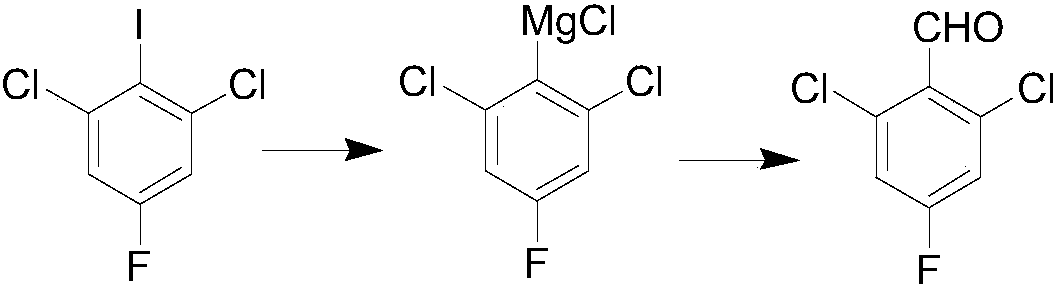
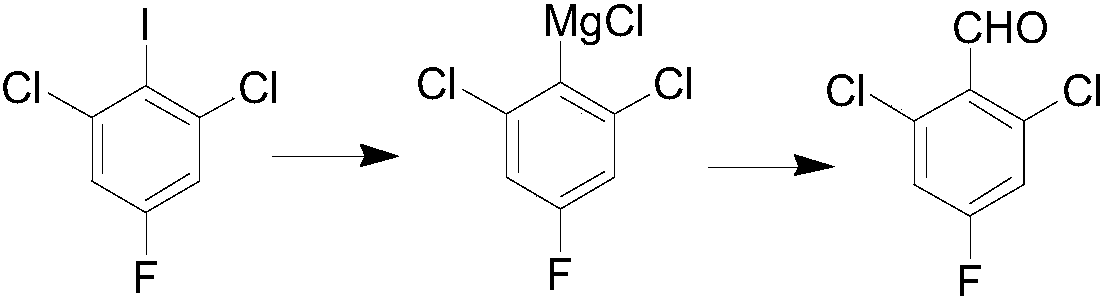
Preparation method for 2,6-dichloro-4-fluorobenzaldehyde
A technology of fluorobenzaldehyde and dichloride, which is applied in the field of preparation of 2,6-dichloro-4-fluorobenzaldehyde, can solve problems such as application limitation, low yield of benzaldehyde, pollute the environment, etc., and achieves simple operation, raw material Easy to obtain, high yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] (1) Preparation of isopropylmagnesium chloride Grignard reagent: first add 20 mL of tetrahydrofuran and 3 g of 2-chloropropane into a 50 mL one-necked flask and stir to make a solution, and set aside. Under the protection of nitrogen, in a 50mL dry three-necked flask, add 1.1g of magnesium chips, a small amount of 2-chloropropane, and a small amount of tetrahydrofuran solvent, raise the temperature to 30-40°C, and initiate the reaction with bromoethane. Then the tetrahydrofuran solution of 2-chloropropane was added dropwise, and after the dropwise addition was completed, an insulation reaction was carried out, and the concentration of the obtained isopropylmagnesium chloride Grignard reagent was 1.7 mol / L.
[0019] (2) Grignard exchange: under the protection of nitrogen, add 10 g of 2,6-dichloro-4-fluoroiodobenzene and 20 ml of tetrahydrofuran into a 100 ml dry three-necked flask. Cool down to 0°C, slowly add the above-prepared isopropylmagnesium chloride Grignard reage...
Embodiment 2
[0023] (1) Preparation of isopropylmagnesium chloride Grignard reagent: first add 20 mL of tetrahydrofuran and 3 g of 2-chloropropane into a 50 mL one-necked flask and stir to make a solution, and set aside. Under the protection of nitrogen, in a 50mL dry three-necked flask, add 1.1g of magnesium chips, a small amount of 2-chloropropane, and a small amount of tetrahydrofuran solvent, raise the temperature to 30-40°C, and initiate the reaction with bromoethane. Then the tetrahydrofuran solution of 2-chloropropane was added dropwise, and after the dropwise addition was completed, an insulation reaction was carried out, and the concentration of the obtained isopropylmagnesium chloride Grignard reagent was 1.7 mol / L.
[0024] (2) Grignard exchange: under the protection of nitrogen, add 10 g of 2,6-dichloro-4-fluoroiodobenzene and 20 ml of tetrahydrofuran into a 100 ml dry three-necked flask. Cool down to -20°C, slowly add the above-prepared isopropylmagnesium chloride Grignard rea...
Embodiment 3
[0028] (1) Preparation of isopropylmagnesium chloride Grignard reagent: first add 20 mL of tetrahydrofuran and 3 g of 2-chloropropane into a 50 mL one-necked flask and stir to make a solution, and set aside. Under the protection of nitrogen, in a 50mL dry three-necked flask, add 1.1g of magnesium chips, a small amount of 2-chloropropane, and a small amount of tetrahydrofuran solvent, raise the temperature to 30-40°C, and initiate the reaction with bromoethane. Then the tetrahydrofuran solution of 2-chloropropane was added dropwise, and after the dropwise addition was completed, an insulation reaction was carried out, and the concentration of the obtained isopropylmagnesium chloride Grignard reagent was 1.7 mol / L.
[0029] (2) Grignard exchange: under nitrogen protection, 7.4 g of 2,6-dichloro-4-fluoroiodobenzene and 20 ml of tetrahydrofuran were added to a 100 ml dry three-necked flask. Cool down to 10°C, slowly add the above-prepared isopropylmagnesium chloride Grignard reage...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com