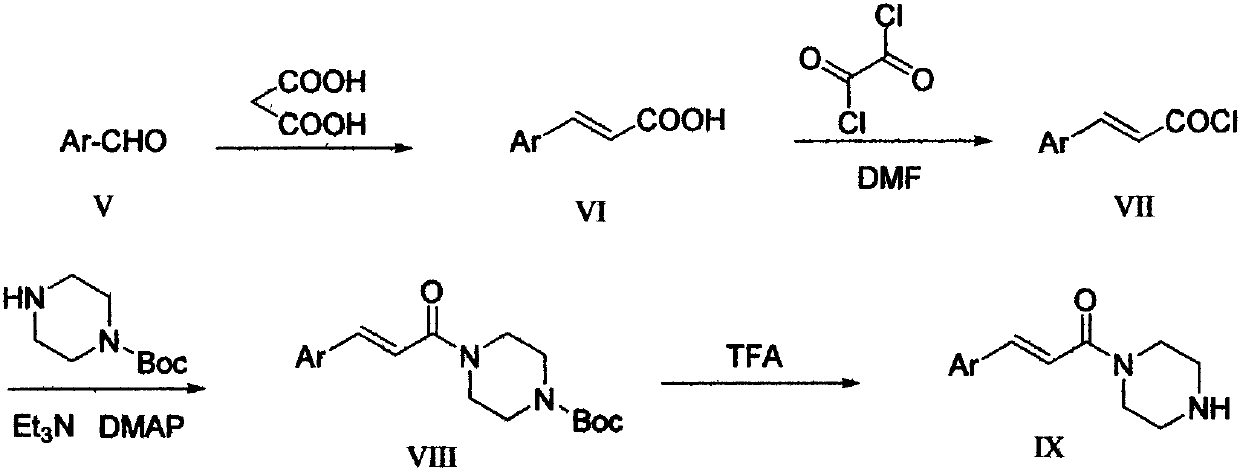
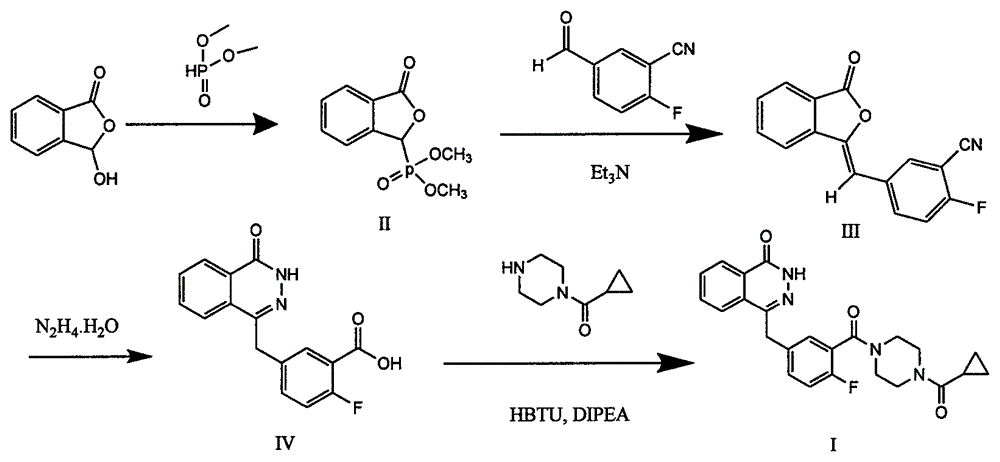
The invention provides a pyridazinone derivative, and a preparation method and a medical application thereof. O-formylbenzoic acid used as a raw material reacts with dimethyl phosphite to obtain dimethyl (3-oxo-1,3-dihydroisobenzofuran-1-yl)phosphonate, the dimethyl (3-oxo-1,3-dihydroisobenzofuran-1-yl)phosphonate reacts with 3-cyano-4-fluorobenzaldehyde in the presence of triethylamine to prepare (Z,E)-2-fluoro-5-[(3-oxoisobenzofuran-1(3H)-ylidene)methyl]benzonitrile, and the (Z,E)-2-fluoro-5-[(3-oxoisobenzofuran-1(3H)-ylidene)methyl]benzonitrile is reduced by hydrazine hydrate to prepare 2-fluoro-5-[(4-oxo-3,4-dihydropyridazin-1-yl)methyl]benzoic acid; and benzaldehyde or substituted aromatic formaldehyde or furfural used as a raw material and malonic acid undergo a Knoevenagel reaction to obtain cinnamic acid or substituted cinnamic acid or furan-2-acrylic acid, the cinnamic acid or substituted cinnamic acid or furan-2-acrylic acid and 1-tert-butoxycarbonylpiperazine undergo an amidation reaction, a tert-butoxycarbonyl group is removed from the obtained amidation product in the presence of trifluoroacetic acid, and the obtained product and the 2-fluoro-5-[(4-oxo-3,4-dihydropyridazin-1-yl)methyl]benzoic acid undergo the amidation reaction to obtain a series of (E)-4-{3-[4-[(3-substituted aryl)acryloyl]piperazin-1-carbonyl]-4-fluorobenzyl}-2H-pyridazin-1-one derivatives. Results of preliminary pharmacological activity screening show that the compound represented by a general formula shown in the present invention has a certain in-vitro PARP-1 inhibition ability and a certain in-vitro tumor cell proliferation resisting activity. The structural general formula of compound is shown in the description; and in the general formula, Ar is selected from two formulas also shown in the description, and R1, R2, R3, R3, R4 and R5 can be the hydrogen atom, the fluorine atom, the chlorine atom, the bromine atom, a methyl group, a methoxy group, a tetrafluoromethyl group and a nitro group.
