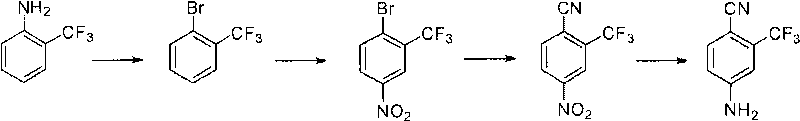
Preparation method of 2-trifluoromethyl-4-aminobenzonitrile
A technology of trifluoromethyl fluorobenzene and trifluoromethyl, which is applied in the field of preparation of 2-trifluoromethyl-4-aminobenzonitrile, can solve the problems of many by-products, the discharge of three wastes affecting environmental protection, and the destruction of waste water.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
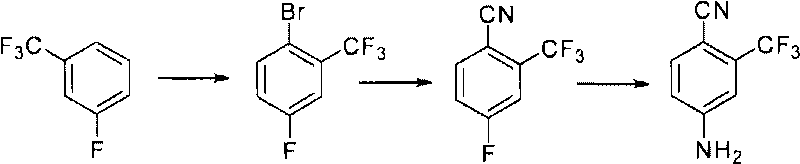
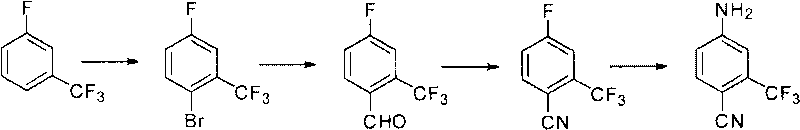
[0073] The inventor also provides a preferred specific embodiment, the preparation method of 2-trifluoromethyl-4-aminobenzonitrile includes using m-trifluoromethyl fluorobenzene as the main raw material, through bromination, grignardization and formylation , cyanide, amino substitution four-step synthesis, the preparation steps are as follows;
[0074] (1) Bromination: react m-trifluoromethylfluorobenzene with brominating agent in acid to generate 2-bromo-5-fluorobenzotrifluoride;
[0075] (2) Grignardization and formylation: under the reaction of magnesium chips and organic solvents, the Grignard reagent is obtained, and then reacted with N,N-dimethylformamide (DMF) through continuous reaction to obtain 2-trifluoro Methyl-4-fluorobenzaldehyde;
[0076] (3) Cyanide: 2-trifluoromethyl-4-fluorobenzaldehyde is reacted with hydroxylamine hydrochloride and Lewis acid to generate 2-trifluoromethyl-4-fluorobenzonitrile;
[0077] (4) Amination: react 2-trifluoromethyl-4-fluorobenzon...
Embodiment 1
[0088] The first step of positioning bromination: add 300g m-trifluoromethylfluorobenzene (1.83mol), 550g concentrated sulfuric acid (5.61mol), and 110g glacial acetic acid (1.83mol) into the reaction kettle, stir and heat up to 20°C, and add in batches 366 g (1.28 mol) of dibromohydantoin was reacted at heat preservation, deiced, and washed with water to obtain 380 g (1.56 mol) of 2-bromo-5-fluoro-trifluorotoluene with a content of more than 95%.
[0089] The second step of Grignard and formylation: add 39g magnesium chips (1.625mol) and 1200mlTHF into the reaction kettle, stir and heat up to 20°C, add dropwise 300g of 2-bromo-5-fluoro-trifluorotoluene (1.23mol) and keep it warm DMF 117g was added dropwise, and hydrochloric acid was added to destroy the reaction after 2 hours, THF was evaporated, and 218g (1.14mol) of 2-trifluoromethyl-4-fluorobenzaldehyde was obtained by layering, with a content of more than 98%.
[0090] The third step cyanation reaction: 200g2-trifluoromet...
Embodiment 2~5
[0093] Proceed in the same manner as in Example 1, except that the type of brominating agent in the first step of positioning bromination, the molar ratio of m-trifluoromethylfluorobenzene: brominating agent, and the reaction temperature are as shown in Table 1. Table 1 also shows the yield and purity of 2-bromo-5-fluoro-benzotrifluoride.
[0094] Table 1
[0095]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com