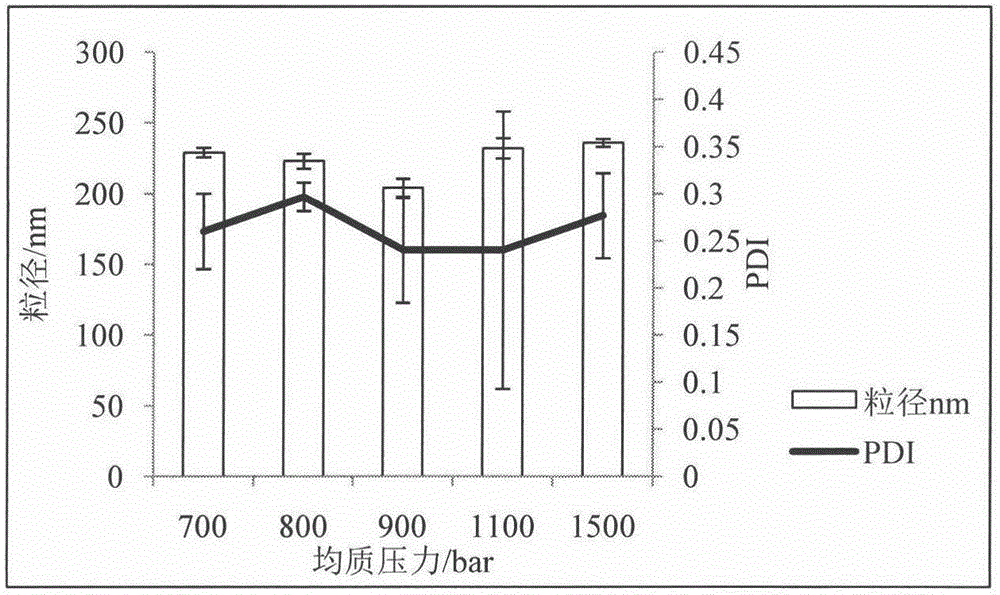
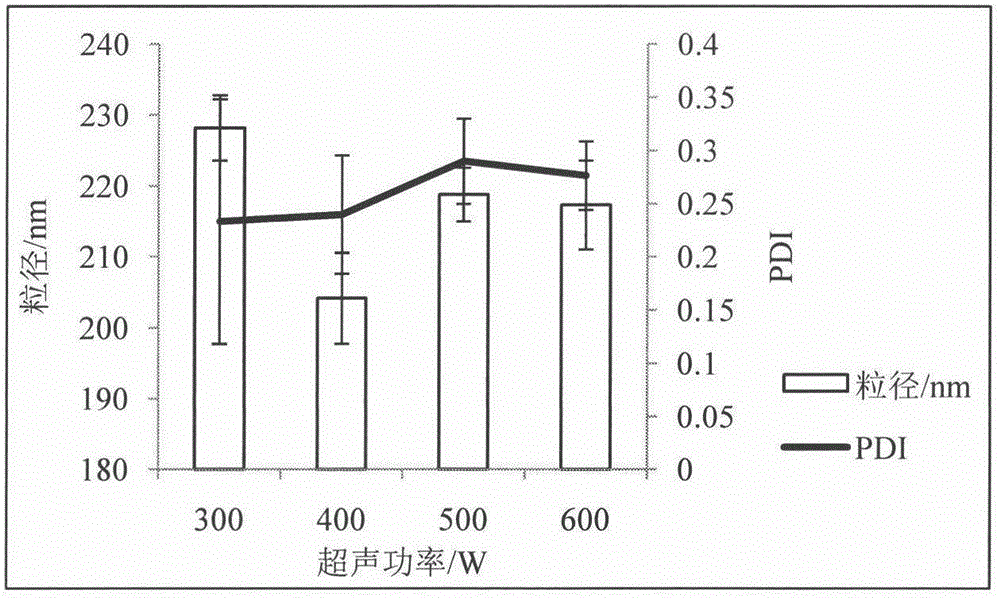
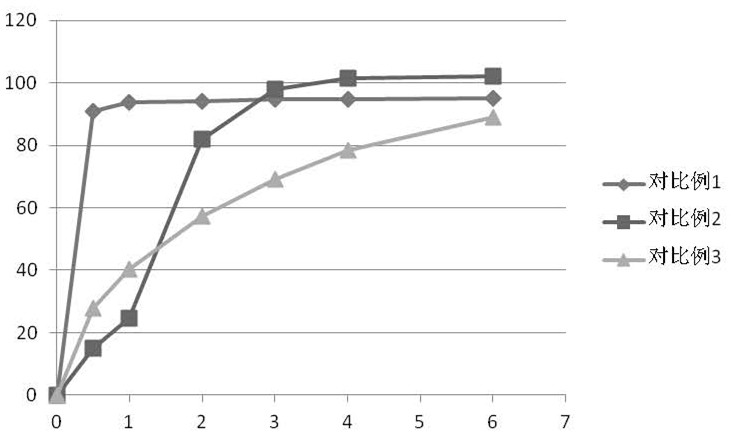
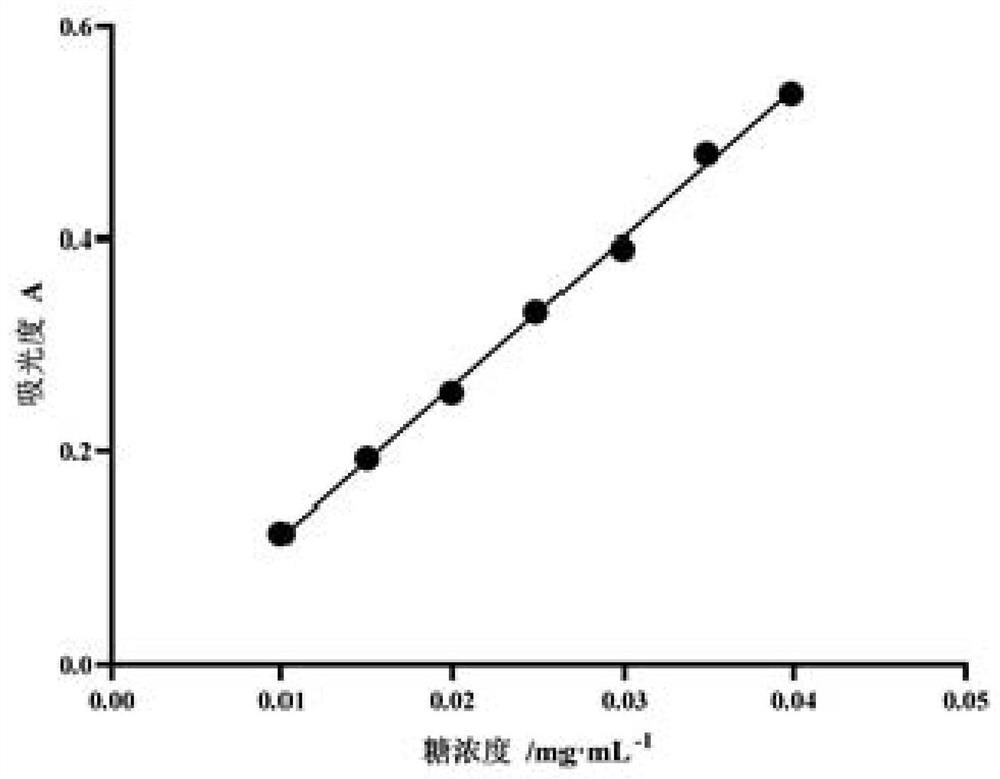
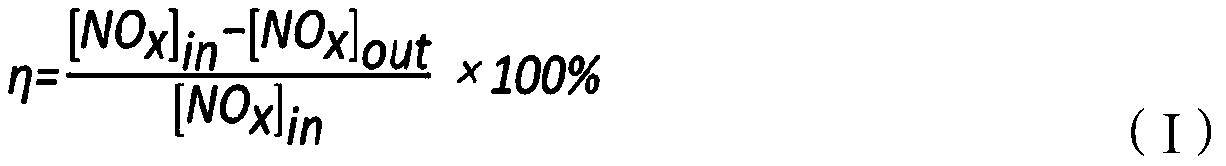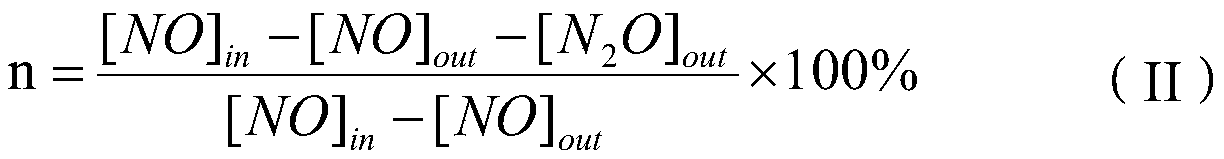Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
36results about How to "Scale up industrial production" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
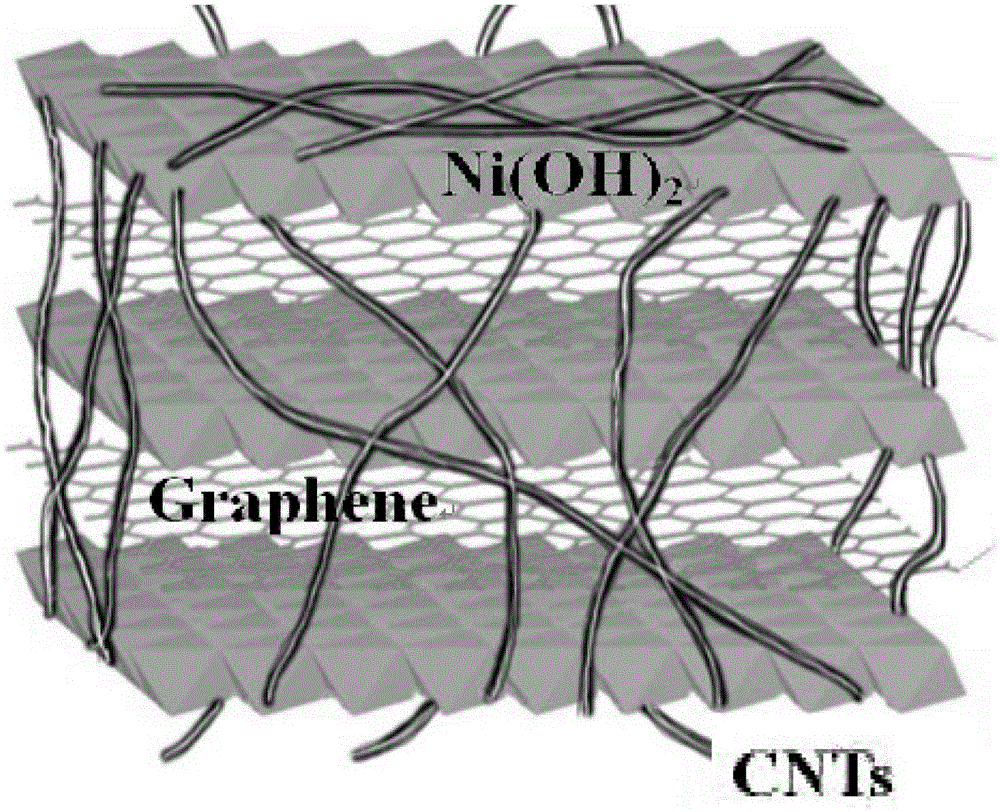
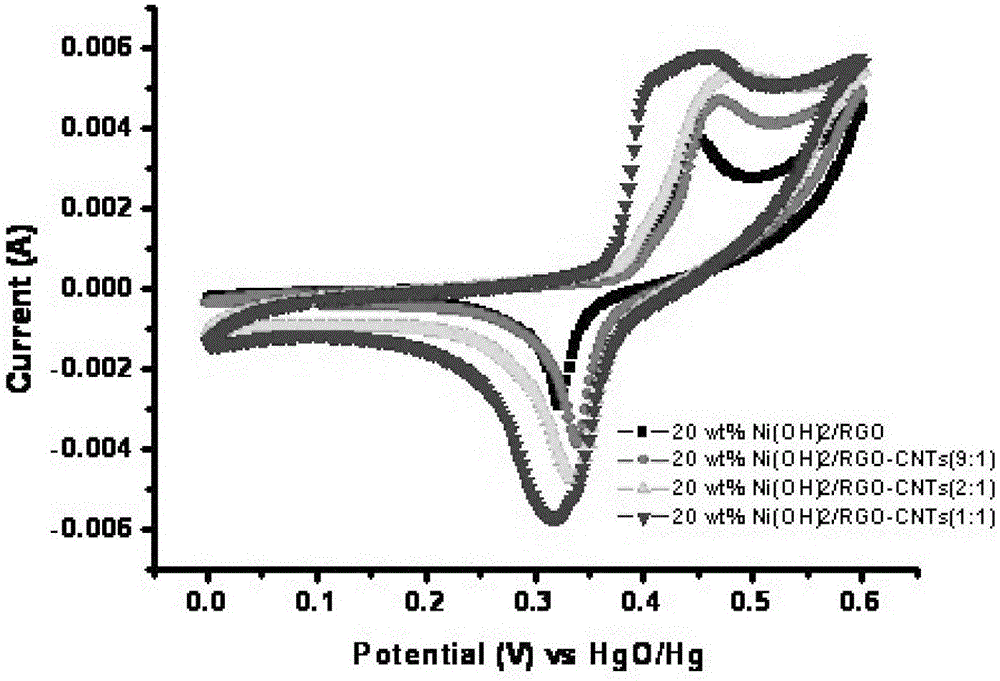
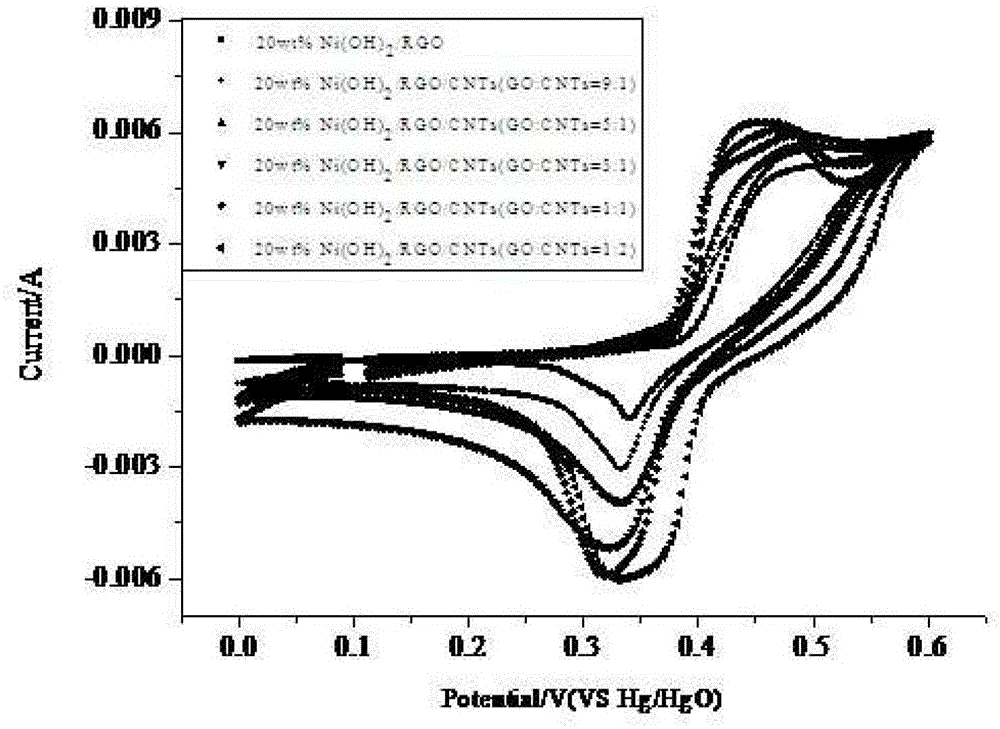
Super-capacitor electrode material and preparation method thereof
InactiveCN103390509ASmall particle sizeEasy to removeHybrid capacitor electrodesHybrid/EDL manufactureMicrowave methodNickel oxide hydroxide
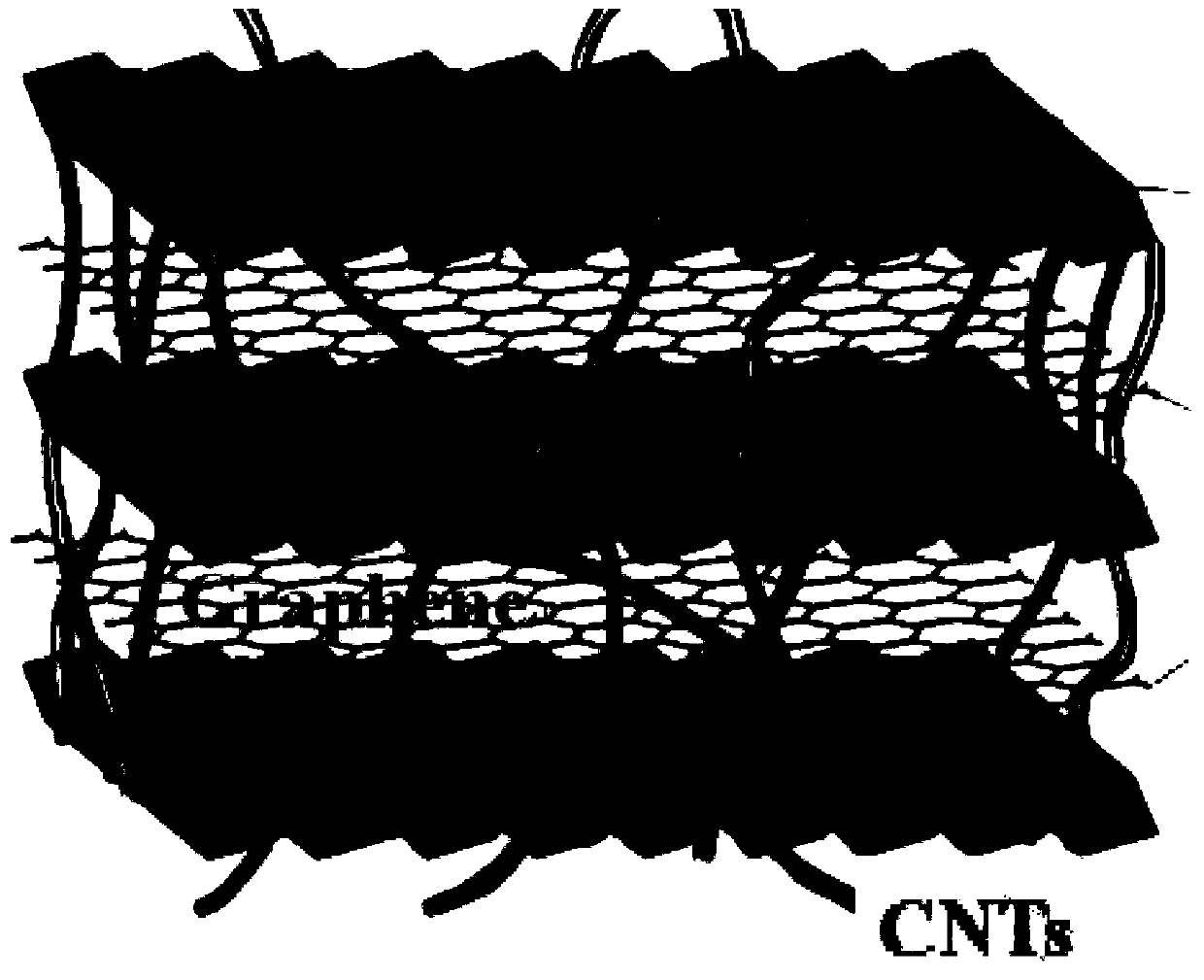
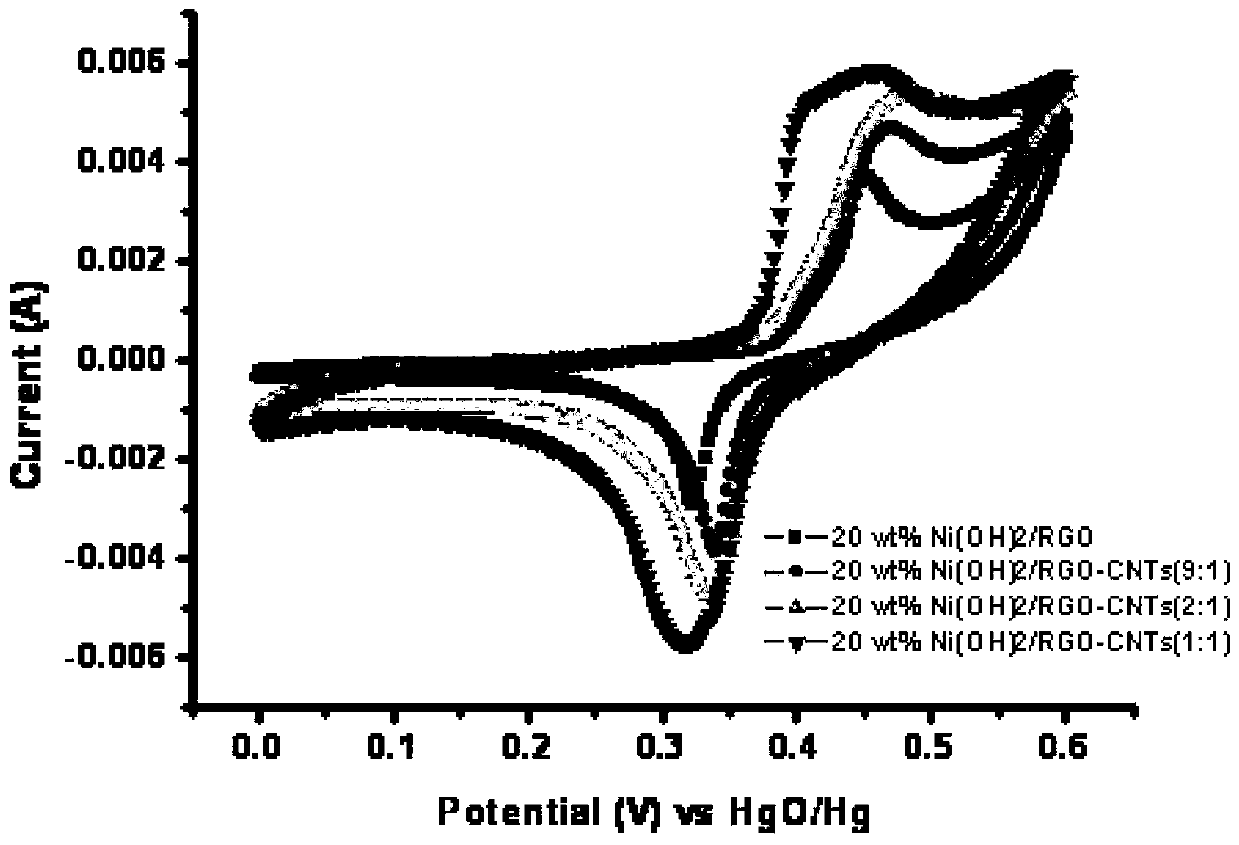
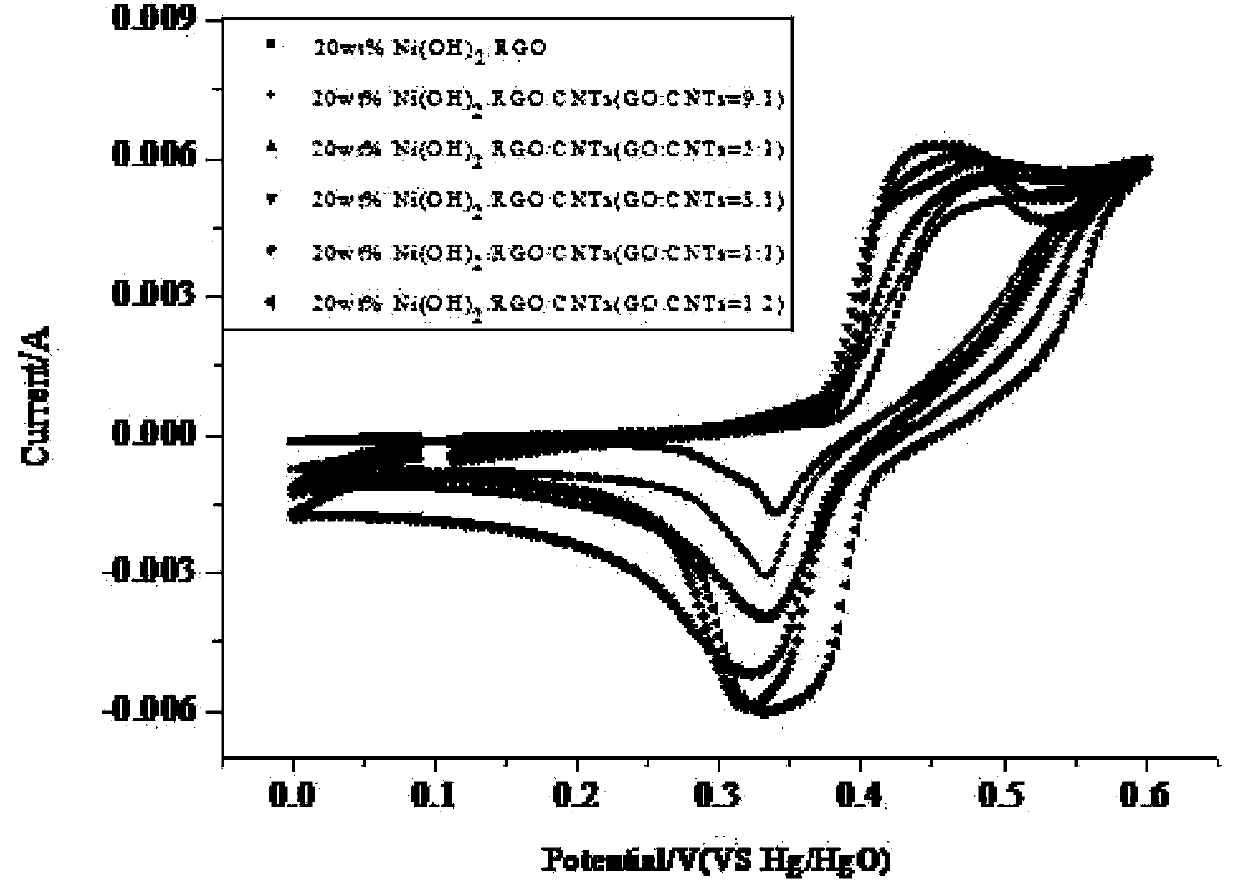
The invention relates to a super-capacitor electrode material and a preparation method thereof. A conventional commercial super-capacitor product still has lower energy density, but the greatest defect of low energy density of the super-capacitor is hopeful to be overcome and the energy density of a carbon nano tube / nickelous hydroxide / graphene composite material can be greatly improved if a carbon nano tube / nickelous hydroxide / graphene composite material with reasonable structure and composition can be manufactured due to the fact that nickelous hydroxide has a large pseudocapacitance value and good reversibility, graphene has a large specific surface area and excellent electrical conductivity, the nickelous hydroxide and the graphene are stacked in a layered manner and can generate an synergistic effect, and a small quantity of conductive carbon nano tubes are doped. The super-capacitor electrode material is a carbon nano tube / nickelous hydroxide / graphene composite prepared with a microwave method. According to the super-capacitor electrode material, micromolecular non-toxic and harmless ethylene glycol is adopted as a reaction medium, microwave heating is adopted to promote fast completion of a reaction, the reaction is finished in one pot, and the prepared composite has a large specific capacity and a large energy density.