Owner:WUHAN UNIV OF SCI & TECH
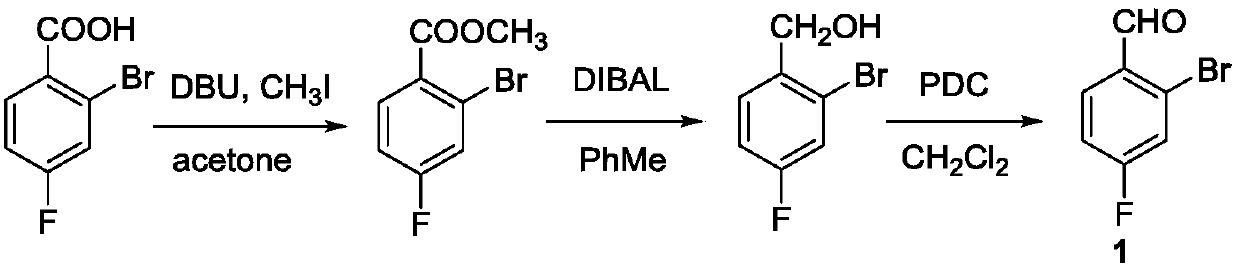
Synthesis method of 3-bromo-4-fluorobenzaldehyde
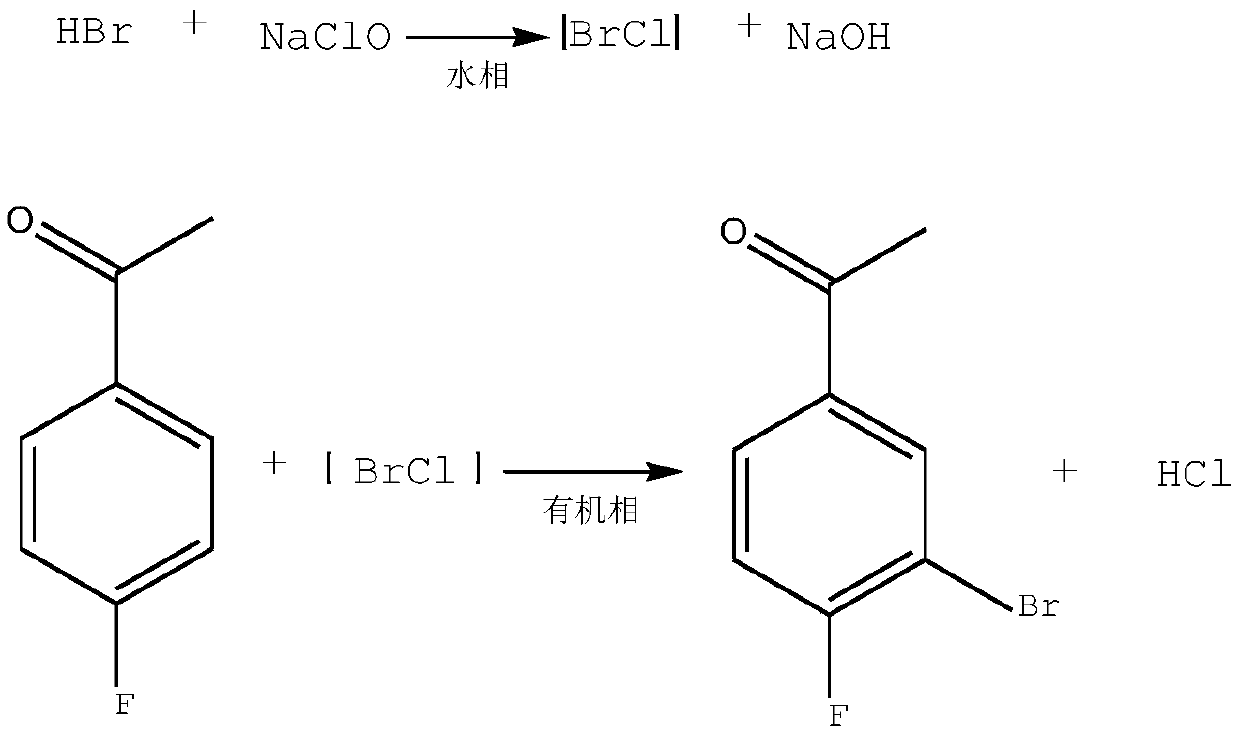
ActiveCN109912396AEasy to operateRaw materials are easy to getOrganic compound preparationCarbonyl compound preparationThermal insulationSynthesis methods
The invention relates to the technical field of synthesis of pesticide and pharmaceutical intermediates, in particular to a synthesis method of 3-bromo-4-fluorobenzaldehyde. The method comprises following steps: (1), 4-fluorobenzaldehyde is dissolved in dichloromethane, and a solution A is obtained; (2), sodium bromide is dissolved in pure water, 35% hydrochloric acid is added during stirring, anda solution B is obtained; (3), after the solution A and the solution B are mixed, ultrasonic waves are started, and an aqueous sodium hypochlorite solution is dropwise added during stirring; (4), after the aqueous sodium hypochlorite solution is dropwise added, thermal insulation is performed under the ultrasonic and stirring condition, and then the solution is left to stand; (5), phase splittingis performed, a methylene dichloride phase is washed to be neutral, and drying and desolvation are performed; (6), a crude product is obtained, body melt crystallization is performed at 31 DEG C, anda pure product is obtained. According to the synthesis method, a catalyst is not needed, and precursor chemical bromine or highly toxic chlorine is not involved in the process; the raw materials areeasily available, dangerousness is lower, and the yield is high; the process is green, easy to operate and environmentally friendly.
Owner:SINOPHARM CHEM REAGENT
Preparation method of rosuvastatin calcium intermediate
InactiveCN107235918AReduce adverse effectsReact cleanOrganic chemistryPotassium persulfateRosuvastatin Calcium
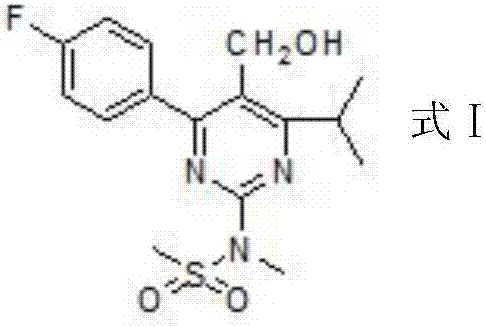
The invention provides a preparation method of a rosuvastatin calcium intermediate. The preparation method of the rosuvastatin calcium intermediate, namely, the compound shown as formula I in the description comprises the following steps: (1) a compound 5 shown as formula II in the description is generated from 4-fluorobenzaldehyde, methyl isobutyrylacetate and urea under the action of a catalyst; (2) a compound 6 shown as formula III in the description is generated from the compound 5 under the action of potassium persulfate as an oxidizing agent; (3) a compound 7 shown as formula IV in the description is generated from the compound 6, tosyl chloride and N-methyl methanesulfonamide under the action of a catalyst; (4) the target compound shown as the formula I is generated from the compound 7 under the action of a vitride solution as a reducing agent and crystallized with a crystallization solution, and a purified target compound is obtained. The preparation method of the rosuvastatin calcium intermediate has the advantages of low production cost, mild condition and simple and convenient operation.
