Preparation method of retigabine and intermediate thereof
A technology of retigabine and its compound, which is applied in the field of pharmaceutical synthesis and can solve problems such as increased raw material costs, low selectivity, and poor selectivity of ethyl chloroformate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
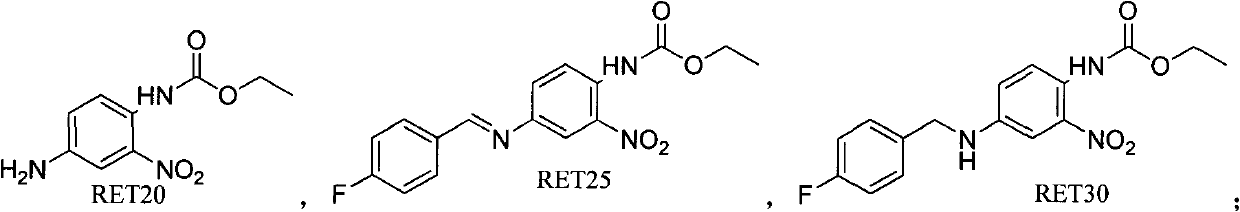
[0066] Embodiment 1: the preparation of formula RET25 compound
[0067]
[0068] Compound of formula RET20 (22.52g, 100mmol) and 4-fluorobenzaldehyde (11.59mL, 110mmol) were dissolved in 400ml of toluene, TsOHH was added 2 O (0.57g, 3mmol), heated to reflux and dewatered with a water separator, reacted for 4 hours, concentrated the solvent to obtain 33.1g of the compound of formula RET25, and the reaction yield was 100%.
Embodiment 2
[0069] Embodiment 2: the preparation of formula RET30 compound
[0070]
[0071] Dissolve the compound of formula RET25 (10 g, 30 mmol) prepared in Example 1 in ethyl acetate, keep the reaction temperature below 10° C., add sodium borohydride (1.7 g, 45 mmol), then drop methanol 15 ml, and react for 4 hours. After the reaction was complete, the reaction was quenched with 100ml of water, extracted with ethyl acetate (100ml×2), the organic phases were combined, and the organic phase was dissolved and washed with 5% sodium chloride water, and the organic phase was concentrated to obtain 9.2g of the compound of formula RET30, with a yield of 92 %.
Embodiment 3
[0072] Embodiment 3: the preparation of Retigabine
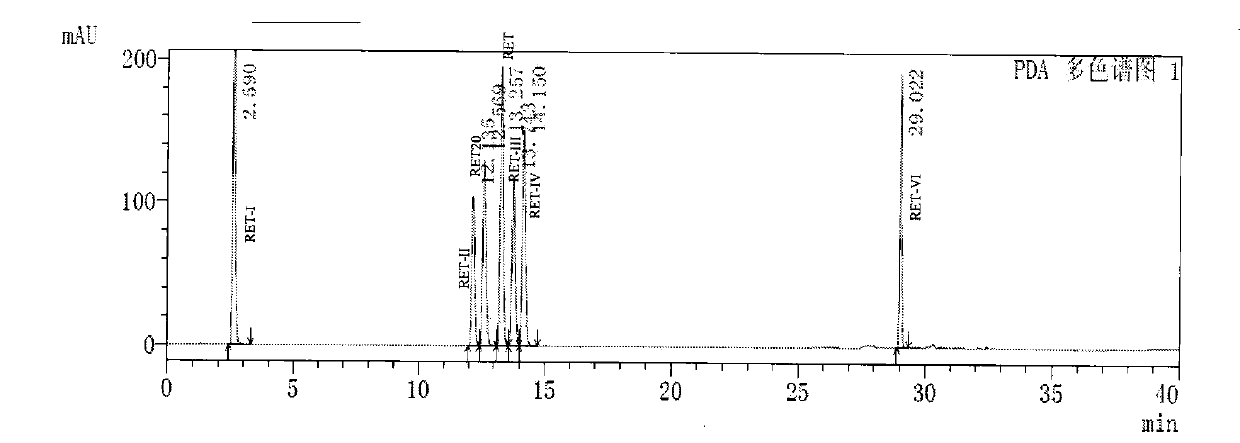
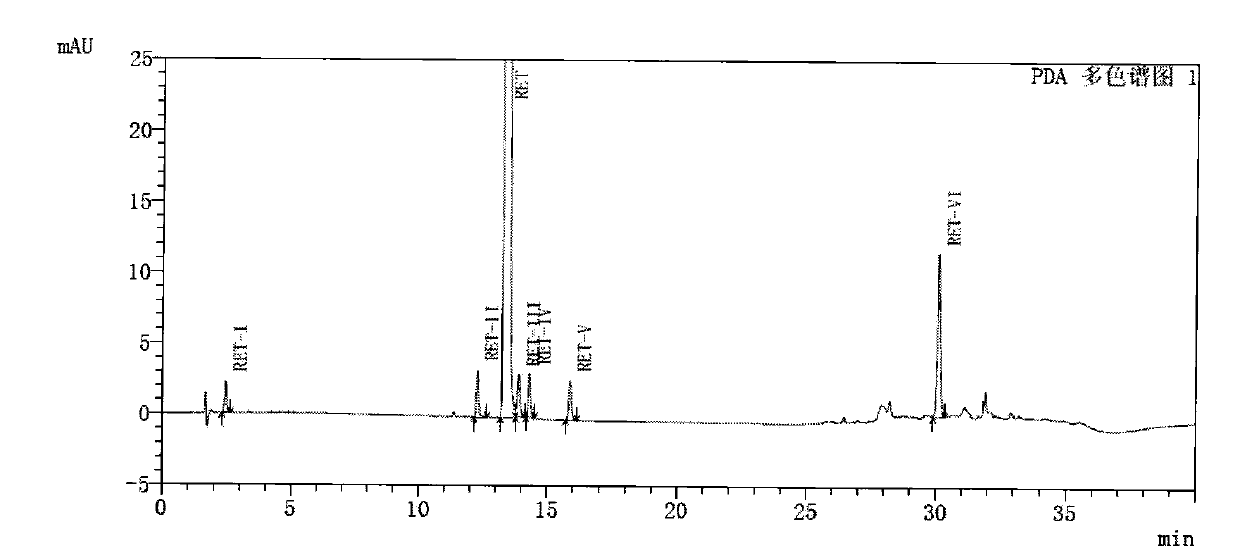
[0073]Dissolve the compound of formula RET30 (5 g, 15 mmol) prepared in Example 2 in ethyl acetate, add 0.6 g of Raney nickel, hydrogen pressure of 1.1 MPa, and react at 55-60° C. for 15 hours. After the reaction is completed, filter and remove Raney nickel, the reaction solution was concentrated, and recrystallized with ethyl acetate to obtain 3.8 g of retigabine with a yield of 83.6%.
[0074] HPLC analysis method:
[0075] Chromatograph: Shimadzu 20AD HPLC or other similar liquid chromatograph;
[0076] Chromatographic column: Agilent ZORBAX SB-C18, 150*4.6mm, 3.5μm;
[0077] Mobile phase A: weigh 1.36g KH 2 PO 4 In 1000ml of water, add 0.5g of sodium heptanesulfonate, adjust the pH to 2.5 with phosphoric acid, filter and degas;
[0078] Mobile phase B: acetonitrile;
[0079] Column temperature: 30°C;
[0080] Flow rate: 1.0ml / min;
[0081] Detection wavelength: UV 250nm;
[0082] Injection volume: 10μl;
[0083...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com