Owner:SOUTHWEST PETROLEUM UNIV
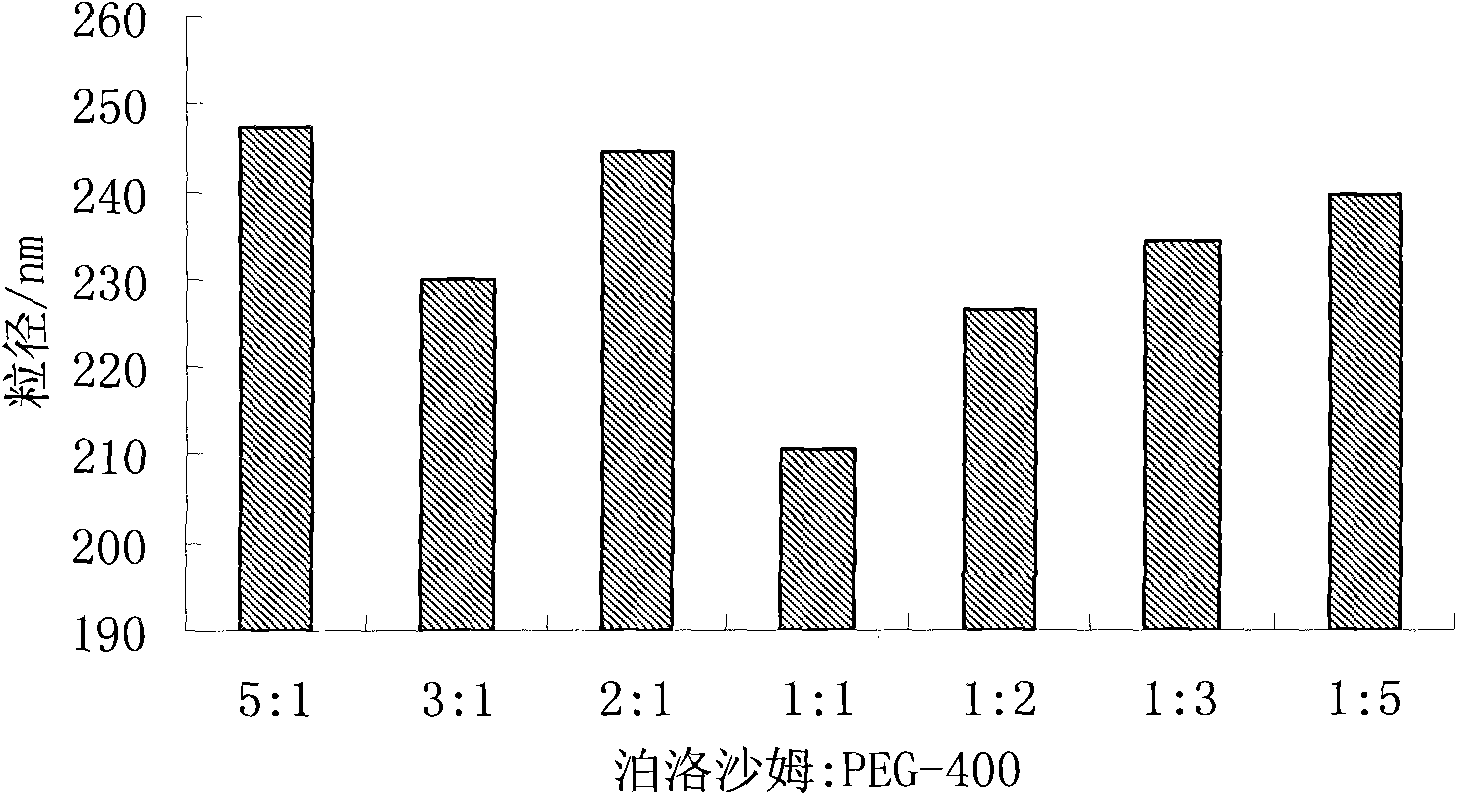
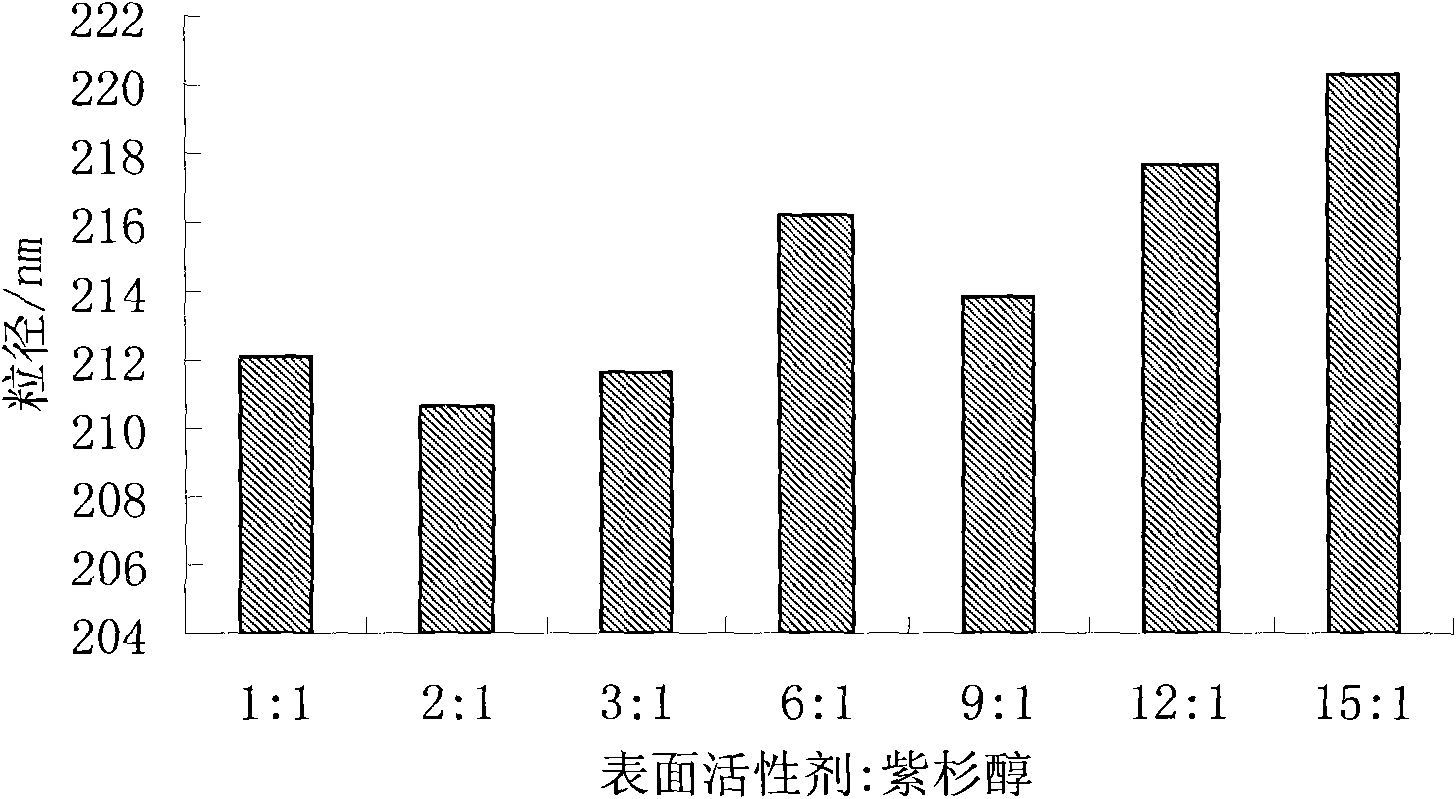
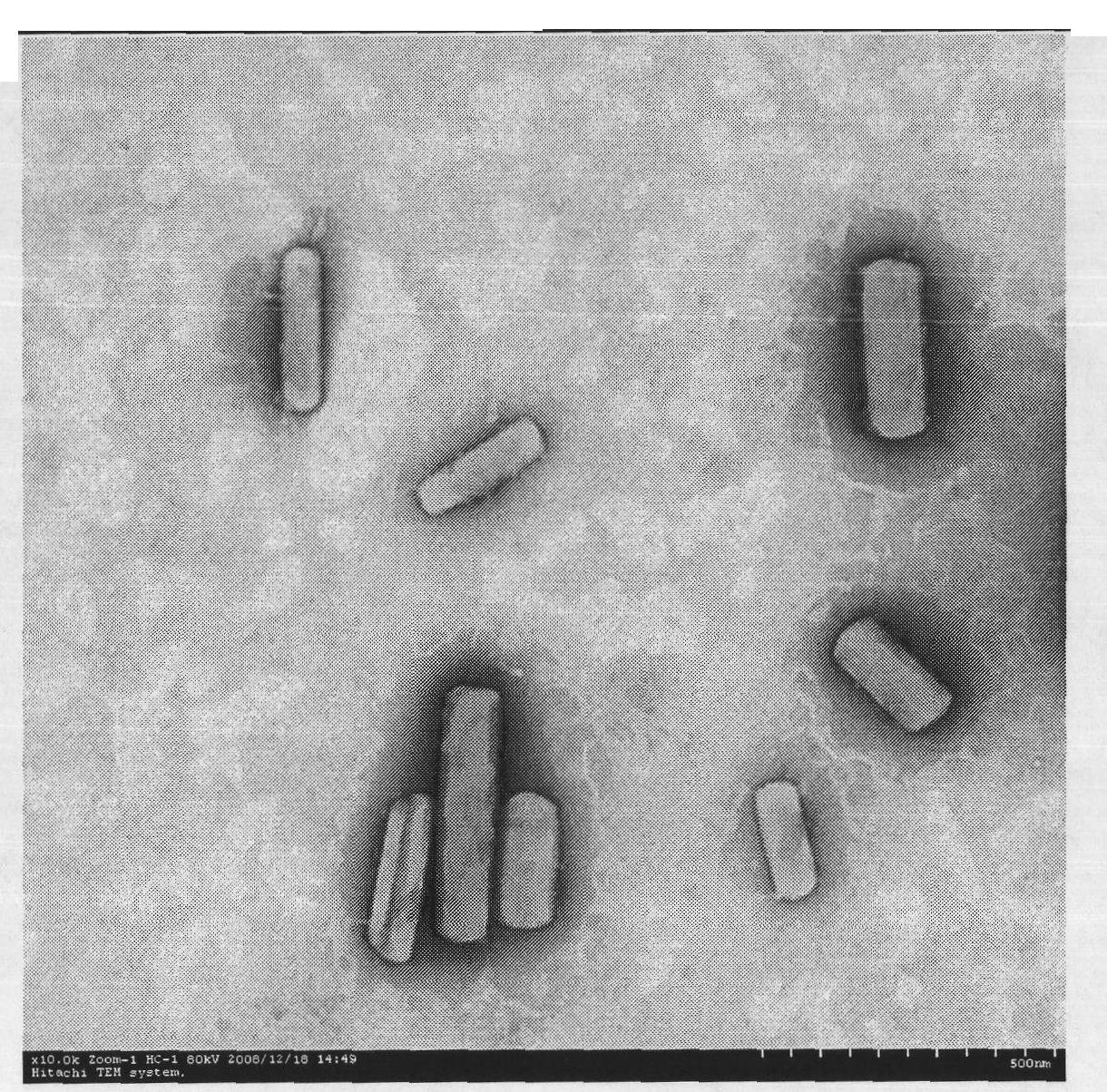
Taxol nanosuspension and preparation method thereof
ActiveCN101843582AHomogeneous pressure dropReduce the number of cyclesOrganic active ingredientsSolution deliveryChemistryToxicity
The invention relates to the field of medicinal preparations, in particular to a taxol nanosuspension and a preparation method thereof. The taxol nanosuspension is characterized in that: a surfactant consists of poloxamer 188 and polyethylene glycol in a weight ratio of 1:1-1:3. According to the invention, the defects of low medicament loading rate, strong toxic or side effect, low stability, complex preparation process, high cost and the like in the conventional taxol preparation are overcome, and the technical breakthrough in the field is achieved; and the taxol nanosuspension has the advantages of increasing medicament content, reducing administration toxicity and production cost and improving the adaptability and compliance of a patient so as to realize clinical use.