Owner:SUZHOU HEALTH COLLEGE
Preparation method of olaparib drug intermediate
InactiveCN108558773AEasy to purifySimple and fast operationOrganic chemistry4-fluorobenzaldehydePetroleum ether
The invention provides a preparation method of an olaparib drug intermediate, and particularly relates to the technical field of preparation of drug intermediates. The method comprises the steps thatS1, 2-carboxybenzaldehyde, triethylamine and dichloromethane are mixed and stirred, then dimethyl phosphite is added for a reaction at the room temperature, methane sulfonic acid is added, a reactionsolution is concentrated to dryness, water is added for beating, filtering and drying are conducted, and beating with petroleum ether is conducted to obtain a white solid; S2, the solid obtained in the first step, 3-cyano-4-fluorobenzaldehyde and dichloromethane are mixed and then cooled, triethylamine is added dropwise for a reaction, a reaction solution is concentrated to dryness, water is addedfor beating, filtering and drying are conducted, and beating with methyl tert-butyl ether is conducted to obtain a white solid; S3, the solid obtained in the second step is mixed with water, coolingis conducted, hydrazine hydrate is added for a reaction, then acetone is added, a NaOH aqueous solution is added for a reaction, cooling is conducted to the room temperature, extraction is conducted,the pH value is adjusted, the white solid is precipitated, and filtering, rinsing with cold water and recrystallization are conducted to obtain the white solid. The preparation method has the advantages that the yield is increased, the production cost is reduced, and the operation is simple and convenient.
Owner:苏州莱克施德药业有限公司
Synthetic method for 4-fluorobenzaldehyde
InactiveCN104098453AHigh reaction yieldMild reaction conditionsCarbonyl compound preparation by hydrolysisHalogenated hydrocarbon preparationChemical synthesisChloride
The invention discloses a synthetic method for 4-fluorobenzaldehyde, and belongs to the technical field of chemical synthesis. The synthetic method comprises: taking 4-fluorotoluene as a raw material, performing a chlorination reaction on 4-fluorotoluene and chlorine, so as to generate a benzal chloride product; then under the effect of a composite catalyst of ferric trichloride and zinc chloride, performing heating hydrolysis on the benzal chloride product; and finally after hydrolysis is finished, performing extraction and rectification to obtain 4-fluorobenzaldehyde. The synthetic method has the advantages of less synthetic steps, high reaction yield (77% or more), mild reaction conditions, simple operation, environment friendliness, no pollution, not high equipment requirement, suitableness for industrialization production, and the like.
Owner:SINO HIGH CHINA
Preparation method of Olaparib
The invention discloses a preparation method of Olaparib. The preparation method comprises the following steps: carrying out a reaction between phthalide (II) and 3-bromo-4-fluorobenzaldehyde (III) toobtain a compound IV, carrying out a reaction between the compound IV and hydrazine monohydrate to obtain a compound V, and carrying out a reaction between the compound V and 1-cyclopropylcarbonylpiperazine to obtain the final product of Olaparib (I). The raw materials used in the reaction are cheap and easy to obtain, and the preparation method is simple in process route, high in total yield andfew in byproducts and is applicable to industrial production.
Owner:SHANDONG YUXIN PHARMA CO LTD
Preparation method for 2,6-dichloro-4-fluorobenzaldehyde
InactiveCN108358766APreparation by organometalhalide reactionOrganic compound preparationN dimethylformamideFormylation reaction
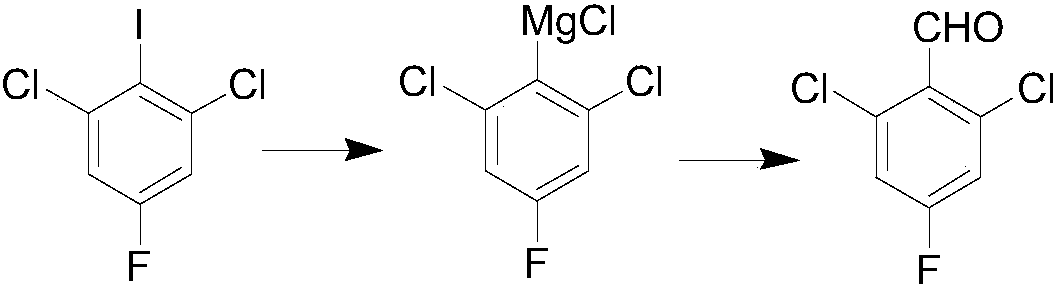
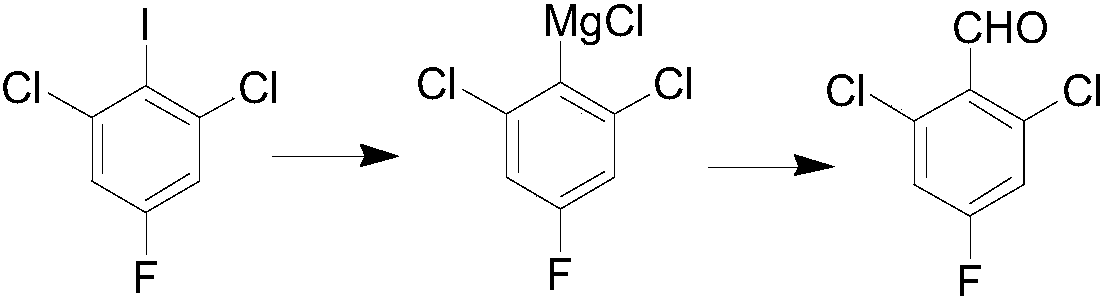
The invention belongs to the technical field of organic synthesis, and particularly relates to a preparation method for 2,6-dichloro-4-fluorobenzaldehyde. The preparation method comprises the following steps: selecting commercially available 1,3-dichloro-2-fluoro-5-iodobenzene as a raw material; under a low-temperature condition, carrying out Grignard exchange reaction on 2,6-dichloro-4-fluoroiodobenzene and isopropylmagnesium chloride to prepare the Grignard reagent of phenylmagnesium chloride; carrying out formylation reaction on the obtained Grignard reagent of phenylmagnesium chloride andN,N-dimethylformamide; after hydrolysis is carried out through diluted hydrochloric acid, obtaining a 2,6-dichloro-4-fluorobenzaldehyde crude product; finally, purifying to obtain a 2,6-dichloro-4-fluorobenzaldehyde finished product. The method has the advantages of abundant raw material, high reaction purity yield, stable technical condition and simpleness in operation, is suitable for mass production and provides a new thought and method for preparing the 2,6-dichloro-4-fluorobenzaldehyde.
