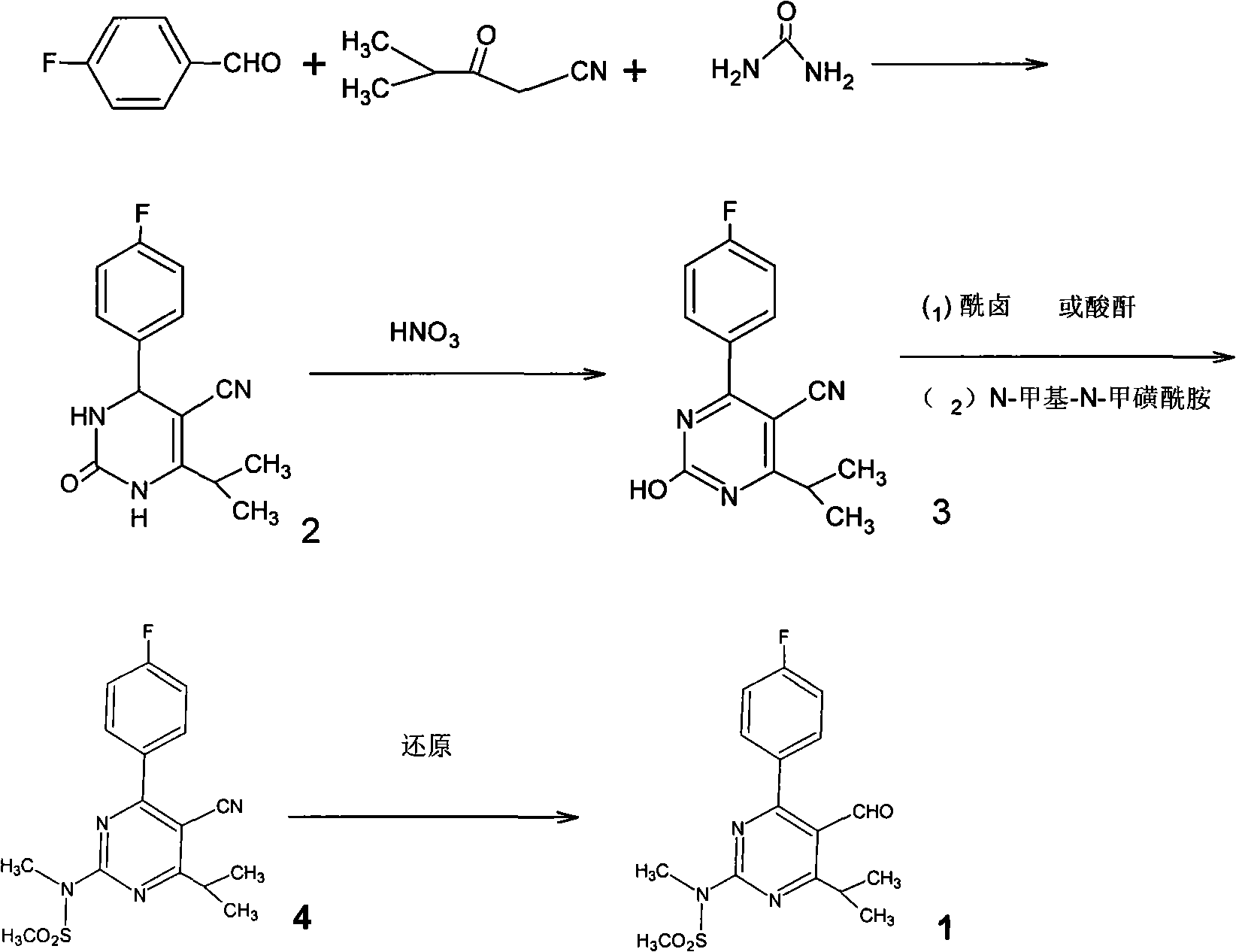
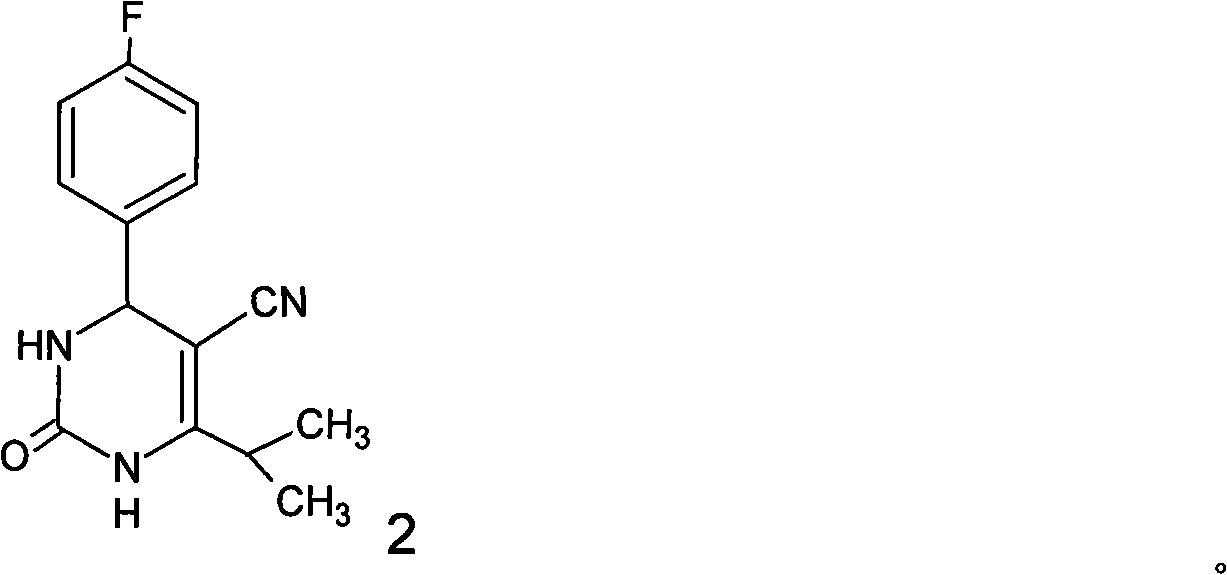Preparation of 4-(4-fluorophenyl)-6-isopropyl-2-(N-methyl-N-methylsulfonyl amido) pyrimidine-5-formaldehyde
A technology of methanesulfonylamino and methanesulfonylamino, which is applied in the field of pharmaceutical compound preparation, can solve the problems of long synthesis route, high energy consumption, unfavorable industrial production and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0107] [Example 1] The preparation method of 4-(4-fluorophenyl)-6-isopropyl-5-cyano-3,4-2(1H)-dihydropyrimidinone (formula 2)
[0108] Add 52.2g (0.47mol) isobutyrylacetonitrile, 58.3g (0.47mol) 4-fluorobenzaldehyde, 49.4g (0.82mol) urea, 0.53g (5.3 mmol) ketone chloride (I), 5.3 ml sulfuric acid and 600 ml methanol. The material in the flask was refluxed for 22 hours under stirring and heating, cooled to room temperature, a solid was produced, filtered, washed the filter cake with methanol, combined the filtrate and washings, and concentrated under reduced pressure to obtain 101.1g of 4-(4-fluorobenzene yl)-6-isopropyl-5-cyano-3,4-2(1H)-dihydropyrimidinone A solid product having the following properties. The yield was 83% (based on the amount of isobutyrylacetonitrile).
[0109] m.p.: 174°C-178°C
[0110] UVλmax (CH3CN, nm): 270
[0111] 1 H-NMR (CDCl3, δ(ppm)): 1.17-1.25 (6H, d, J=6.8Hz), 3.0-3.2 (1H, m), 5.15 (1H, m), 5.6 (1H, m), 7.0 -7.3(2H, m), 7.2-7.4(2H, m), 8.0(...