Owner:南京百思福医药科技有限公司
SCR (Selective Catalytic Reduction) denitration catalyst as well as preparation method and application of SCR denitration catalyst
ActiveCN106732531AScale up industrial productionHigh activityGas treatmentHeterogenous catalyst chemical elementsCeriumSulfur trioxide
The invention provides an SCR (Selective Catalytic Reduction) denitration catalyst as well as a preparation method and application of the SCR denitration catalyst. The SCR denitration catalyst is prepared from 0.8 weight percent to 1.2 weight percent of vanadium pentoxide, 2.5 weight percent to 10 weight percent of tungsten trioxide, 1 weight percent to 20 weight percent of cerium dioxide, 2.5 weight percent to 10 weight percent of molybdenum trioxide, 0.1 weight percent to 5 weight percent of sulfur trioxide, 0 to 2 percent of antimony trioxide and the balance of anatase type titanium dioxide, wherein the denitration rate of the SCR denitration catalyst at the air speed of 30000h<-1> under the temperature range of 240 DEG C to 400 DEG C is more than 94 percent. The SCR denitration catalyst provided by the invention has high activity and good selectivity. The preparation method of the catalyst is the improvement of an existing commercial catalyst preparation process method; amplified industrial production can be carried out by existing equipment.
Owner:DATANG INT CHEM TECH RESINST
Nano-suspension of Hsp90 inhibitor by using benzamide as basic skeleton and preparation method of nano-suspension
ActiveCN104173283AHigh drug contentImprove stabilityOrganic active ingredientsSolution deliveryDrug contentActive agent
The invention discloses a nano-suspension of an Hsp90 inhibitor by using benzamide as a basic skeleton and a preparation method of the nano-suspension. In the nano-suspension, the weight ratio of the Hsp90 inhibitor by using benzamide as the basic skeleton to a surfactant is 1 to 0.1-1 to 10. The nano-suspension has the characteristics of high drug content, small auxiliary dose, high stability, stable dissolution rate and the like, is applicable to multiple administration routes, in particular injection, is capable of overcoming the defects of poor water solubility, difficult administration, low bioavailability, strong toxic and side effects and the like, realizes technical breakthrough in the field, and provides a novel administration preparation with high drug content, high bioavailability and passive targeting to the Hsp90 inhibitors for the first time so as to accelerate the progress of the nano-suspension in clinical application.
Owner:广州少伯控股集团有限公司 +1
Method for producing high-purity capsaicin by using capsicine as raw material
InactiveCN1477098AScale up industrial productionCarboxylic acid amide separation/purificationAqueous solutionOrganic solvent
The present invention relates to a method for purifying capsicine. It uses commercial capsaicine as raw material, and adopts the following steps: dissolving capsaicine in hydrophilic organic solvent,and making it implement complexation reaction with aqueous solution containing silver ion, then using hydrophobic organic solvent to treat silver ion complex, after the silver ion is removed, using alkali and acid to treat concentrate, finally, recovering high-purity capsicine.
Owner:GUANGZHOU INST OF GEOCHEMISTRY - CHINESE ACAD OF SCI
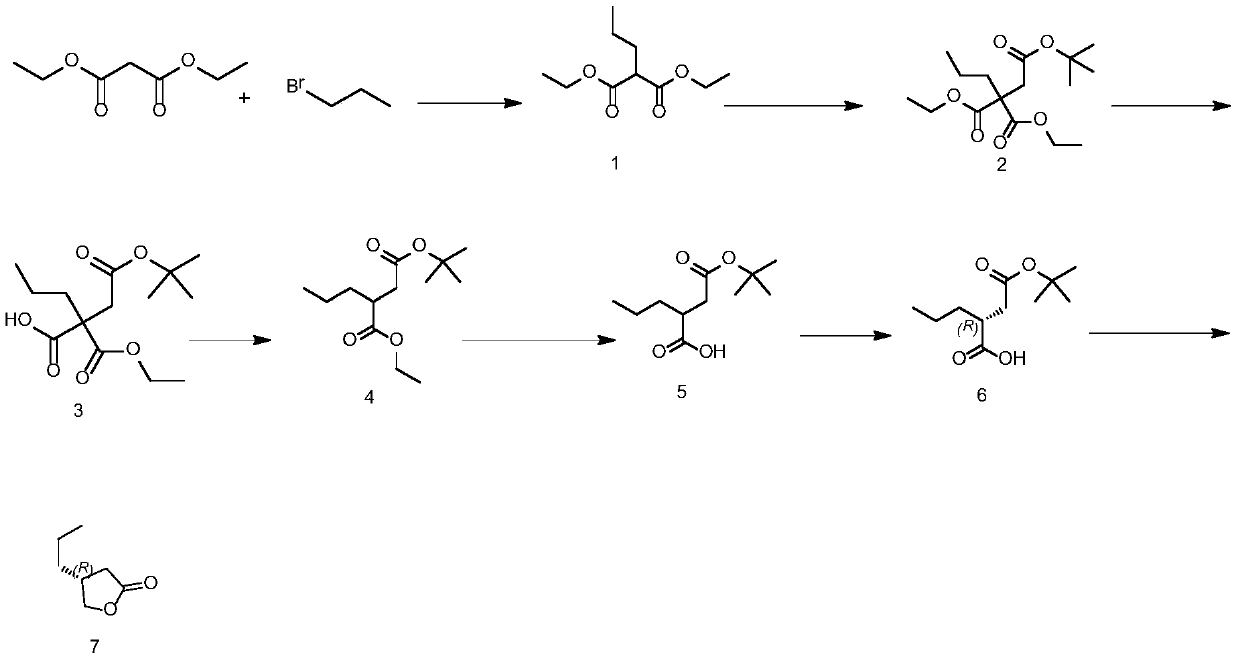
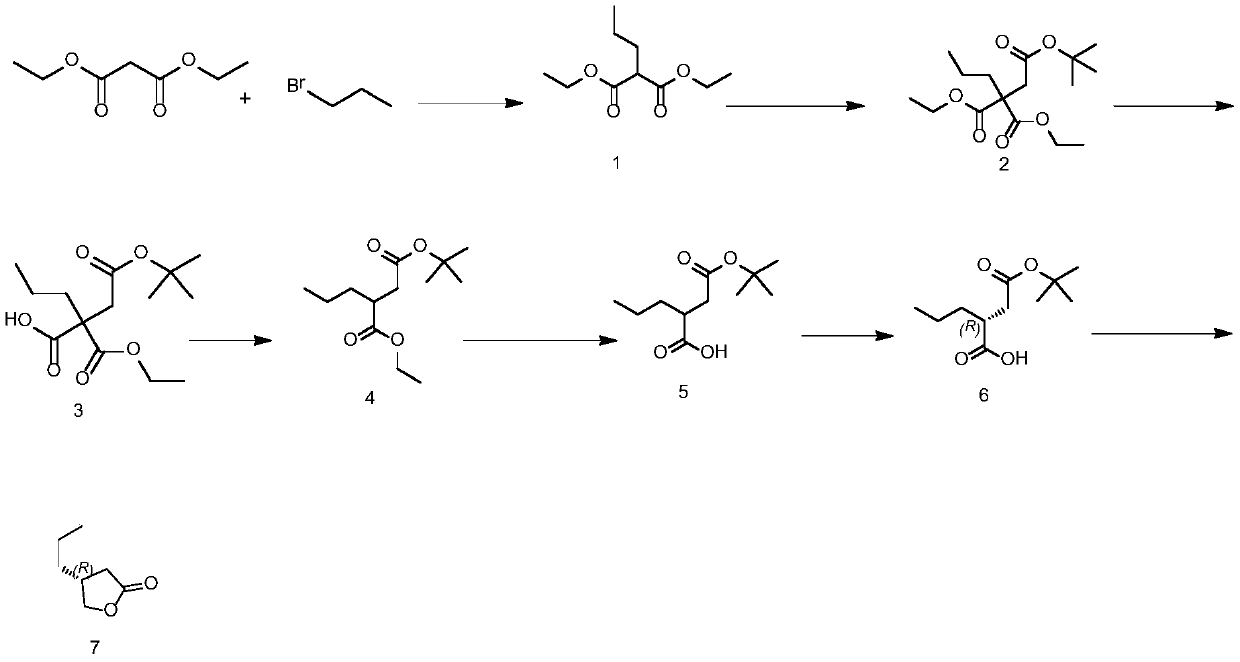
Preparation method of Brivaracetam intermediate
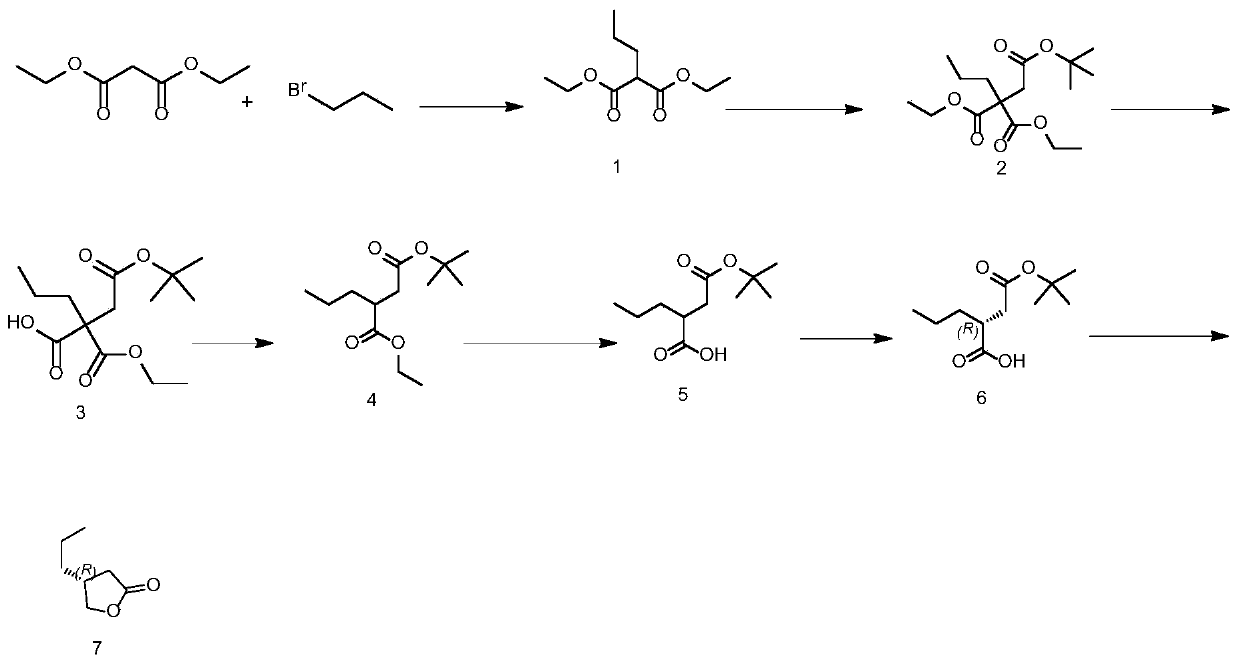
The invention discloses a preparation method of a Brivaracetam intermediate, wherein the reaction equation is shown as follows: diethyl malonate, bromopropane and an aprotic polar solvent are used forpreparing a compound of formula (1) under the action of an alkali; the compound of formula (1), tert-butyl bromoacetate and an aprotic polar solvent are used to prepare a compound of formula (2) under the action of an alkali; the compound of formula (2) and a polar solvent are used to prepare a compound of formula (3) under the action of an alkali; the compound of formula (3) is used to prepare acompound of formula (4) at high temperature; the compound of formula (4) and a polar solvent are used to prepare a compound of formula (5) under the action of an alkali; the compound of formula (5) and the resolving agent prepare the compound of formula (6) under the resolving solvent; the compound of formula (6) and a reducing agent are subjected to reduction reaction and hydrolysis reaction toprepare a compound of the formula (7). According to the invention, conventional raw materials are selected and can be prepared through conventional reactions, the optical purity materials are easier to purify, and the method is suitable for large-scale industrial production.
Owner:重庆经致制药技术开发有限公司
Food-grade high-content lutein ester and preparation method thereof
The invention provides a food-grade high-content lutein ester and a preparation method thereof. The preparation method comprises the following steps: adding C2-4 alcohol solvent into a marigold extractum, stirring and washing for 3-6 hours or carrying out ultrasonic treatment for 1-2 hours at 30-60 DEG C, cooling to 30-50 DEG C, and filtering to obtain a filter cake I; adding methanol or ethanol into the filter cake I to wash the filter cake I 1-3 times, and filtering to obtain a filter cake II; and drying the filter cake II to remove the solvent, thereby obtaining the high-content lutein ester crystal. The lutein ester content in the lutein ester product obtained by the method is at least 80%, wherein the all-trans lutein ester content is at least 90%; and the n-hexane solvent residue is less than 1.0 ppm. The method provided by the invention has the advantages of simple steps, environmental protection, high product content, low solvent residue, short time consumption and low cost, can directly reutilize the recovered solvent, and can easily implement industrial production.