Owner:珠海奥博凯生物医药技术有限公司
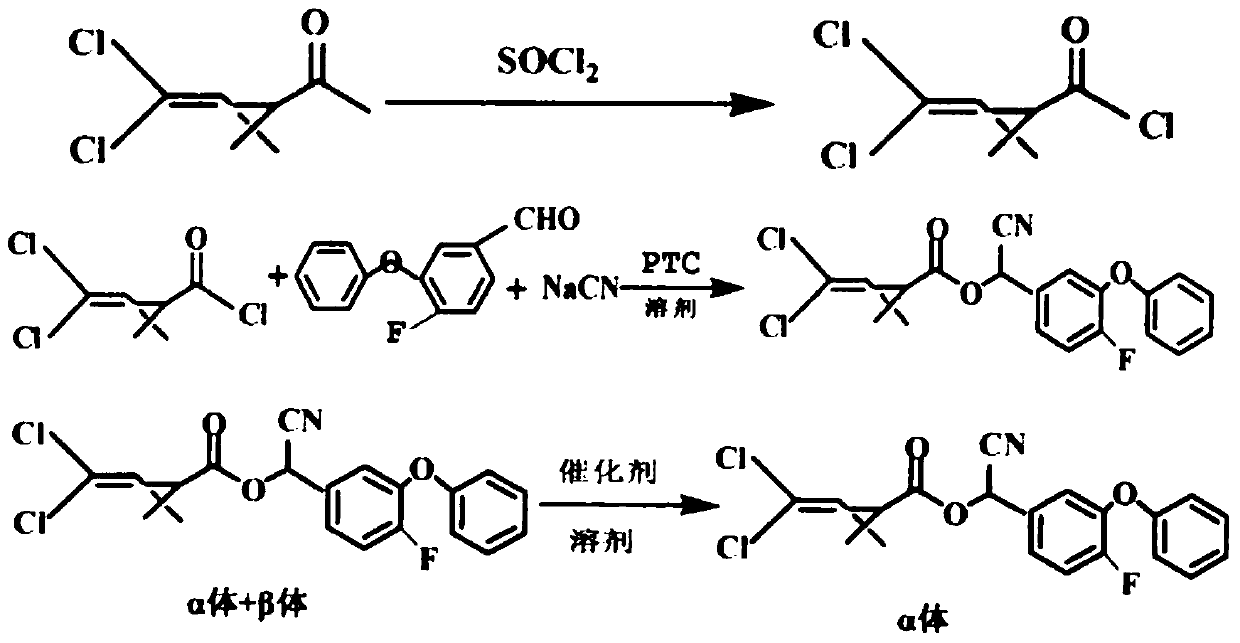
Preparation method of lambda-cyhalothrin
InactiveCN103420872AHigh crude oil contentHigh yieldCarboxylic acid nitrile preparationOrganic compound preparationIsomerizationDistillation
The invention discloses a preparation method of lambda-cyhalothrin. The preparation method of the lambda-cyhalothrin comprises the steps that 3-(2,2-dichloroethenyl)-2,2-dimethylcyclopropanecarboxylic acid serves as an initial raw material, DMF serves as a catalyst, n-hexane serves as solvent, an n-hexane solution of 3-(2,2-Dichlorovinyl)-2,2-dimethylcyclopropanecarbonyl chloride is obtained, a reaction among the n-hexane solution of the 3-(2,2-Dichlorovinyl)-2,2-dimethylcyclopropanecarbonyl chloride, 3-phenoxy-4-fluoro-benzaldehyde and sodium cyanide is carried out, methyl trioctyl ammonium chloride serves as a catalyst, a condensation reaction is carried out to obtain a cyhalothrin condensation compound, washing and desalting are carried out on the cyhalothrin condensation compound to obtain a cyhalothrin n-hexane solution, a composite catalyst is directly added to the cyhalothrin n-hexane solution, and an epimerization reaction is carried out to obtain the lambda-cyhalothrin. Compared with the prior art, the technological process is simple, the solvent does not need to be replaced in the process of preparation, the situation that isopropanol is used for carrying out working procedures such as rectification and dewatering is avoided, and the same solvent is adopted; due to the fact that the composite catalyst is adopted, the rate of the epimerization reaction is improved, and due to the facts that the n-hexane serves as epimerization solvent, and the isopropanol is not adopted, the working procedure of distillation recycling of the isopropanol and the working procedure of dewatering of the isopropanol are omitted, material loss is reduced, production cost is reduced, industrial production can be easily carried out, and popularization prospect and application prospect are wide.
