Perazopanib-quercetin eutectic crystal as well as preparation method and application thereof
A technology for pazopanib and quercetin, applied in the field of pazopanib-quercetin co-crystal and its preparation, can solve the problem of pazopanib co-crystal is less, achieve obvious curative effect and good stability , Significant effect of medical application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Preparation of pazopanib-quercetin co-crystals:
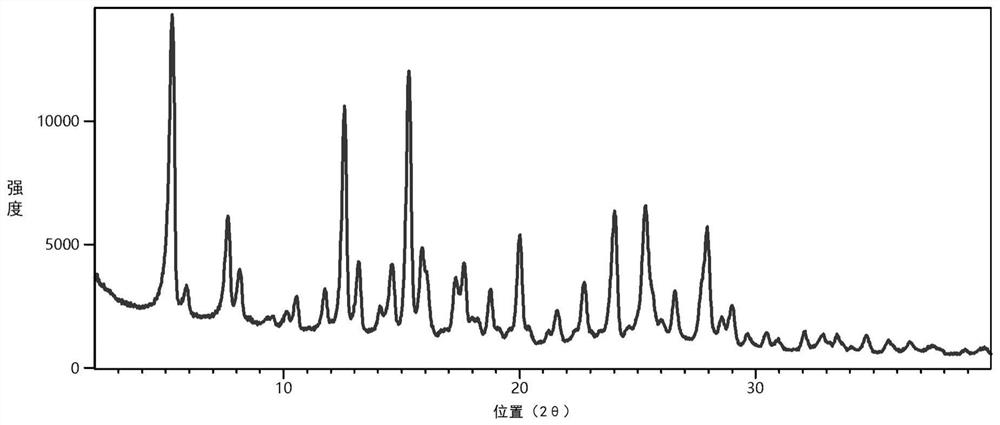
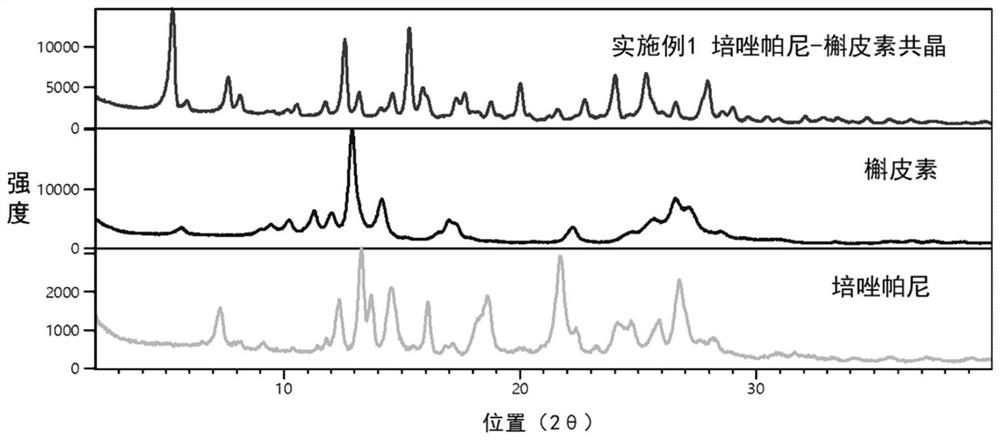
[0027] The co-crystal was prepared according to the molar ratio of pazopanib and quercetin as 1:1, 21.88 mg of pazopanib and 15.11 mg of quercetin were weighed, and 5 mL of acetonitrile was added. At room temperature (25°C), the slurry was magnetically stirred for 3 days, filtered, and the filter cake was dried at 60°C for 2 hours to obtain a yellow solid, which was the pazopanib-quercetin co-crystal. The product was characterized using powder X-ray diffractometer (PXRD) and the results were as follows figure 1 shown. The PXRD pattern of the co-crystal was compared with pazopanib and quercetin, such as figure 2 shown. from figure 2 It can be seen that the PXRD pattern of the co-crystal is significantly different from that of pazopanib and quercetin. The characteristic peaks of the three are further compared, as shown in Table 1. It can be seen from Table 1 that the co-crystal has characteristic diffraction peaks ...
Embodiment 2
[0031] Preparation of pazopanib-quercetin co-crystals:
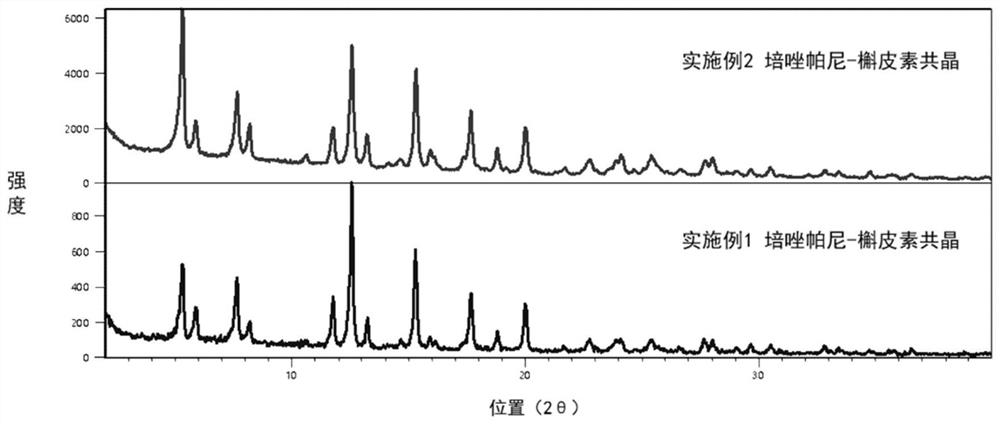
[0032] Prepare a co-crystal according to the molar ratio of pazopanib and quercetin as 1:2, weigh 21.88 mg of pazopanib and 30.22 mg of quercetin, add 5 mL of acetonitrile, and stir magnetically at room temperature (25°C). Beat for 3 days, filter, and place the filter cake to dry at 60°C for 2h. A yellow solid was obtained and characterized using a powder X-ray diffractometer (PXRD), and the results were as follows image 3 shown. from image 3 It can be seen that the characteristic diffraction peaks of this product are basically consistent with those of the product obtained in Example 1, that is, the pazopanib-quercetin co-crystal.
Embodiment 3
[0034] Stability of pazopanib-quercetin co-crystal:
[0035] The pazopanib-quercetin co-crystal obtained in Example 1 was placed in an artificial climate chamber under accelerated conditions (40°C, 75% RH), and samples were taken on the 5th and 10th days and X-rays were used It was characterized by powder diffractometer (PXRD), and the results were as follows Figure 4 shown. It can be seen from the figure that the pazopanib-quercetin co-crystal does not undergo crystal transformation under accelerated conditions for at least 10 days.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com