Owner:INNOBIO CORP LTD
Separation and preparation process of high-purity typhaneoside
InactiveCN102241717ALarge amount of preparationHigh puritySugar derivativesSugar derivatives preparationTyphaneosideSilica gel
The invention discloses a separation and preparation process of high-purity typhaneoside. The separation and preparation process comprises: carrying out supercritical CO2 extraction to obtain flavones, carrying out solvent extraction and silica gel column chromatography separation and enrichment to obtain crude typhaneoside, and separating and purifying the crude typhaneoside with a preparative reversed-phase high-performance liquid chromatograph to obtain a typhaneoside crystal. Through the separation and preparation method disclosed in the invention, not only can flavones be continuously extracted from Pollen typhae, but also high-purity typhaneoside monomers can be prepared.
Owner:NANJING ZELANG MEDICAL TECH
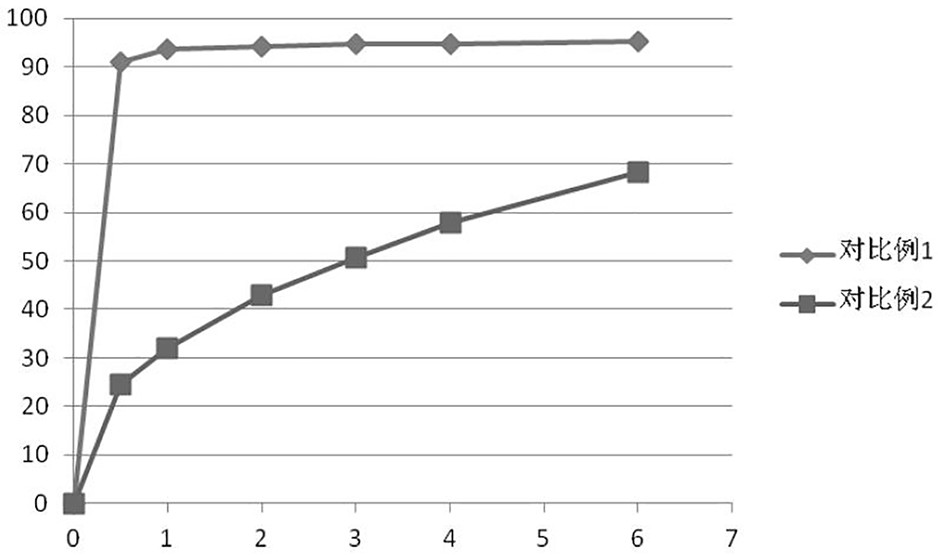
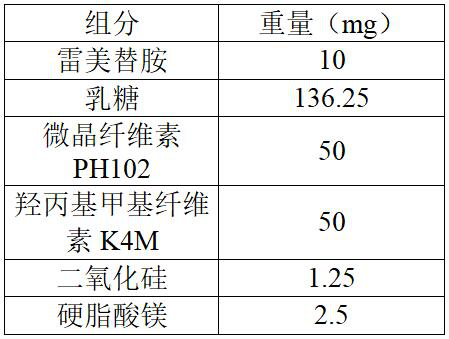
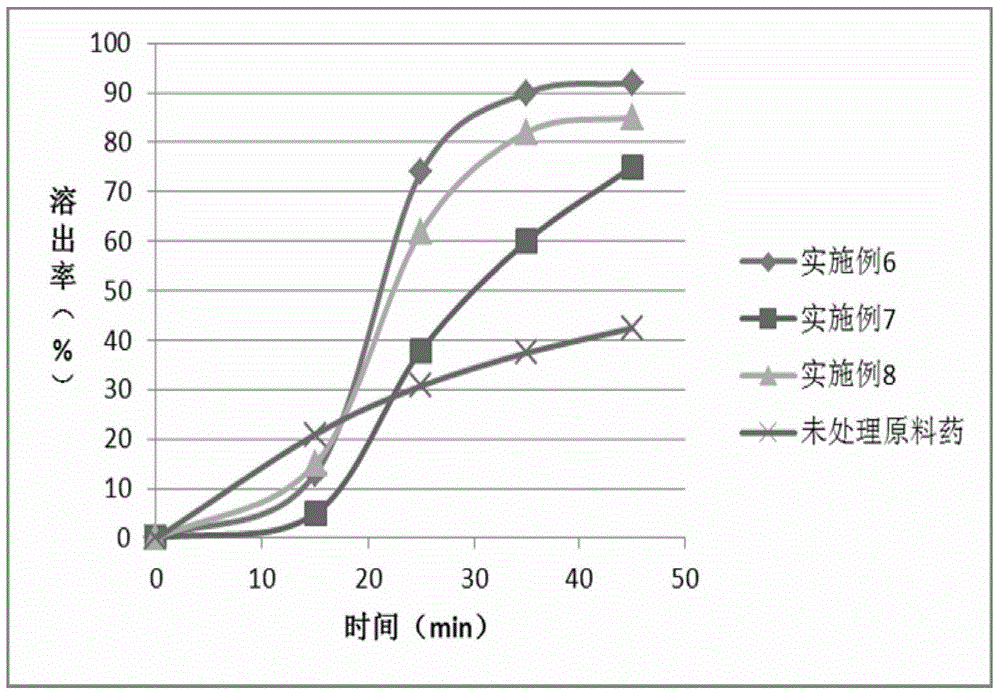
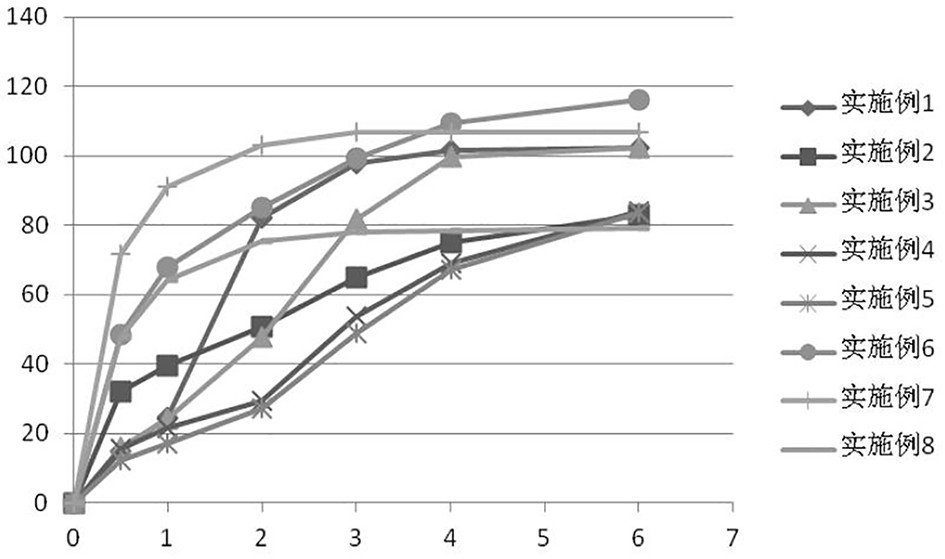
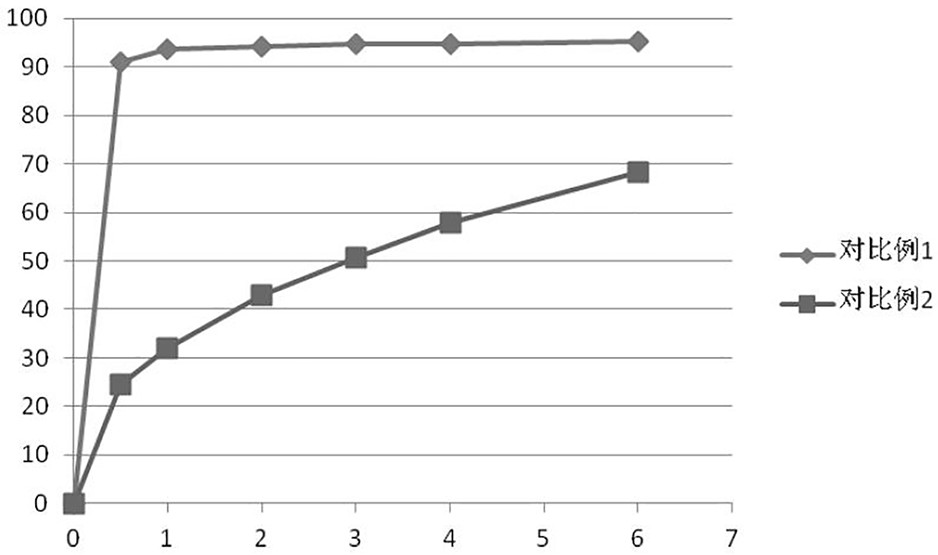
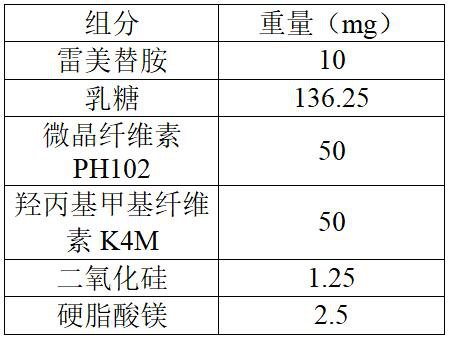
Ramelteon sustained release preparation and preparation method thereof
ActiveCN113274364ASustained releaseAdequate sleep timeOrganic active ingredientsNervous disorderSucrosePyrrolidinones
The invention provides a Ramelteon sustained release preparation. The dosage form of the Ramelteon sustained release preparation is a tablet, a granule or a capsule. The Ramelteon sustained-release preparation comprises the following raw materials in parts by weight: 1 to 30 parts of Ramelteon, 50 to 250 parts of a filling agent, 5 to 50 parts of a sustained-release material and 0.2 to 10 parts of a lubricating agent; the sustained-release material is selected from at least one of hydroxypropyl methylcellulose, polyvinylpyrrolidone, hydroxypropyl cellulose, sodium alginate, calcium alginate, guar gum and xanthan gum; and the filling agent is selected from at least one of lactose, mannitol, sorbitol, cane sugar, microcrystalline cellulose and calcium hydrophosphate. The Ramelteon sustained release preparation can maintain enough drug concentration in a long time, prolongs the drug action time, and is completely dissolved out.
Owner:广东科泰鼎润药业科技有限公司
Method for improving water solubility of acetylisovaleryltylosin tartrate
ActiveCN107936073AEasy to operateScale up industrial productionSugar derivativesSugar derivatives preparationSolubilityAluminium chlorohydrate
The invention provides a technical method for improving the water solubility of acetylisovaleryltylosin tartrate through extraction, purification and impurity removal. The method comprises the following steps: firstly stirring and washing an acetylisovaleryltylosin crude extract with purified water, heating the crude extract to 30 to 50 DEG C while stirring is performed, and filtering, so as to obtain an acetylisovaleryltylosin washed product, repeating the step again, and ensuring that the yield of the crude extract is greater than 99 percent after two times of purification and washing; thendissolving the acetylisovaleryltylosin washed product with purified water, adding tartaric acid while dissolving is performed, to adjust the pH to be 3 to 5, cooling the washed product to 10 to 20 DEGC, adding aluminum polychlorid after the washed product is completely dissolved, ensuring that a small amount of precipitate appears, and performing solid-liquid separation through a decolorizing agent after the washed product is left to stand, so as to obtain clear and transparent bright yellow solution; and finally performing drying and water removal, so as to obtain an acetylisovaleryltylosintartrate finished product, and ensuring that the finished product is clear and transparent faint yellow after the finished product is dissolved in tap water.
Owner:湖北回盛生物科技有限公司
Three-high danshu capsule and production and preparation process thereof
ActiveCN101703670ANo side effectsEasy to useMetabolism disorderCapsule deliverySalvia miltiorrhizaAlcohol
The invention relates to a three-high danshu capsule and a preparation process thereof. The three-high danshu capsule is characterized by comprising the following components in parts by weight: 10-20 parts of hawthorn, 8-15 parts of prepared multiflower knotweed root, 5-10 parts of cassia seed, 5-10 parts of rhizoma alismatis, 3-8 parts of ginseng, 3-8 parts of bulbus allii macrostemi and 5-10 parts of radix salviae miltiorrhizae; the preparation process comprises the following steps: (1) extracting the ginseng, the bulbus allii macrostemi and the radix salviae miltiorrhizae by ethanol, filtering an extract, recovering the ethanol from filtrate, decompressing and concentrating; (2) after extracting by the ethanol, adding water into the medicine residues of the ginseng, the bulbus allii macrostemi and the radix salviae miltiorrhizae, the hawthorn, prepared multiflower knotweed root, the cassia seed and the rhizoma alismatis, extracting, filtering, decompressing and concentrating the filtrate; (3) after concentrating, merging the ethanol extract and the water extract and concentrating continuously; (4) drying the extract in vacuum, pulverizing, sieving and storing dry powder after pulverizing for later use; (5) adding the auxiliary materials of talcum powder and magnesium stearate with a conventional quantity into the obtained extract powder and mixing; and (6) preparing the capsule. Experiments show that the three-high danshu capsule has a better health-care function of regulating blood fat and has no adverse reaction.
Owner:TIANJIN TIANSHI BIOLOGICAL DEV
Nanometer iron-series catalyst and preparation method thereof
InactiveCN101099930AGood stabilitySimple preparation processMetal/metal-oxides/metal-hydroxide catalystsNanometreHeating time
The present invention discloses one kind of nanometer iron catalyst and its preparation process. The nanometer iron catalyst is prepared through the following steps: preparing nanometer iron particle through introducing iron pentacarbonyl vapor into heated liquid medium via stirring to heat decompose iron pentacarbonyl, or heating the mixture of liquid medium and iron pentacarbonyl to heat decompose iron pentacarbonyl; cooling the mixture of nanometer iron particle and liquid medium, condensating and shunting to obtain nanometer iron catalyst. The present invention utilizes liquid medium to block the aggregation of iron particle for obtain nanometer iron particle and controls the quantity of iron pentacarbonyl vapor and the liquid medium and heating time to control the particle size. The nanometer iron catalyst has high activity, high dispersivity, high stability and simple industrial production process.