Owner:LIANYUNGANG CCA CHEM CO LTD
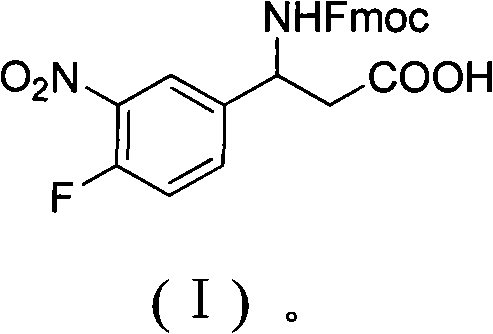
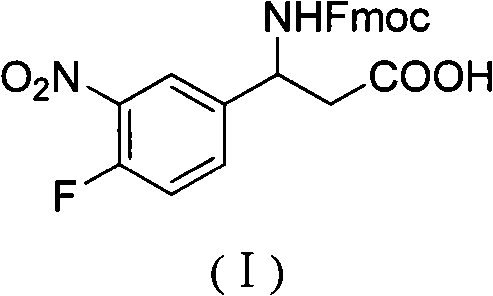
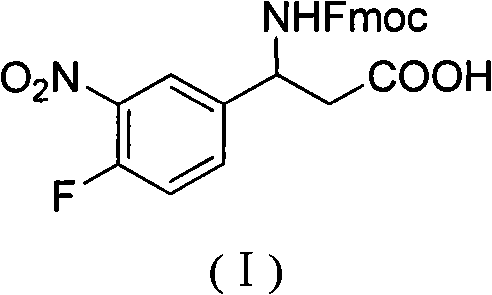
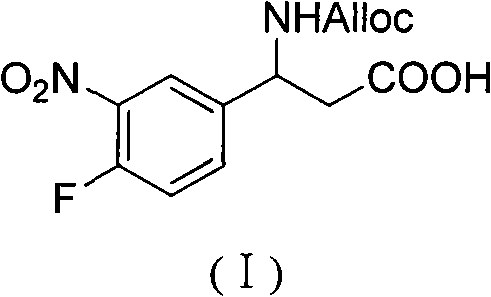
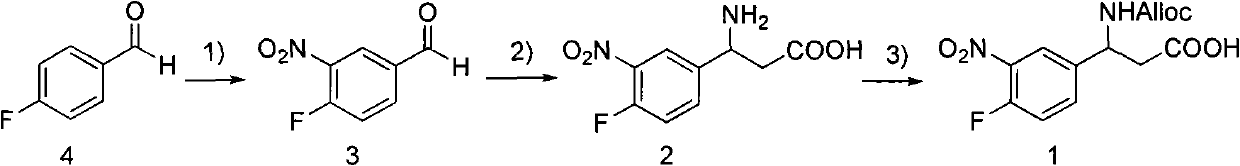
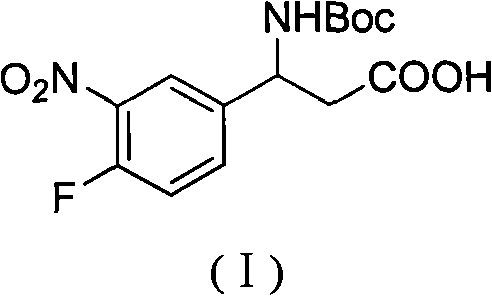
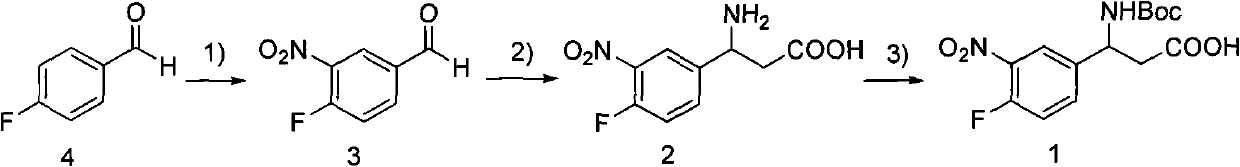
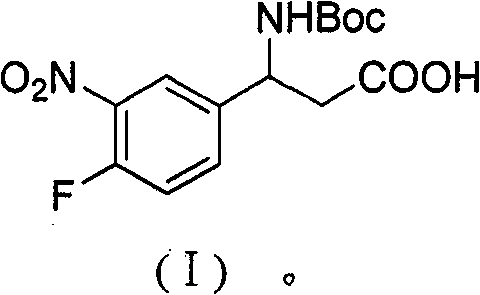
3-Fmoc amino-3-(3-nitro-4-fluophenyl) propionic acid and preparation method thereof
ActiveCN101985432AHas inhibitory activityCarbamic acid derivatives preparationOrganic compound preparationAmino acid synthesisPropanoic acid
The invention relates to a compound in the formula (I) and a preparation method thereof. The method comprises the following steps: 1) 4-fluorobenzaldehydes reacts to generate 3-nitro-4-fluophenyl fluorobenzaldehydes under the action of concentrated sulfuric acid and concentrated nitric acid; 2) the 3-nitro-4-fluophenyl fluorobenzaldehydes, malonic acid and ammonium acetate reflux in an alcohol solvent for 6 to 8 hours to prepare 3-amino-3-(3-nitro-4-fluophenyl) propionic acid; and 3) the 3-amino-3-(3-nitro-4-fluophenyl) propionic acid, a sodium carbonate solution with the weight percentage concentration of 8 to 12% and dioxane are mixed to form a suspension and the solution of Fmoc Cl or Fmoc-Osu and the dioxane is added in the suspension and reacts with the suspension to generate 3-Fmoc amino-3-(3-nitro-4-fluophenyl) propionic acid. The 3-Fmoc amino-3-(3-nitro-4-fluophenyl) propionic acid of the invention is combined with other amino acids to obtain a series of polypeptide compounds with certain inhibition activity on histone deacetylase (HDAC), and has the advantages of simple preparation method, easy obtainment of the raw materials, and high yield.
Owner:合肥华纳生物医药科技有限公司
Preparation method of 2-bromine-4-fluorobenzaldehyde
InactiveCN109809977AOptimize purification stepsSimple purification methodOrganic compound preparationCarbonyl compound separation/purificationIce waterPurification methods
The invention belongs to the technical field of organic synthesis, and particularly relates to a preparation method of 2-bromine-4-fluorobenzaldehyde. The method comprises the following steps that (1)4-fluorobenzaldehyde is dissolved in an acid solution to be prepared into 0.1 to 100mol / L of 4-fluorobenzaldehyde acid solution; (2) the temperature of the solution is raised to 30 to 100 DEG C; bromination reagents are added while stirring is performed; stirring reaction is performed for 1 to 24h; (3) the bromination reagents are added again; stirring reaction is performed for 24 to 72h; (4) after the reaction is finished, reaction liquid is poured into ice water; (5) water phases are extracted by alkane solvents; organic phases are merged and are washed; pressure reduction concentration isperformed to remove organic solvents; 2-bromine-4-fluorobenzaldehyde crude products are obtained; the crude products are refined; the target product of 2-bromine-4-fluorobenzaldehyde is obtained. Theraw materials are cheap and can be easily obtained; the used bromination reagents are environment-friendly water treatment agents; the purification method of the target product is simple; the industrial production is facilitated.
Owner:JIANGSU UNIV OF TECH
Preparation of 4-(4-fluorophenyl)-6-isopropyl-2-(N-methyl-N-methylsulfonyl amido) pyrimidine-5-formaldehyde
The invention provides a method for preparing a Rosuvastatin intermediate, 4-Fluorophenyl-6-isopropyl-2-(N-methyl-N-methylsulfonyl amino) pyrimidinyl-5-formyl. The method uses isobutyryl acetonitrile, 4-Fluorobenzaldehyde and carbamide as raw materials and is obtained by steps of cyclization, oxidation, substitution and reduction etc. The method of the invention dose not need expensive raw materials and has the advantages of low technological cost, simple reaction, high product yield and being applicable to industrialized production.
