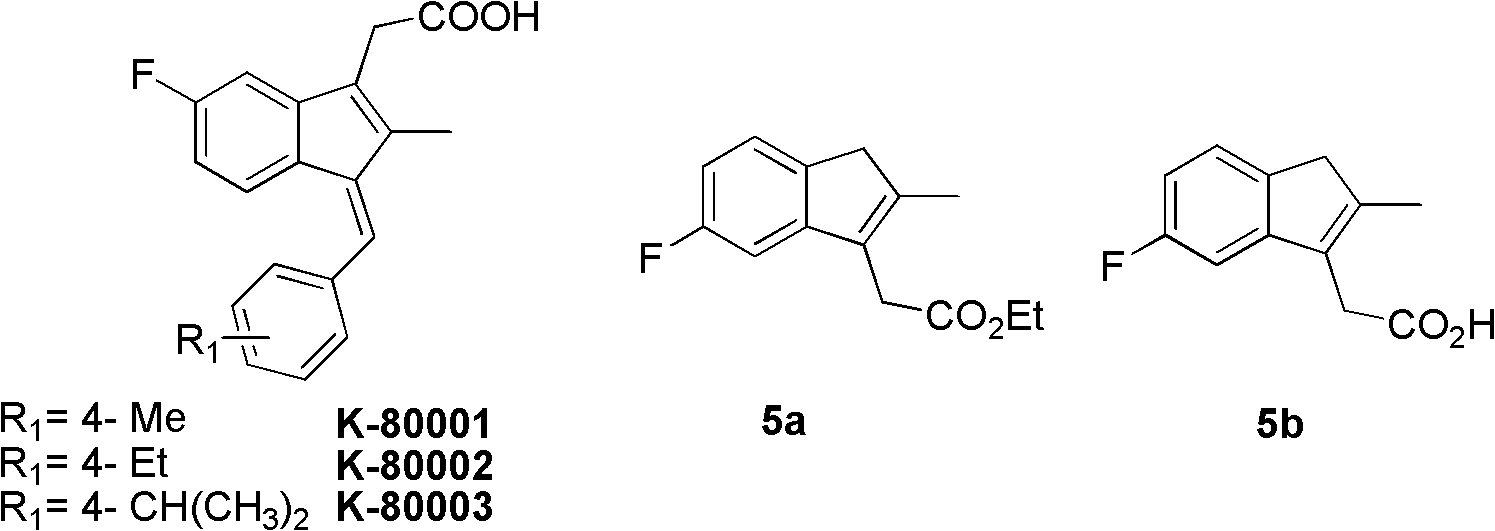
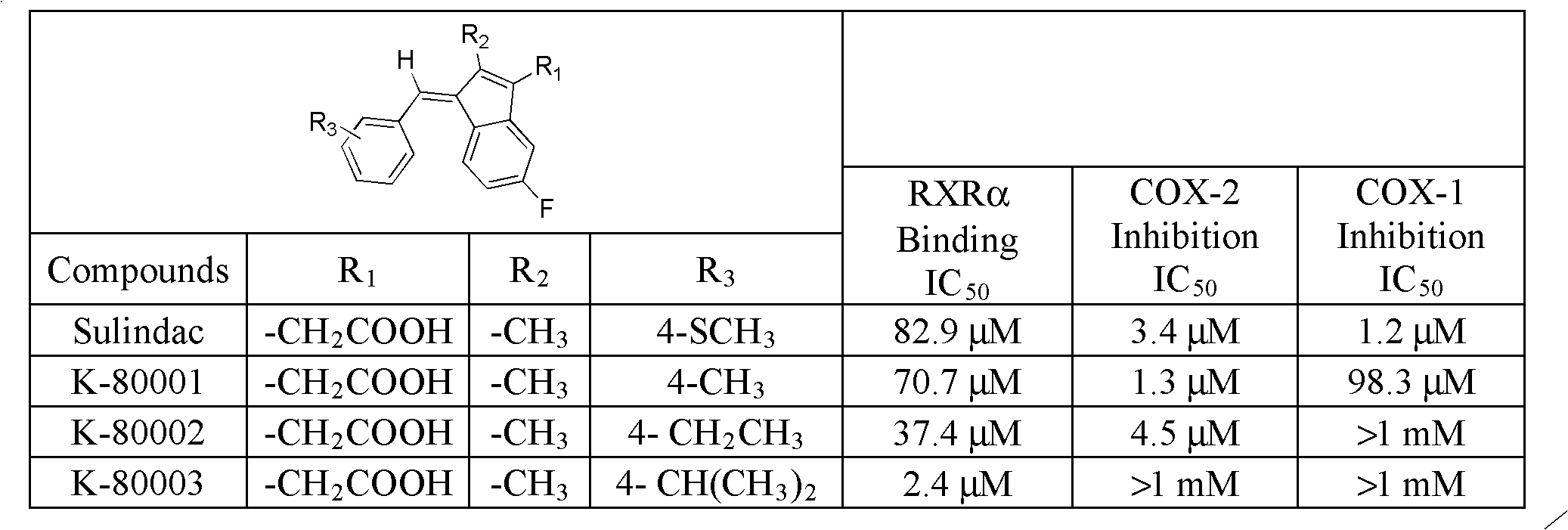
Synthesis method and application of intermediate of sulindac analogue
A synthesis method and analog technology, which are applied in the field of synthesis and application of intermediates of sulindac analogs, can solve problems such as damage to the environment, corrode equipment and air pollution, and achieve reduced equipment corrosion, reduced air pollution, and high yield. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
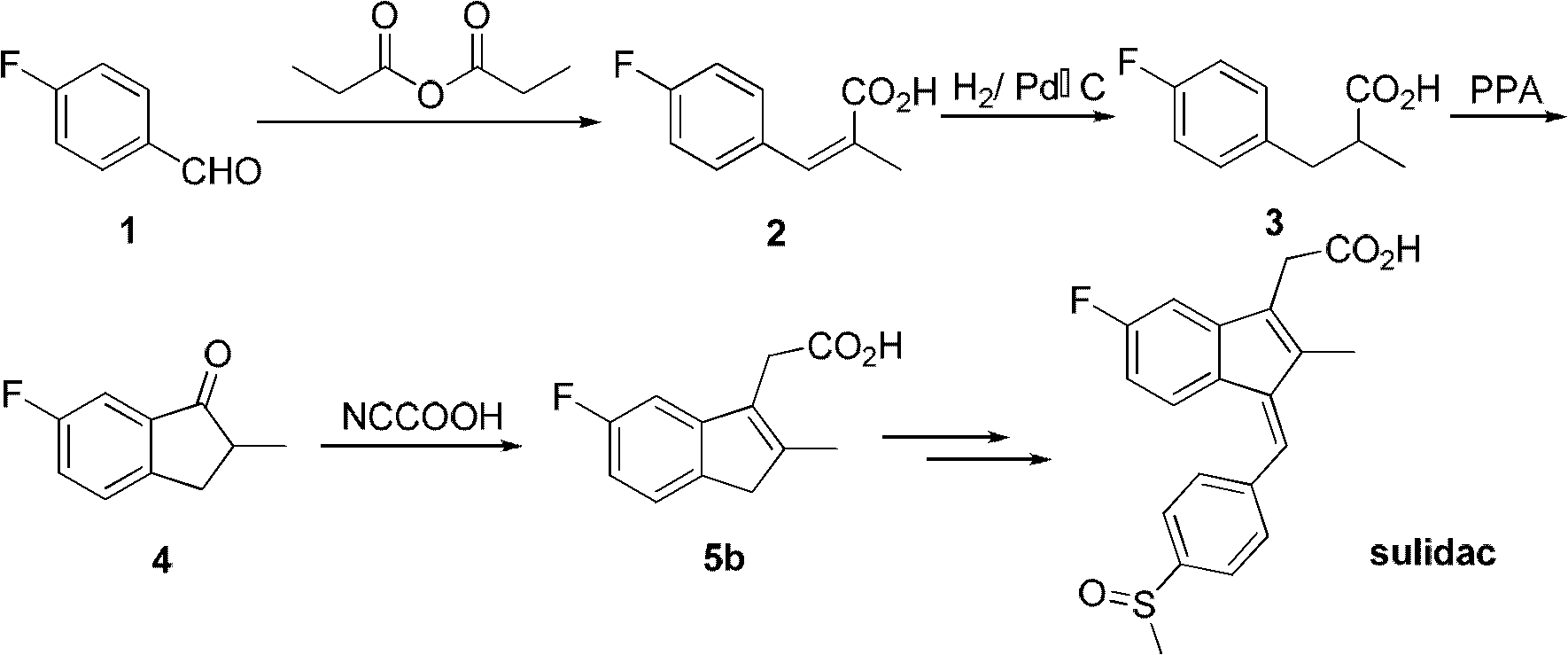
[0037] Embodiment 1: Preparation of 4-fluoro-2-methylcinnamic acid (2)
[0038] In a 500 mL three-necked flask equipped with a condenser, add 52.0 mL (480 mmol) of 4-fluorobenzaldehyde (1), 72.8 g (528 mmol) of anhydrous potassium carbonate powder and 124.0 mL of propionic anhydride. After stirring evenly, slowly heat to 160°C and keep for 12h. After cooling, add 50mL of water, and then add about 10g of sodium carbonate to make the solution alkaline. After slight heating, filter, and carefully acidify the filtrate to pH=2-3 with concentrated hydrochloric acid under the condition of ice-water bath. After the crystals were completely precipitated, suction filtration was performed, and the light yellow crude product was purified to obtain 82.1 g of 4-fluoro-2-methylcinnamic acid (2).
Embodiment 2
[0039] Example 2: Preparation of 3-(4-fluorophenyl)-2-methylpropionic acid (3)
[0040] Dissolve 15.0 g of 4-fluoro-2-methylcinnamic acid (2) in methanol, heat slightly until fully dissolved and add it to a 100 mL autoclave, add 1.2 g of palladium carbon with 10% palladium content, then seal and ventilate hydrogen in 0.8Mpa, stirred at room temperature for 10h. After the reaction, the catalyst was filtered off (recovered and used more than twice), the filter cake was washed with a small amount of methanol, and the filtrate was combined to remove the solvent under reduced pressure to obtain 3-(4-fluorophenyl)-2-methylpropane Acid (3) 14.7g, two-step reaction yield 93%.
Embodiment 3
[0041] Example 3: Preparation of 3-(4-fluorophenyl)-2-methylpropionic acid (3)
[0042] The preparation process of 4-fluoro-2-methylcinnamic acid (2) is the same as in Example 1, except that the base is replaced by sodium propionate, and the reaction time is 16 hours. Then the catalytic hydrogenation reaction is the same as in Example 2 to obtain 3-(4-fluorophenyl)-2-methylpropionic acid (3), and the two-step reaction yield is 87.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com