Owner:JIANGSU TIANYI ULTRA FINE METAL POWDER
Brain-targeting nimodipine nano-suspension and preparation method thereof
InactiveCN105596293AReduce solubilityReduce wearOrganic active ingredientsSenses disorderMedicineNimodipine
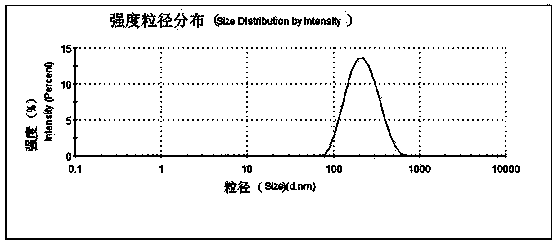
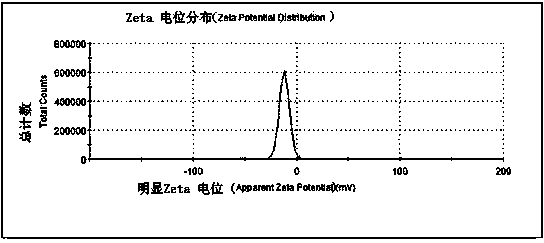
The invention discloses a brain-targeting nimodipine nano-suspension and a preparation method thereof. According to the preparation method, nimodipine serving as a model drug is subjected to ultrasonic supersonic coprecipitation and high pressure homogenization by using poloxamer 188 and tween 80 as stabilizing agents to prepare the brain-targeting nimodipine nano-suspension. The nano-suspension has a grain size of 200-230nm, a polydispersity index of 0.24+ / -0.056 and Zeta electric potential of (-23.8+ / -1.05)mV. The nano-suspension can be used for achieving the effect of brain-targeting administration by utilizing poloxamer and tween as the stabilizing agents.
Owner:CHINA PHARM UNIV
Extraction method of purslane extract and obtained product
InactiveCN113662976AGood solubility compatibilityQuick extractionPlant ingredientsFiltrationPurslane extract
The invention discloses a preparation method of a purslane extract and the obtained purslane extract. The method comprises the following steps: (1) taking a clean purslane raw material, and crushing the purslane raw material to 10-100 mesh; (2) adding 5-15 times of an extraction solvent into the purslane powder obtained in the step (1), and carrying out extraction at the temperature of 30-80 DEG C, wherein the adopted extraction solvent is ammonia water and / or a sodium carbonate solution; (3) cooling the mixture obtained in the step (2), and then carrying out filtering; and (4) carrying out vacuum concentration on the filtrate obtained in the step (3) to obtain a dry purslane extract. The novel extraction solvent disclosed by the invention is good in dissolving compatibility on portulaca oleracea polysaccharides, flavonoids and alkaloids, low in cost, safe and efficient.
Owner:广东丽研生物科技有限公司
Ramelteon quick-release and slow-release double-release preparation and preparation method thereof
ActiveCN113274365AFast releaseAdequate sleep timeOrganic active ingredientsNervous disorderCelluloseImmediate release
The invention provides a Ramelteon quick-release and slow-release dual-release preparation. The Ramelteon quick-release and slow-release dual-release preparation comprises a quick-release part and a slow-release part; and the dosage form of the Ramelteon quick-release and slow-release double-release preparation is a double-layer tablet, a granule or a capsule, wherein the quick release part comprises the following raw materials in parts by weight: 1 to 30 parts of Ramelteon, 20 to 160 parts of a first filling agent, 0.5 to 10 parts of a disintegrating agent and 0.1 to 5 parts of a first lubricating agent; the slow-release part comprises the following raw materials in parts by weight: 1 to 30 parts of Ramelteon, 20 to 160 parts of a second filling agent, 5 to 30 parts of a sustained-release material and 0.2 to 10 parts of a second lubricating agent; the slow-release material is selected from at least one of hydroxypropyl methylcellulose, polyvinylpyrrolidone, hydroxypropyl cellulose, sodium alginate, calcium alginate, guar gum and xanthan gum. The preparation has a relatively high release speed in the initial stage, and meanwhile, the action time of the medicine can be prolonged.
Owner:海南慧谷药业有限公司
Rapid extraction method of high-purity lentinan
The invention relates to the field of lentinan, in particular to a rapid extraction method of high-purity lentinan. According to the invention, fresh shiitake mushrooms are subjected to freeze drying, smashing, soaking, flash extraction, centrifugation of an extract, alcohol precipitation, ultrasonic redissolution, centrifugation and freeze drying, so the lentinan is obtained. The method comprises the following specific steps: raw material preparation and pretreatment: weighing fresh shiitake mushrooms, freeze-drying the fresh shiitake mushrooms, and conducting crushing and soaking; flash extraction: putting a mushroom soaking solution into a flash extractor for extraction; centrifuging: centrifuging the extract to obtain a supernatant; alcohol precipitation: adding absolute ethyl alcohol into the extract, and conducting centrifuging to obtain a polysaccharide precipitate; redissolving: adding deionized water into the polysaccharide precipitate, and carrying out ultrasonic dissolving to obtain extractum; and freezing and drying: freezing and drying the extractum to obtain the lentinan. According to the invention, extraction time is short, product purity is high, the extraction efficiency of the lentinan is improved, and application and popularization of the lentinan are facilitated.
Owner:EAST CHINA UNIV OF SCI & TECH +1
Preparation method of water-soluble acanthopanax powder capsule
ActiveCN113318137AGood effectBiodegradableAntinoxious agentsPharmaceutical non-active ingredientsBiologyRhizome
The invention discloses a preparation method of a water-soluble acanthopanax powder capsule, and belongs to the technical field of extraction and processing of plant rhizomes. According to the preparation method, the acanthopanax is taken as a raw material, saponin is dissolved out by adopting ethanol reflux extraction, and the water-soluble acanthopanax powder capsule is obtained through spray drying after being wrapped by chitosan oligosaccharide, so that the technological process is green, environment-friendly, simple and convenient to operate and good in dissolution property, and can be easily amplified to industrial production; a new thought is provided for development and utilization of water-soluble preparation of other Chinese herbal medicines insoluble in water, and the administration route and the use object are further widened; and in addition, the clathrate compound prepared by the invention is high in bioavailability, so that the curative effect of the clathrate compound is greatly improved, and the treatment cost is greatly reduced. Therefore, the technical scheme disclosed by the invention has great market application and popularization values.
Owner:NORTHEAST FORESTRY UNIVERSITY
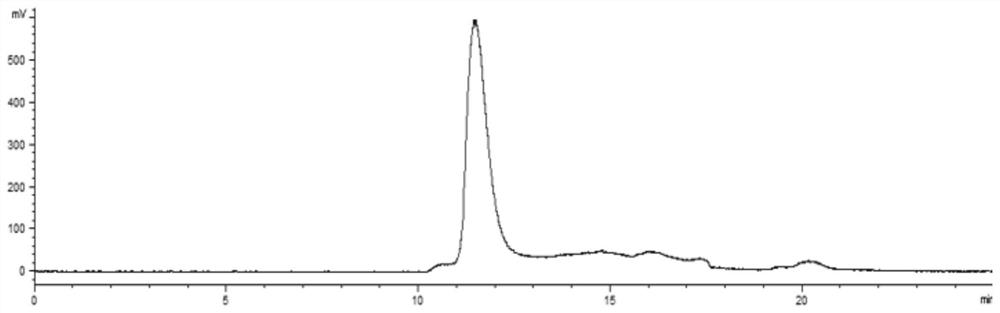
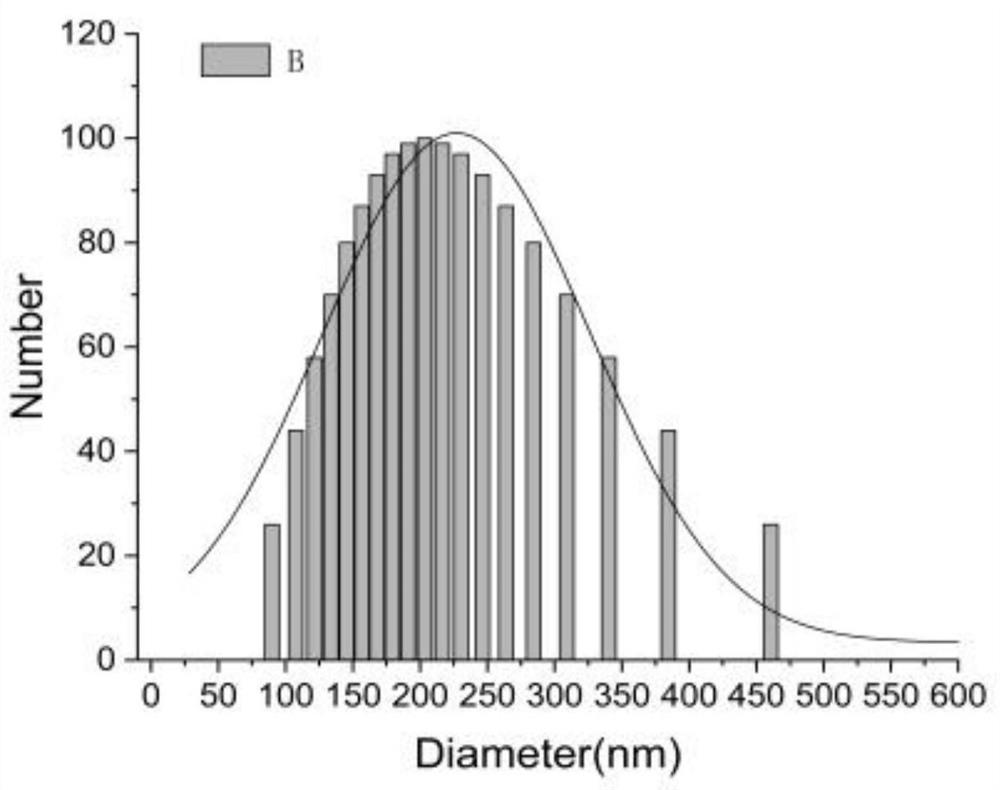
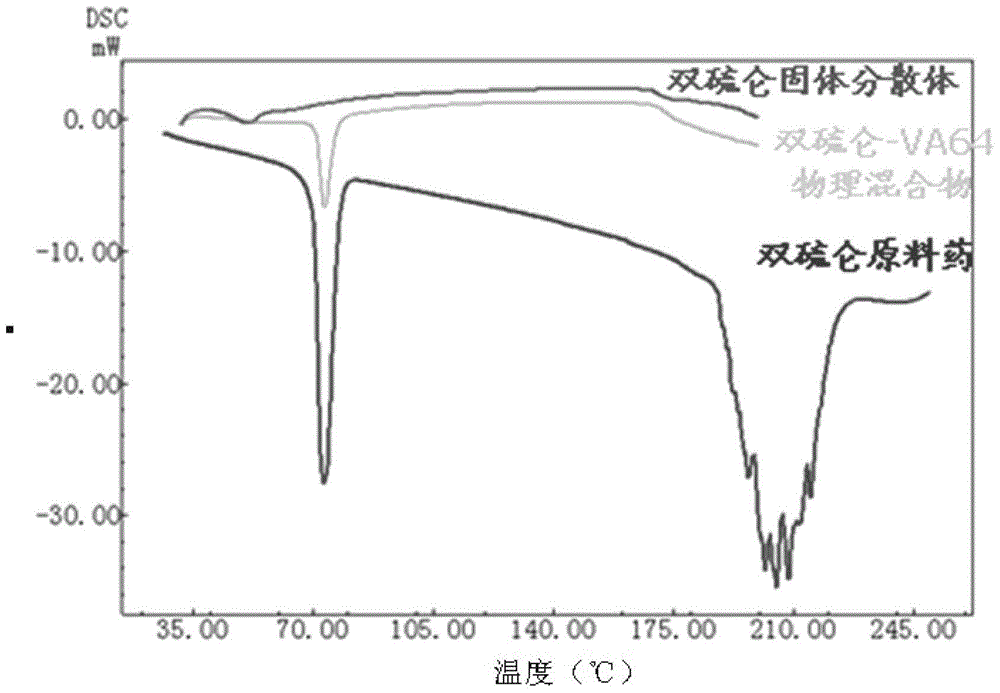
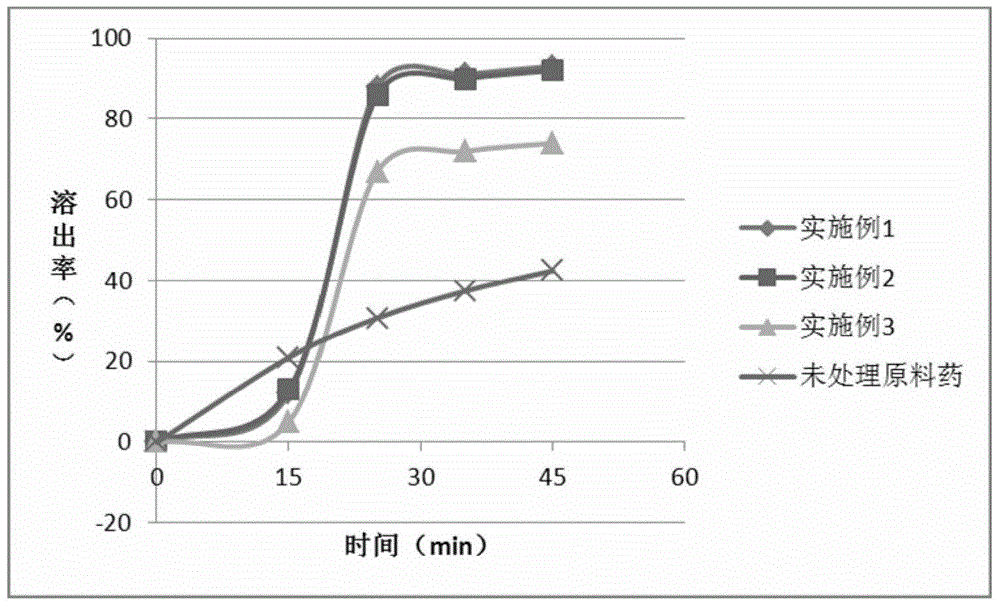
A kind of disulfiram enteric-coated tablet and preparation method thereof
InactiveCN104146978BImprove stabilityDisintegrates quicklyOrganic active ingredientsPharmaceutical delivery mechanismCross-linkSolubility
The purpose of the present invention is to provide an anti-tumor disulfiram enteric-coated tablet with good stability, high bioavailability and little side effects by using modern pharmaceutical technology. The disulfiram enteric-coated tablet is composed of the following components in turn from the inside to the outside: a. a tablet core composed of disulfiram and pharmaceutically acceptable excipients; b. an intermediate isolation layer composed of an isolation material; c. an enteric-coated An enteric-coated layer composed of raw materials and pharmaceutically acceptable excipients; it is characterized in that: the tablet core is made of disulfiram bulk drug, diluent, and disintegrant that have been processed to increase solubility, and are directly compressed into tablets or made into granules before adding The disintegrating agent is compressed; wherein, the diluent is selected from one or more of LubriTose lactose series, sucrose, lactose, mannitol, precrossified starch, microcrystalline cellulose, and calcium carbonate in traditional excipients, and the dosage is 30% to 50% of the total weight of the tablet core; the disintegrant is selected from one or more of sodium carboxymethyl starch, low-substituted hydroxypropyl cellulose, cross-linked sodium carboxymethyl cellulose, and cross-linked povidone kind.