Owner:滁州市庆云医药有限公司
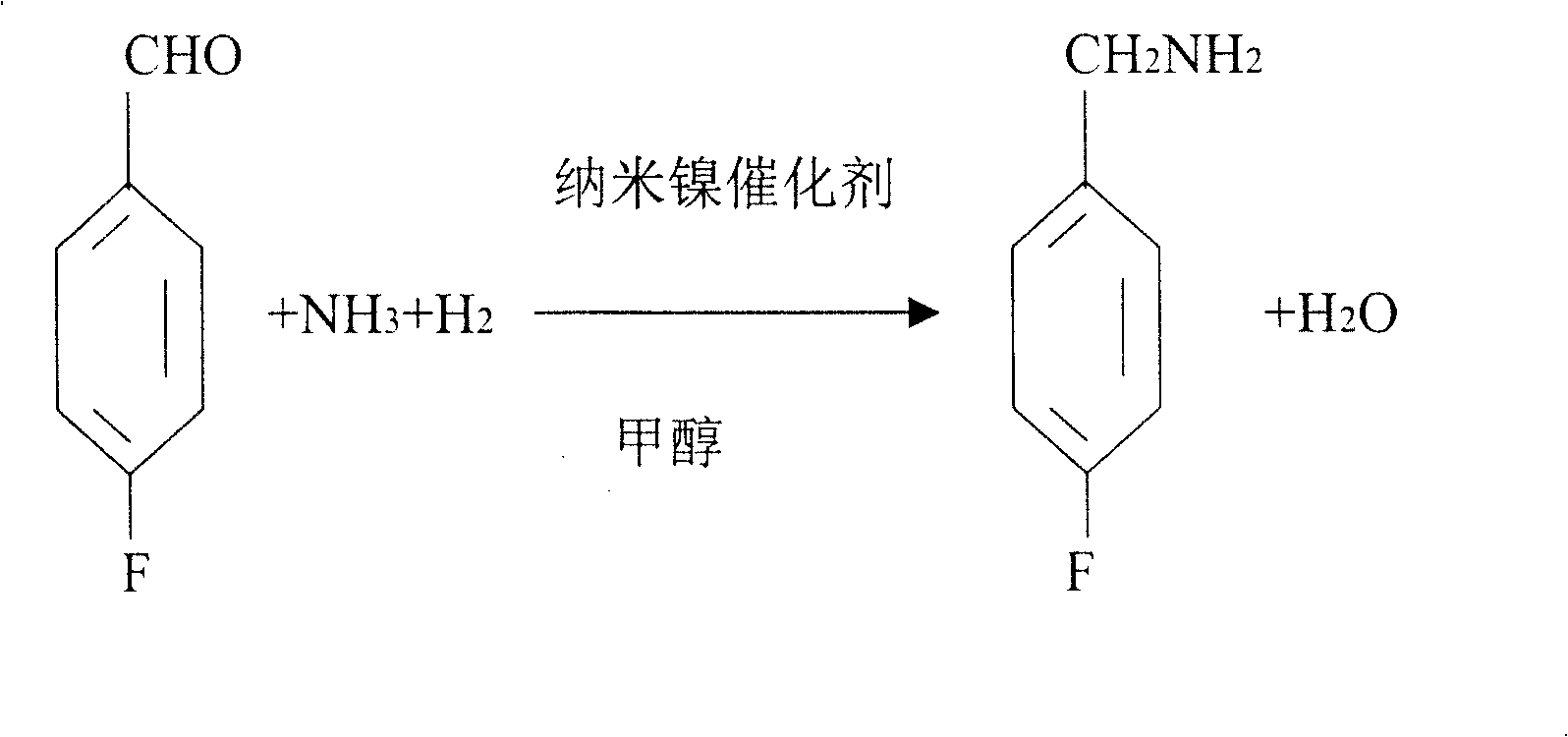
Process for preparing 4-fluorobenzylamine with nano nickel as catalyst
InactiveCN100453527CReduce dosageLow costPreparation by reductive alkylationHydrogen4-fluorobenzylamine
The invention provides a process for preparing 4-fluorobenzylamine with nano nickel as catalyst, which employs 4-fluorobenzaldehyde, hydrogen, absolute methanol, liquid ammonia as raw material. Test results show that, under the same reaction conditions, the process of the present invention can realize an average yield of above 95%, and a purity above 99%.
Owner:张炳庚
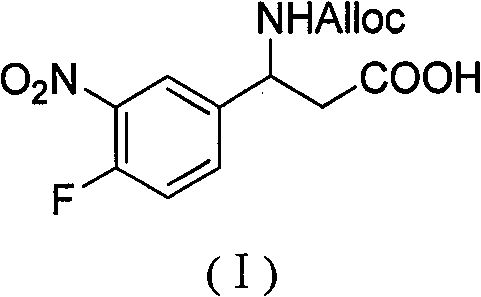
3-alloc amido-3-(3- nitryl-4-fluorophenyl) propionic acid and preparation method thereof
InactiveCN101967113BHas inhibitory activityCarbamic acid derivatives preparationOrganic compound preparationAmino acid synthesisPropanoic acid
The invention relates to a 3-alloc amido-3-(3- nitryl-4-fluorophenyl) propionic acid and a preparation method thereof. The method comprises the following steps of: (1) generating 3-nitryl-4-fluorobenzaldehyde by reacting 4-fluorobenzaldehyde under the action of concentrated sulfuric acid and concentrated nitric acid; (2) refluxing the 3-nitryl-4-fluorobenzaldehyde, malonic acid and ammonium acetate in an alcoholic solvent for 6-8 h to obtain 3-amido-3-(3- nitryl-4-fluorophenyl) propionic acid; and (3) mixing the 3-amido-3-(3- nitryl-4-fluorophenyl) propionic acid with 8-12% sodium carbonate solution in percentage by weight and dioxane to form a suspension, adding a AllocCl and dioxane solution to the suspension and reacting to generate 3-alloc amido-3-(3- nitryl-4-fluorophenyl) propionic acid. A series of peptide compounds obtained by synthesizing the 3-alloc amido-3-(3-nitryl-4-fluorophenyl) propionic acid with other amino acids have a certain inhibitory activity on HDAC (Histone Deacetylase). The invention has the advantages of simple preparation method, easily obtained raw materials and high yield.
