Preparation method of lambda-cyhalothrin
A technology of high-efficiency cyfluthrin and cyfluthrin, which is applied in the field of pesticide chemical industry, can solve the problems of difficult industrialized production, harsh reaction conditions, complicated preparation process, etc., and achieves the effects of reducing distillation recovery, improving reaction rate and simple process flow
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
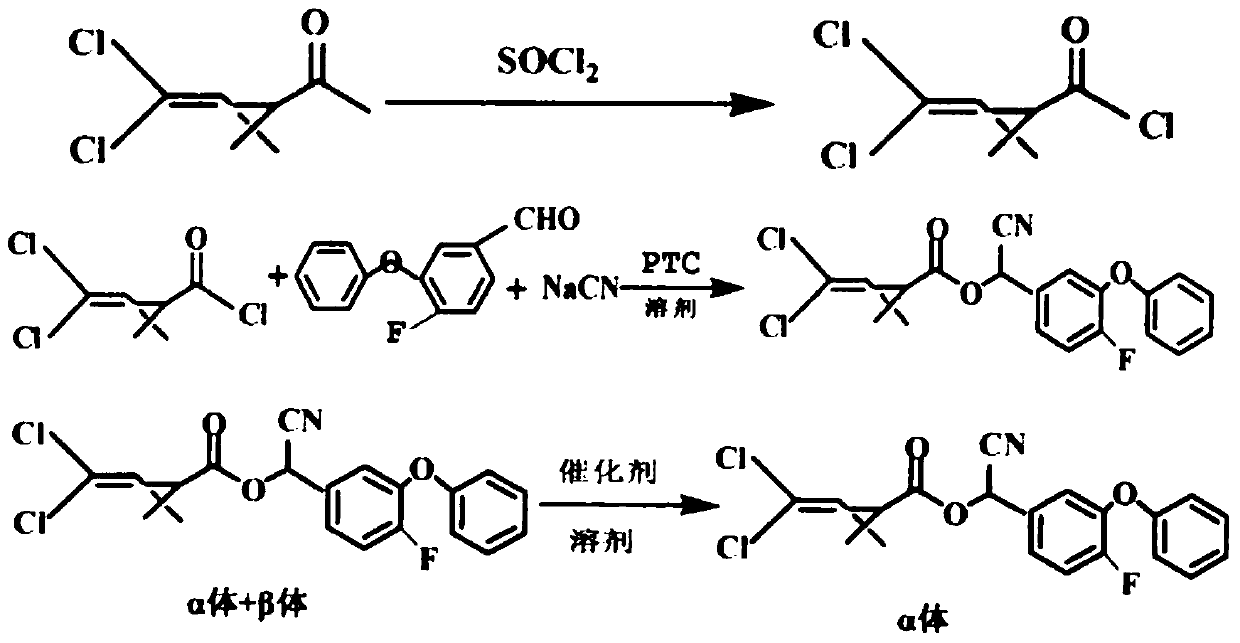
[0019] (1) Synthesis of Dichlorochrysanthemum Acid Chloride:
[0020] In a 1000ml four-necked flask equipped with a thermometer, ventilation duct, and tail gas exhaust pipe, put 400ml of n-hexane, add 210g of permethrin, add 1.2g of DMF as a catalyst under stirring, add 136g of thionyl chloride dropwise under heating, and dropwise add After completing the heat preservation reaction for 6 hours, GC tracked and detected the complete conversion of permethrin, and recovered excess thionyl chloride by distillation under reduced pressure to obtain 404 g of permethrin-chloride n-hexane solution.
[0021] (2) Synthesis of cyfluthrin:
[0022] Put 28.5g of sodium cyanide and 130g of water into a 1000ml reaction bottle, stir until completely dissolved, add 0.108g of trioctylmethyl ammonium chloride and 108g of fluoroether aldehyde, stir and add 202g of dichlorochrysanthemum chloride-n-hexane solution drop by drop Add temperature at 25°C, add dropwise evenly for 3 hours, take samples fo...
Embodiment 2
[0026] Change the reaction condition of the first step, other steps are as embodiment 1, namely:
[0027] (1) Synthesis of Dichlorochrysanthemum Acid Chloride:
[0028] In a 1000ml four-necked flask equipped with a thermometer, a ventilation duct, and an exhaust pipe, put 400ml of n-hexane, add 210g of permethrin, add 3.0g of DMF as a catalyst under stirring, add 160g of thionyl chloride dropwise under heating, and dropwise add After completing the heat preservation reaction for 2 hours, GC tracked and detected the complete conversion of permethrin, and recovered excess thionyl chloride by distillation under reduced pressure to obtain 405 g of permethrin-chloride n-hexane solution.
Embodiment 3
[0030] Change the reaction condition of the 3rd step, other steps are as embodiment 1, namely:
[0031] (3) Synthesis of beta-cyfluthrin:
[0032] Add 390g of cyfluthrin n-hexane solution into a stirred four-necked flask equipped with a thermometer, stir and add 39g of epimerization catalyst (that is, a composite catalyst of triethylamine:diisopropylamine=4:6) at 25°C, and then Keep warm for 8 hours, add 19.5g seed crystals at 20°C and keep warm for 12 hours, keep warm at 15°C for 48 hours, and keep warm at 10°C for 24 hours. Sampling was performed to detect that the α-body content reached 95%, then the reaction was terminated, and 203 g of lambda-cyfluthrin was obtained by suction filtration and drying.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com