Owner:SHENYANG PHARMA UNIVERSITY
High-efficient iron-series catalyst and its preparation method
InactiveCN101099932BGood dispersionImprove stabilityMetal/metal-oxides/metal-hydroxide catalystsLiquid ironNitrogen
The present invention discloses one kind of nanometer iron catalyst and its preparation process and apparatus. The nanometer iron catalyst is prepared through the following steps: adding liquid iron pentacarbonyl; heating iron pentacarbonyl in a reactor for the iron pentacarbonyl to infiltrate into the pores of catalyst carrier; further heating or introducing high temperature pressurized high purity nitrogen or other inert gas to decompose iron pentacarbonyl to obtain nanometer level iron particle; cooling, filling the prepared nanometer iron catalyst into package filled with high purity nitrogen and sealing for preserving. The present invention utilizes solid carrier to block the aggregation of iron particle for obtain nanometer iron particle. The nanometer iron catalyst has high activity, high dispersivity, high stability and simple preparation process, and is suitable for industrial production.
Owner:JIANGSU TIANYI ULTRA FINE METAL POWDER
A nanosuspension for oral protein immunization and its preparation method
InactiveCN105919937BLow encapsulation efficiencyHigh encapsulation efficiencySsRNA viruses negative-senseViral antigen ingredientsZeta potentialAntigen
The invention relates to a nano suspension for oral protein immunization and a preparation method thereof, and belongs to the field of medicine. Bovine serum albumin (BSA) is taken as the model drug, PLGA is taken as the carrier, and a solvent volatilization method is adopted to prepare a BSA loaded MCS / PLGA / BSA nano suspension. The particle size of the suspension is 532.8 nm, the zeta potential is 28.92 mv, and the encapsulation rate is 89.34%. Mannosylated chitosan (MCS) is adsorbed on the surface of PLGA / BSA nano particles through a static effect, thus the stability of nano particles in gastrointestinal liquid becomes more stable, moreover, the stay time in intestinal cavity is prolonged, and the nano particles with positive charges can be absorbed by cells more easily. Nano particles are transferred by epithelium related with follicle to PP nodes; through the combination between mannose ligands and mannose acceptors on antigen-presenting cells (APCs), the intake of APCs is increased, and thus the immunization reactions are induced.
Owner:CHINA PHARM UNIV
Ramelteon sustained-release preparation and preparation method thereof
ActiveCN113274364BSustained releaseAdequate sleep timeOrganic active ingredientsNervous disorderSucroseCaplet Dosage Form
Owner:广东科泰鼎润药业科技有限公司
Method for preparing meliternin
InactiveCN102875539AReduce environmental pollutionHigh product purityOrganic chemistryPolyamideColumn chromatography
The invention discloses a method for preparing meliternin. The method comprises the steps as follows: taking melicope triphylla as a raw material, carrying out heat reflux extraction, two aqueous phase extraction, column chromatography under polyamide resin, and separating at high-speed counter-current chromatography, so as to obtain meliternin. The method disclosed by the invention is simple in operation, and high in yield and product purity.
Owner:NANJING ZELANG AGRI DEV
Carvedilol solid self-emulsifying sustained release tablets and preparing method thereof
InactiveCN107049977AReduce solubilityImprove shortcomingsOrganic active ingredientsPill deliveryMesoporous silicaCarvedilol
The invention discloses a method for preparing carvedilol solid self-emulsifying sustained release tablets. The method comprises the steps of synthesizing hollow mesoporous silica with a uniform particles size and an obvious hollow structure; conducting liquid self-emulsifying with the drug-loaded hollow mesoporous silica as a solid carrier, so that carvedilol solid self-emulsifying particles are obtained; finally, evenly mixing the carvedilol solid self-emulsifying particles with proper auxiliary materials for wet granulation, and conducting tabletting to obtain the carvedilol solid self-emulsifying sustained release tablets. The preparing process is simple, operation is easy, and the cost is low. The prepared carvedilol solid self-emulsifying sustained release tablets have an obvious slow release effect and broad application prospects.
Owner:CHINA PHARM UNIV
Taxol nanosuspension and preparation method thereof
InactiveCN101843582BFacilitated DiffusionSmall particle sizeOrganic active ingredientsSolution deliveryDrug contentSide effect
The invention relates to the field of medicinal preparations, in particular to a taxol nanosuspension and a preparation method thereof. The taxol nanosuspension is characterized in that: a surfactant consists of poloxamer 188 and polyethylene glycol in a weight ratio of 1:1-1:3. According to the invention, the defects of low medicament loading rate, strong toxic or side effect, low stability, complex preparation process, high cost and the like in the conventional taxol preparation are overcome, and the technical breakthrough in the field is achieved; and the taxol nanosuspension has the advantages of increasing medicament content, reducing administration toxicity and production cost and improving the adaptability and compliance of a patient so as to realize clinical use.
Owner:南京百思福医药科技有限公司
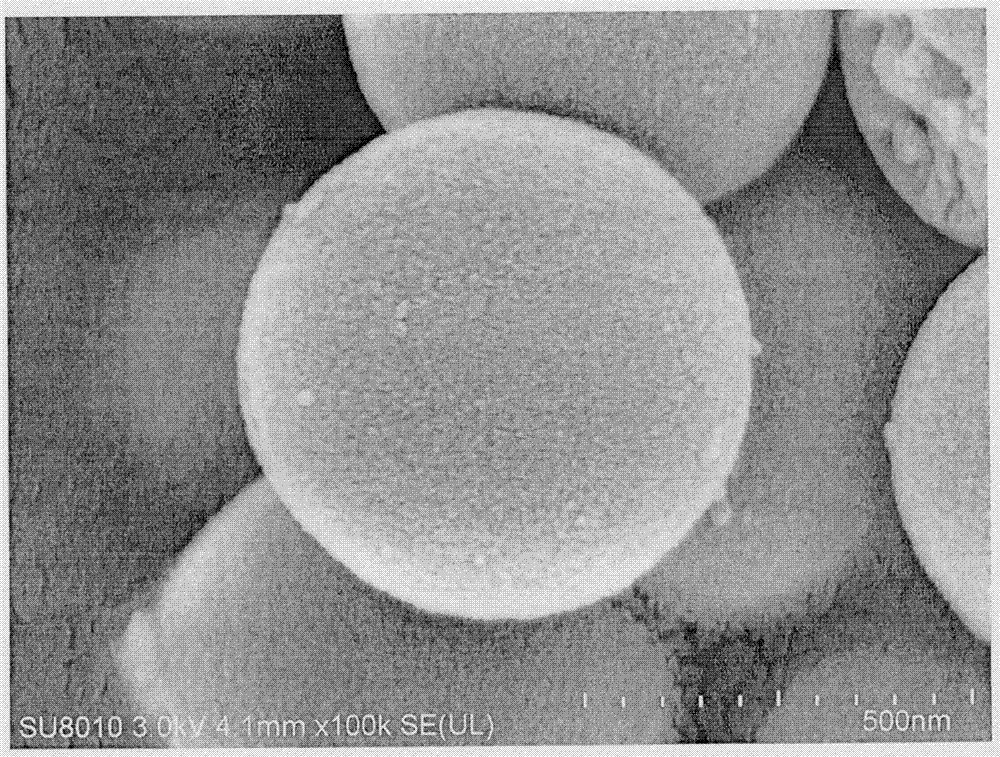
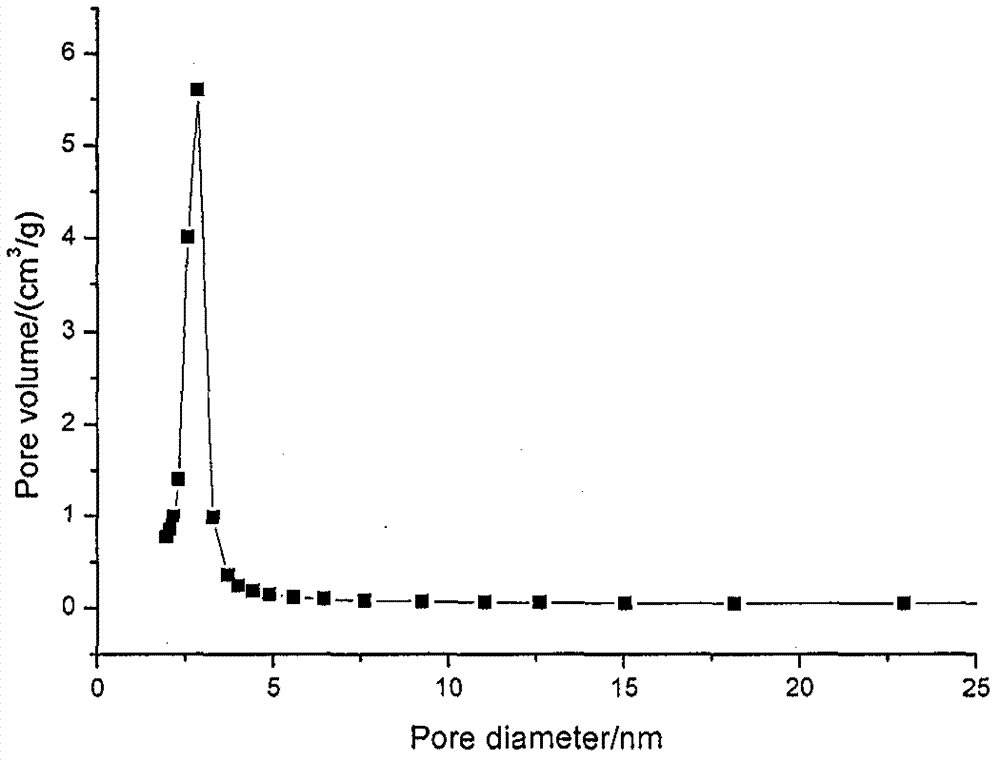
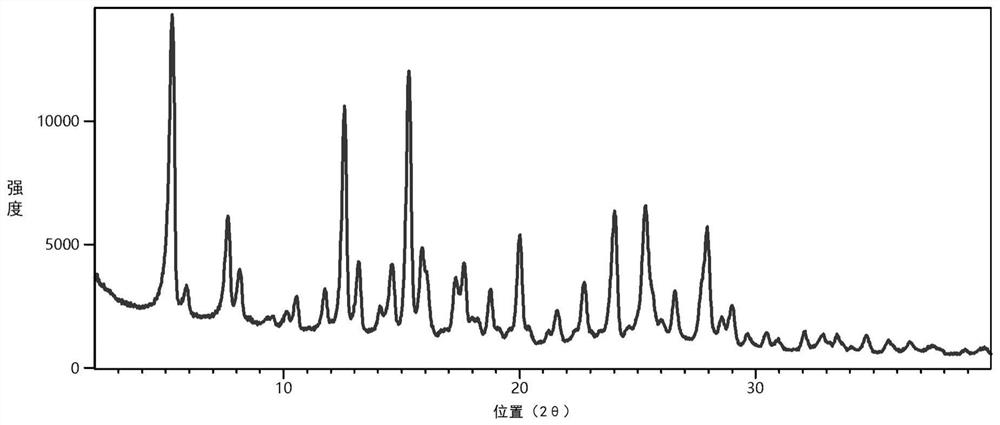
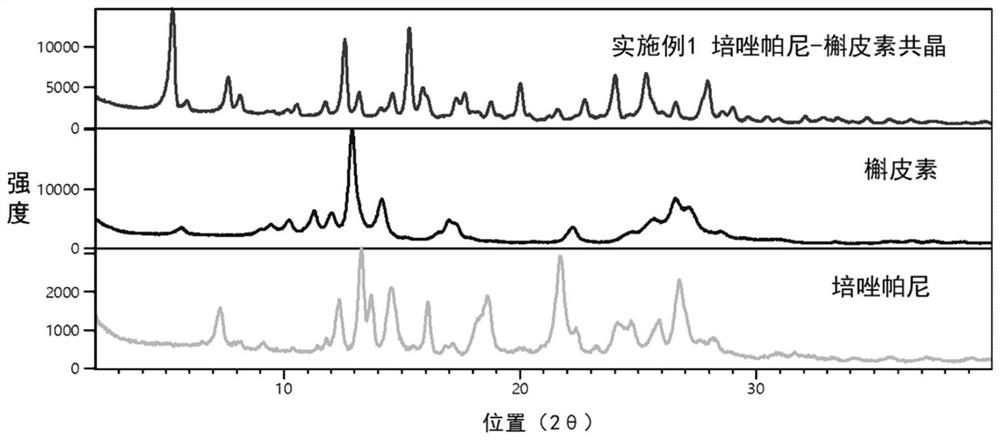
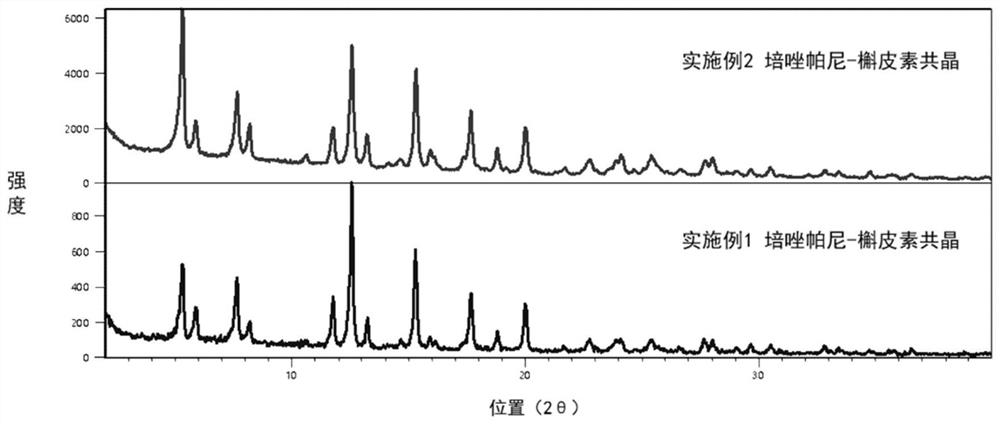
Perazopanib-quercetin eutectic crystal as well as preparation method and application thereof
ActiveCN114751893AImprove bioavailabilityReduces hepatotoxic effectsOrganic active ingredientsOrganic chemistry methodsPhysical chemistryQuercitrin