Owner:BEIJING OKEANOS TECH
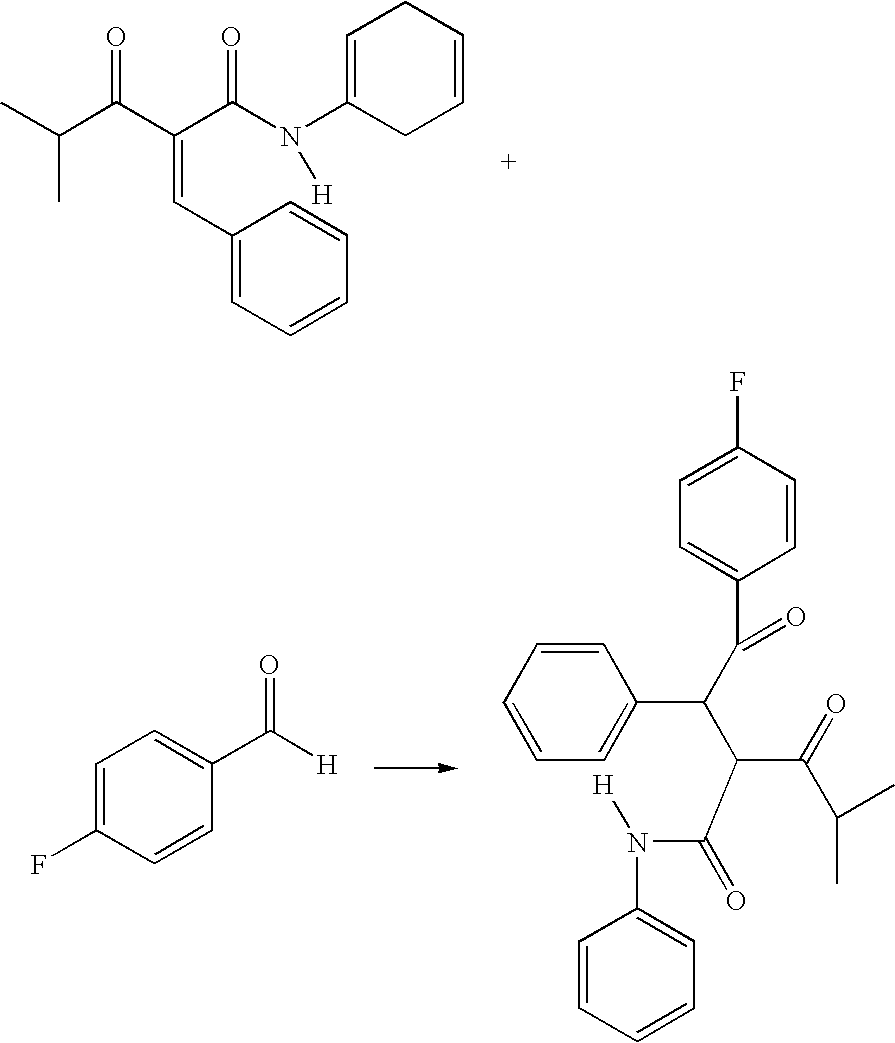
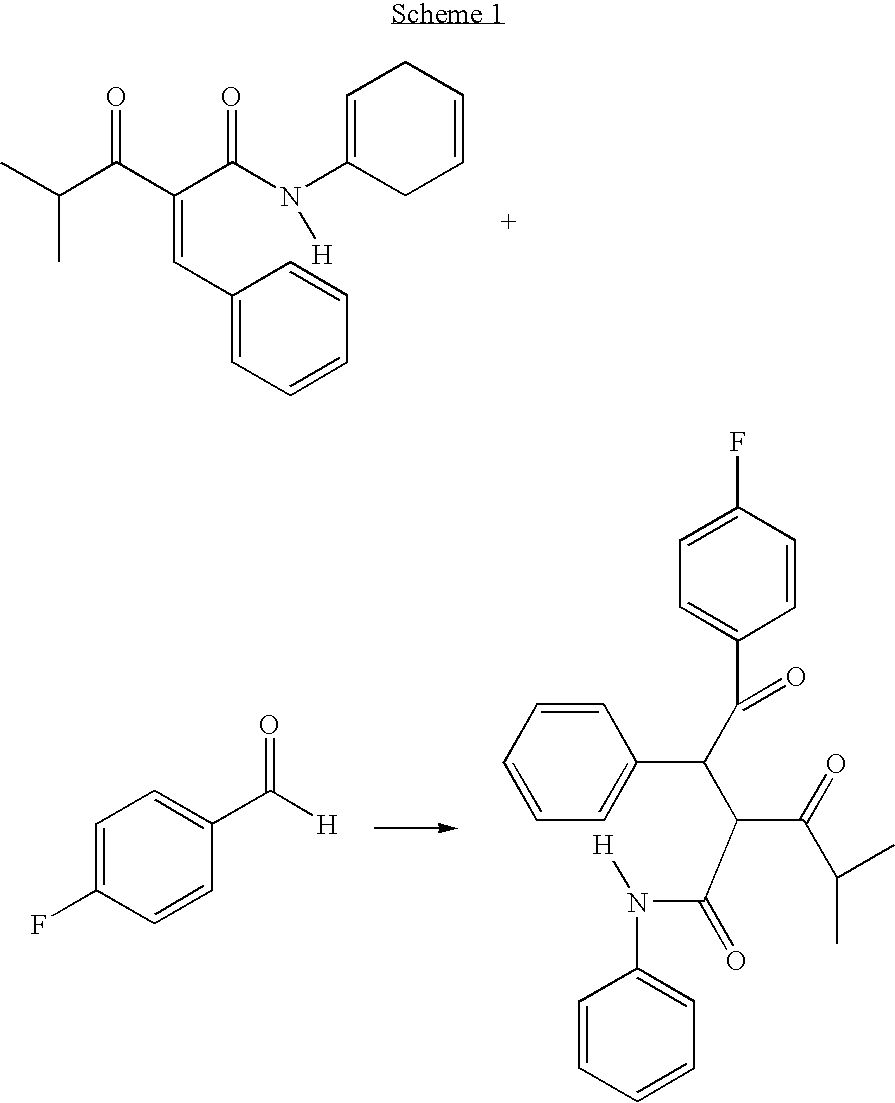
Preparation of an Atorvastatin Intermediate
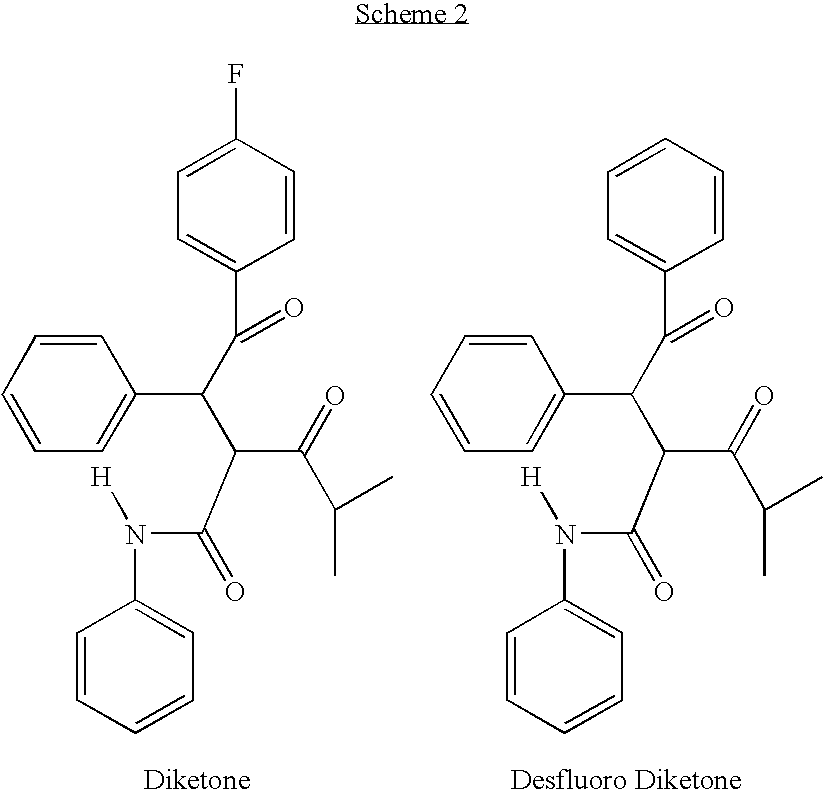
InactiveUS20090221852A1Minimized impurityMinimize formationOrganic compound preparationCarboxylic acid amides preparationDiketoneOxygen
The diketone of atorvastatin is prepared by first washing a reaction vessel with a non-ketonic solvent, especially tetrahydrofuran, to remove water. 4-fluorobenzaldehyde is then reacted with benzylidine isobutyryl acetanilide in the reaction vessel to form 4-fluoro-alpha-(2-methyl-1-oxopropyl)-gamma-oxo-N,beta-diphenylbenzene-butanainide
Owner:PFIZER INC
3-Boc amidocyanogen-3-(3-nitryl-4-fluorophenyl) monoprop and preparation method thereof
ActiveCN101962349BHas inhibitory activityCarbamic acid derivatives preparationMetabolism disorderAmino acid synthesisHistone deacetylase
The invention relates to a compound disclosed as a formula (I) and a preparation method thereof. The preparation method comprises the following steps of: (1) reacting 4-fluorobenzaldehyde to generate 3-nitryl-4-fluorobenzaldehyde under the actions of oil of vitriol and strong nitric acid; (2) refluxing the 3-nitryl-4-fluorobenzaldehyde, propane