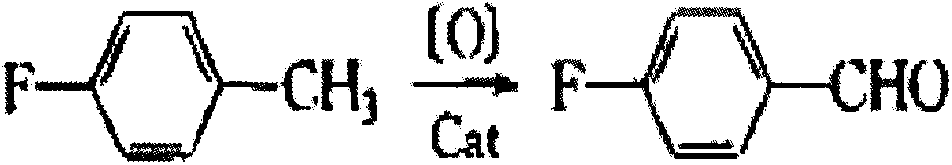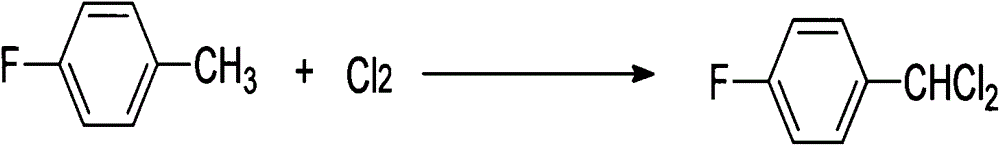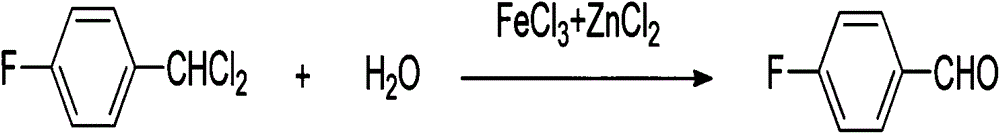Synthetic method for 4-fluorobenzaldehyde
A technology for p-fluorobenzaldehyde and a synthesis method, which is applied in chemical instruments and methods, preparation of carbonyl compounds by hydrolysis, preparation of halogenated hydrocarbons, etc., can solve the problems of low recovery rate and difficult requirements for process equipment, and achieves easy operation and environmental protection. The effect of no pollution equipment requirements and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] (1) 110.5g of p-fluorotoluene is added to 500ml of chloroform, continue feeding chlorine gas in the middle, carry out chlorination reaction, control the progress of the reaction with GC tracking method, make the content of monochlorobenzyl product less than 2%, and the content of trichlorobenzyl product less than 10%,
[0037] (2) Add FeCl to it 3 +ZnCl 2 Catalyst 6.9g, then heat up to 130°C, add 1.7ml of water to initiate the reaction for hydrolysis, control the temperature to reflux at 130°C,
[0038] (3) Add water dropwise in the there-necked flask, make the complete hydrolysis of dichlorobenzyl product,
[0039] (4) After the reaction finishes, adjust the pH value to 8.0~9.0 with a concentration of 30% sodium hydroxide solution, then leave to stand for stratification,
[0040] (5) adding 10% hydrochloric acid to adjust the pH value to 5.0-7.0, adding toluene to extract the organic layer,
[0041] (6) Finally, the solvent was spin-dried, the product was dried, an...
Embodiment 2
[0045] (1) 69g of p-fluorotoluene is added to 500ml of difluorotoluene, continue to feed chlorine gas in the middle, carry out chlorination reaction, control the progress of the reaction with GC tracking method, make the content of monochlorobenzyl product less than 2%, and the content of trichlorobenzyl product less than 2%. 10%,
[0046] (2) Add FeCl to it 3 +ZnCl 2 Catalyst 6.9g, then heat up to 100°C, add 0.5ml of water to initiate the reaction for hydrolysis, control the temperature to reflux at 100°C,
[0047] (3) Add water dropwise in the there-necked flask, make the complete hydrolysis of dichlorobenzyl product,
[0048] (4) After the reaction finishes, adjust the pH value to 8.0~9.0 with a concentration of 30% sodium hydroxide solution, then leave to stand for stratification,
[0049] (5) adding 10% hydrochloric acid to adjust the pH value to 5.0-7.0, adding THF to extract the organic layer,
[0050] (6) Finally, the solvent was spin-dried, the product was dried, ...
Embodiment 3
[0054] (1) 138g of p-fluorotoluene is added to 500ml of difluorotoluene, continue to feed chlorine gas in the middle, carry out chlorination reaction, control the progress of reaction with GC tracking method, make the monochlorobenzyl product content be less than 2%, the trichlorobenzyl product content is less than 10%,
[0055] (2) Add FeCl to it 3 +ZnCl 2 Catalyst 6.9g, then heat up to 150°C, add 5ml of water to initiate the reaction for hydrolysis, control the temperature to reflux at 150°C,
[0056] (3) Add water dropwise in the there-necked flask, make the complete hydrolysis of dichlorobenzyl product,
[0057] (4) After the reaction finishes, adjust the pH value to 8.0~9.0 with a concentration of 30% sodium hydroxide solution, then leave to stand for stratification,
[0058] (5) adding 10% hydrochloric acid to adjust the pH value to 5.0-7.0, adding DMF to extract the organic layer,
[0059] (6) Finally, the solvent was spin-dried, the product was dried, and the organ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com