Embodiment 2
[0112] [Example 2] The preparation method of 4-(4-fluorophenyl)-6-isopropyl-5-cyano-3,4-2(1H)-dihydropyrimidinone
[0113] Repeat the method of Example 1 just to replace 530mg (5.3mmol) ketone chloride (I) with 14.33g (53mmol) iron chloride (III). Hexahydrate, obtain 79.2g4-(4-fluorophenyl)-6- Isopropyl-5-cyano-3,4-2(1H)-dihydropyrimidinone is a colorless crystalline product having the following properties. The yield was 65% (based on the amount of isobutyrylacetonitrile).
Embodiment 3
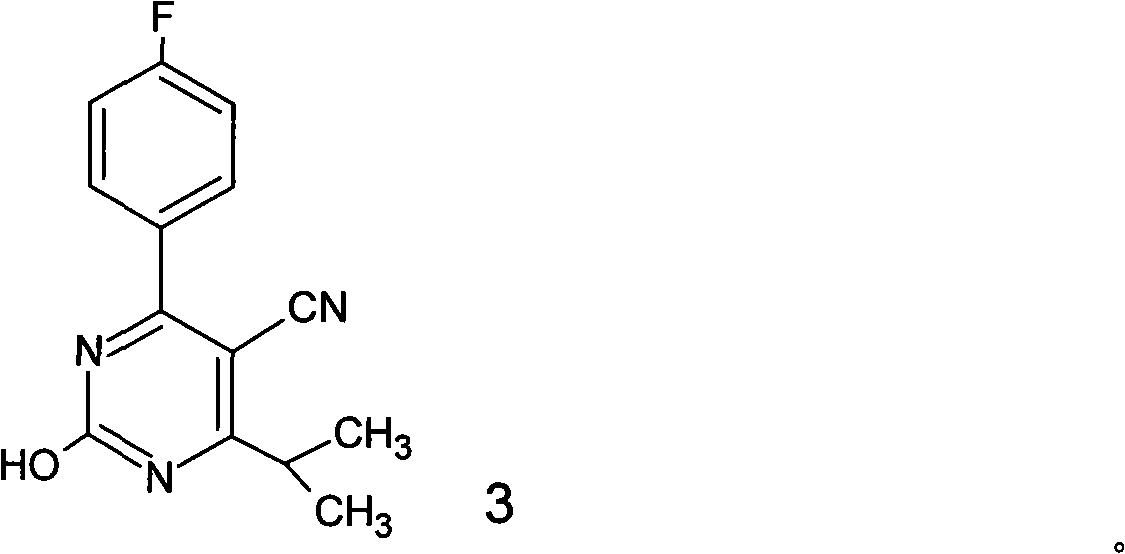
[0114] [Example 3] The preparation method of 4-(4-fluorophenyl)-2-hydroxyl-6-isopropyl-5-cyanopyrimidine (formula 3)
[0115] 120 ml (1.57 mol) of nitric acid (60-61%, sp.gr.: 1.38) were added to a 250 ml glass flask equipped with a stirrer and a thermometer. Slowly add 33 g (0.127 mol) of 4-(4-fluorophenyl)-6-isopropyl-5-cyano-3,4-2 prepared by the same method as in Example 1 to nitric acid below 10°C (1H)-Dihydropyrimidinone, the mixture was reacted at room temperature for 50 minutes. After the reaction was completed, the reaction mixture was neutralized by adding the mixture to 1500 ml of saturated aqueous sodium bicarbonate solution to produce a white solid, which was filtered, washed with water, and dried. 29.26 g of 4-(4-fluorophenyl)-2-hydroxy-6-isopropyl-5-cyanopyrimidine as a white solid product having the following properties were obtained. The yield was 89% (based on the amount of 4-(4-fluorophenyl)-6-isopropyl-5-cyano-3,4-2(1H)-dihydropyrimidinone).
[0116] m.p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com