The invention discloses a pezopanib-quercetin eutectic crystal as well as a preparation method and application of the pezopanib-quercetin eutectic crystal. The eutectic crystal is formed by pezopanib and quercetin, and in an X-ray powder diffraction pattern obtained through Cu-K alpha radiation measurement, the eutectic crystal has characteristic diffraction peaks when the diffraction angles 2 theta are 5.3 degrees + / -0.2 degrees, 7.6 degrees + / -0.2 degrees, 12.6 degrees + / -0.2 degrees, 15.3 degrees + / -0.2 degrees, 17.7 degrees + / -0.2 degrees and 20.0 degrees + / -0.2 degrees. The pezopanib-quercetin eutectic crystal is good in stability, and does not generate crystal form transformation in at least 10 days under an accelerated condition (40 DEG C, 75% RH). The preparation method is simple, and industrial production can be amplified. Quercetin is introduced, so that the solubility of the pezopanib-quercetin eutectic crystal is obviously higher than that of a pezopanib monomer.
Owner:SHENZHEN NYCRIST TECH CO LTD
Ramelteon quick-release sustained-release double-release preparation and preparation method thereof
ActiveCN113274365BFast releaseAdequate sleep timeOrganic active ingredientsNervous disorderCelluloseCaplet Dosage Form
The invention provides a ramelteon immediate-release sustained-release double-release preparation, comprising an immediate-release part and a sustained-release part; the dosage form of the ramelteon immediate-release sustained-release double-release preparation is a double-layer tablet, granule or capsule wherein, in parts by weight, the immediate-release part includes the following raw materials: 1-30 parts of ramelteon, 20-160 parts of the first filler, 0.5-10 parts of disintegrant , 0.1-5 parts of the first lubricant; in parts by weight, the slow-release part includes the following raw materials: 1-30 parts of ramelteon, 20-160 parts of the second filler, 5 parts Parts ~ 30 parts of slow-release material, 0.2 part ~ 10 parts of the second lubricant; the slow-release material is selected from hypromellose, polyvinylpyrrolidone, hypromellose, sodium alginate, calcium alginate, At least one of guar gum and xanthan gum. The preparation has a higher release rate in the initial stage and can prolong the action time of the drug at the same time.
Owner:海南慧谷药业有限公司
A kind of supercapacitor electrode material and preparation method thereof
InactiveCN103390509BSmall particle sizeEasy to removeHybrid capacitor electrodesHybrid/EDL manufactureMicrowave methodNickel oxide hydroxide
Owner:SOUTHWEST PETROLEUM UNIV
A method for improving the water solubility of acetylisovaleryl tylosin tartrate
ActiveCN107936073BEasy to operateScale up industrial productionSugar derivativesSugar derivatives preparationAqueous solubilityTYLOSIN TARTRATE
The invention provides a process for extracting, purifying, and removing impurities from acetylisovaleryl tylosin tartrate to improve its water solubility. The steps include: first stirring and washing the crude acetylisovaleryl tylosin extract with purified water, Heat up to 30‑50°C while stirring, filter to obtain acetylisovaleryl tylosin water wash, repeat the above steps again, and the yield of the crude extract of 2 times of purified water wash is greater than 99%; then dissolve acetylisovaleryl with purified water Tylosin was washed with water, and while dissolving, tartaric acid was added to adjust the pH to 3-5, and the temperature was lowered to 10-20°C. After it was completely dissolved, polyaluminum chloride was added and a small amount of precipitation appeared. After standing still, it was obtained by solid-liquid separation with a decolorizing agent Clear, transparent and bright yellow solution; finally, dry and remove water to obtain the finished product of acetylisovaleryl tylosin tartrate, which is clear, transparent and light yellow after dissolving in tap water.
Owner:湖北回盛生物科技有限公司
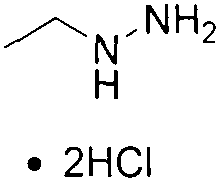
New method for synthesizing ethylhydrazine dihydrochloride
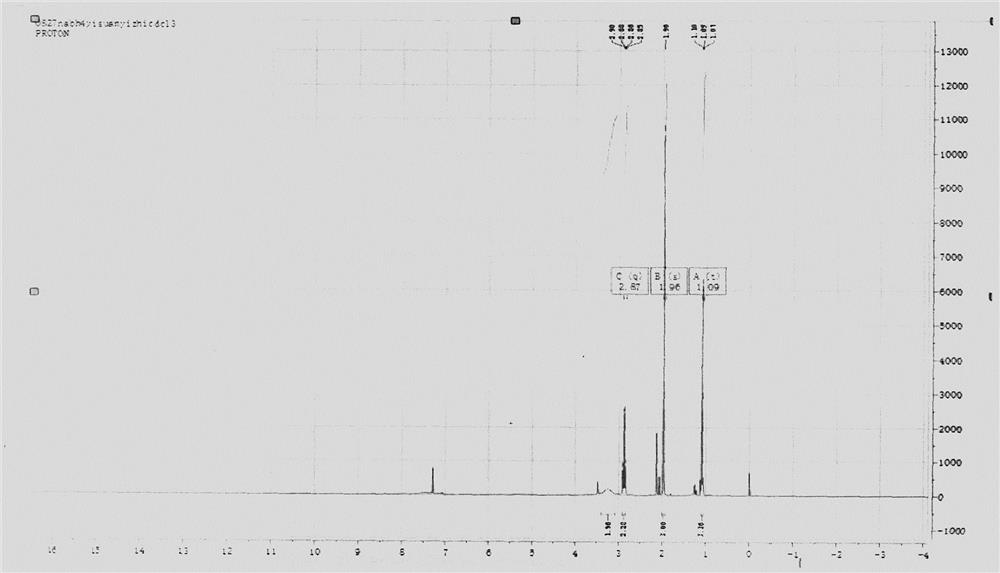
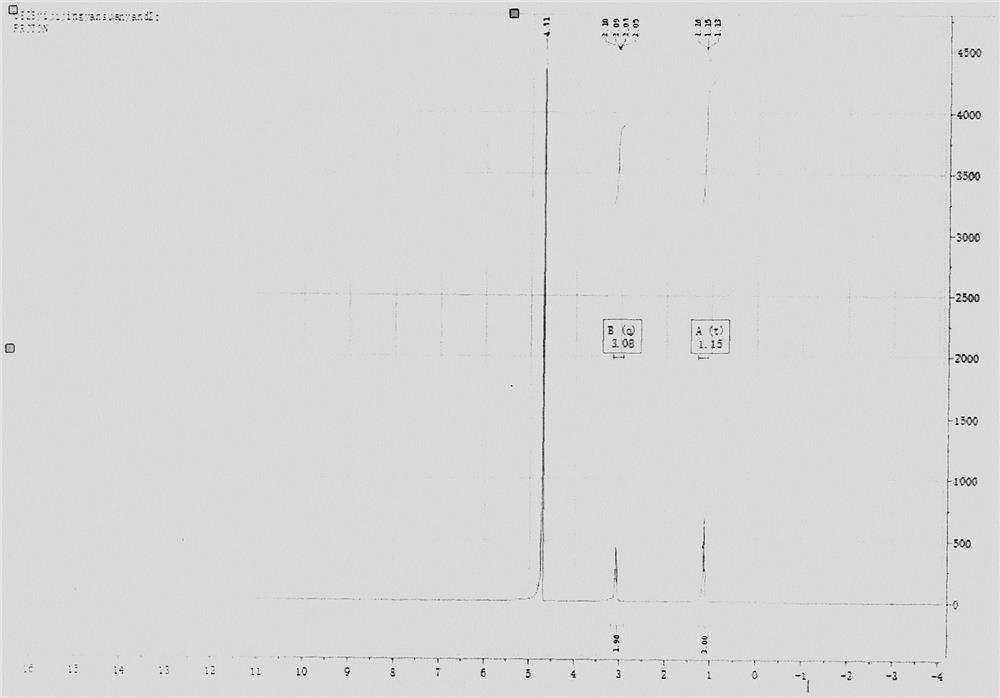
ActiveCN112624938AReduce generationScale up industrial productionHydrazine preparationHydrazide preparationPtru catalystEthyl group
The invention provides a new synthesis method of ethylhydrazine dihydrochloride, and the method comprises the following steps: using acethydrazide which is simple and easy to prepare as a raw material, reacting acethydrazide with bromoethane in the presence of a catalyst to generate N-acetyl-N'-ethylhydrazine, and removing acetyl in hydrochloric acid under a strongly acidic condition to obtain the ethylhydrazine dihydrochloride product. The product structure is confirmed by 1H NMR and single crystal, the raw materials of the method are cheap and easily available, the production cost can be greatly reduced, and the method is suitable for industrial production.
Owner:NANKAI UNIV
A kind of scr denitrification catalyst and its preparation method and application
ActiveCN106732531BScale up industrial productionHigh activityGas treatmentHeterogenous catalyst chemical elementsPtru catalystCerium(IV) oxide
The invention provides an SCR (Selective Catalytic Reduction) denitration catalyst as well as a preparation method and application of the SCR denitration catalyst. The SCR denitration catalyst is prepared from 0.8 weight percent to 1.2 weight percent of vanadium pentoxide, 2.5 weight percent to 10 weight percent of tungsten trioxide, 1 weight percent to 20 weight percent of cerium dioxide, 2.5 weight percent to 10 weight percent of molybdenum trioxide, 0.1 weight percent to 5 weight percent of sulfur trioxide, 0 to 2 percent of antimony trioxide and the balance of anatase type titanium dioxide, wherein the denitration rate of the SCR denitration catalyst at the air speed of 30000h<-1> under the temperature range of 240 DEG C to 400 DEG C is more than 94 percent. The SCR denitration catalyst provided by the invention has high activity and good selectivity. The preparation method of the catalyst is the improvement of an existing commercial catalyst preparation process method; amplified industrial production can be carried out by existing equipment.
Owner:DATANG INT CHEM TECH RESINST
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com