diacid and ammonium acetate for 6-8 hours in an alcoholic solvent to obtain 3-nitryl-3-(3-nitryl-4-fluorophenyl) monoprop; (3) mixing the 3-nitryl-3-(3-nitryl-4-fluorophenyl) monoprop, a sodium carbonate solution with mass percent concentration of 8-12 percent and dioxane to form suspending liquid, adding Boc2O and a dioxane solution into the suspending liquid, and reacting to generate the 3-Boc amidocyanogen-3-(3-nitryl-4-fluorophenyl) monoprop.The 3-Boc amidocyanogen-3-(3-nitryl-4-fluorophenyl) monoprop is synthesized with other amino acids to obtain a series of polypeptide compounds which have certain inhibitory activity with histone deacetylase (HDAC). The invention has simple preparation method and high yield, and raw materials are easy to obtain.
Owner:合肥华纳生物医药科技有限公司
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com

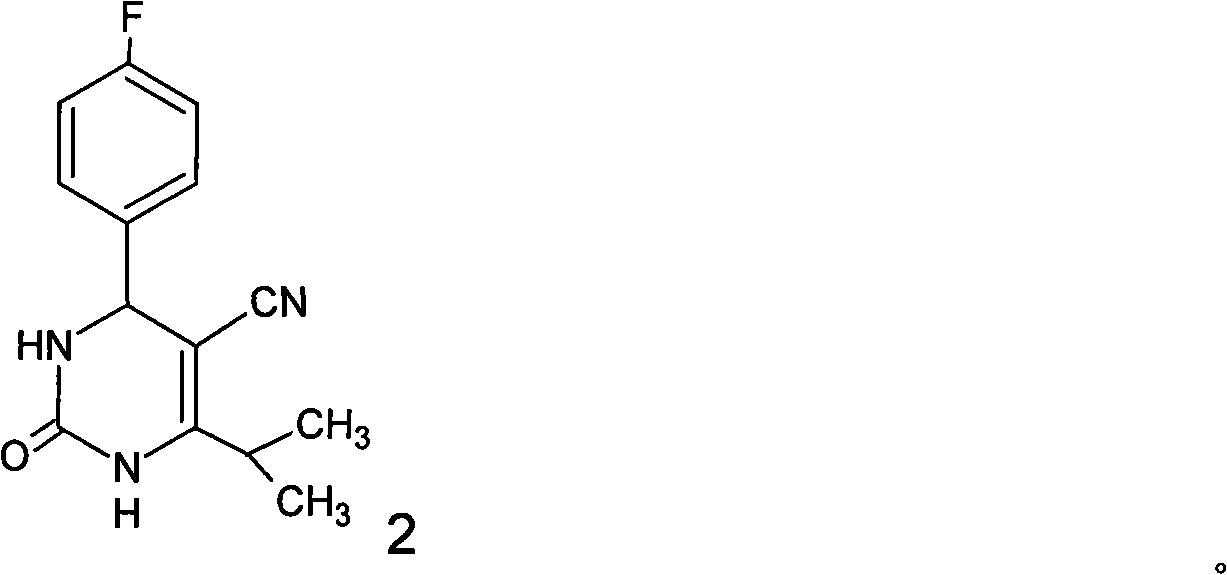


![Process for the preparation of 5-[4-[2-[N-methyl-N-(2-pyridyl) amino] ethoxy] phenyl methyl] thiazolidine-2, 4-dione maleate Process for the preparation of 5-[4-[2-[N-methyl-N-(2-pyridyl) amino] ethoxy] phenyl methyl] thiazolidine-2, 4-dione maleate](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/c18dcf06-a4a8-4073-9c43-844b563778e7/US20050043539A1-20050224-C00001.png)