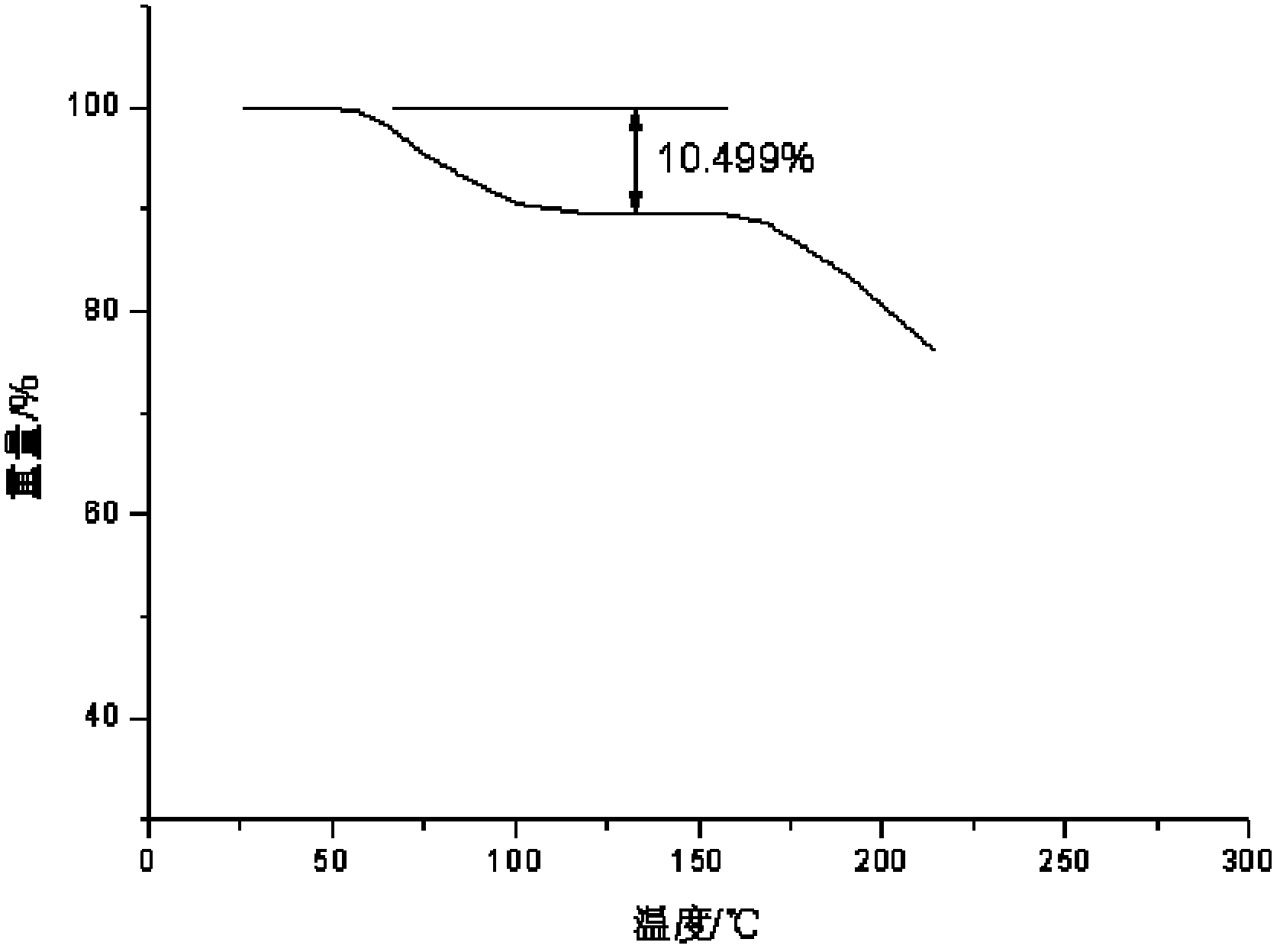
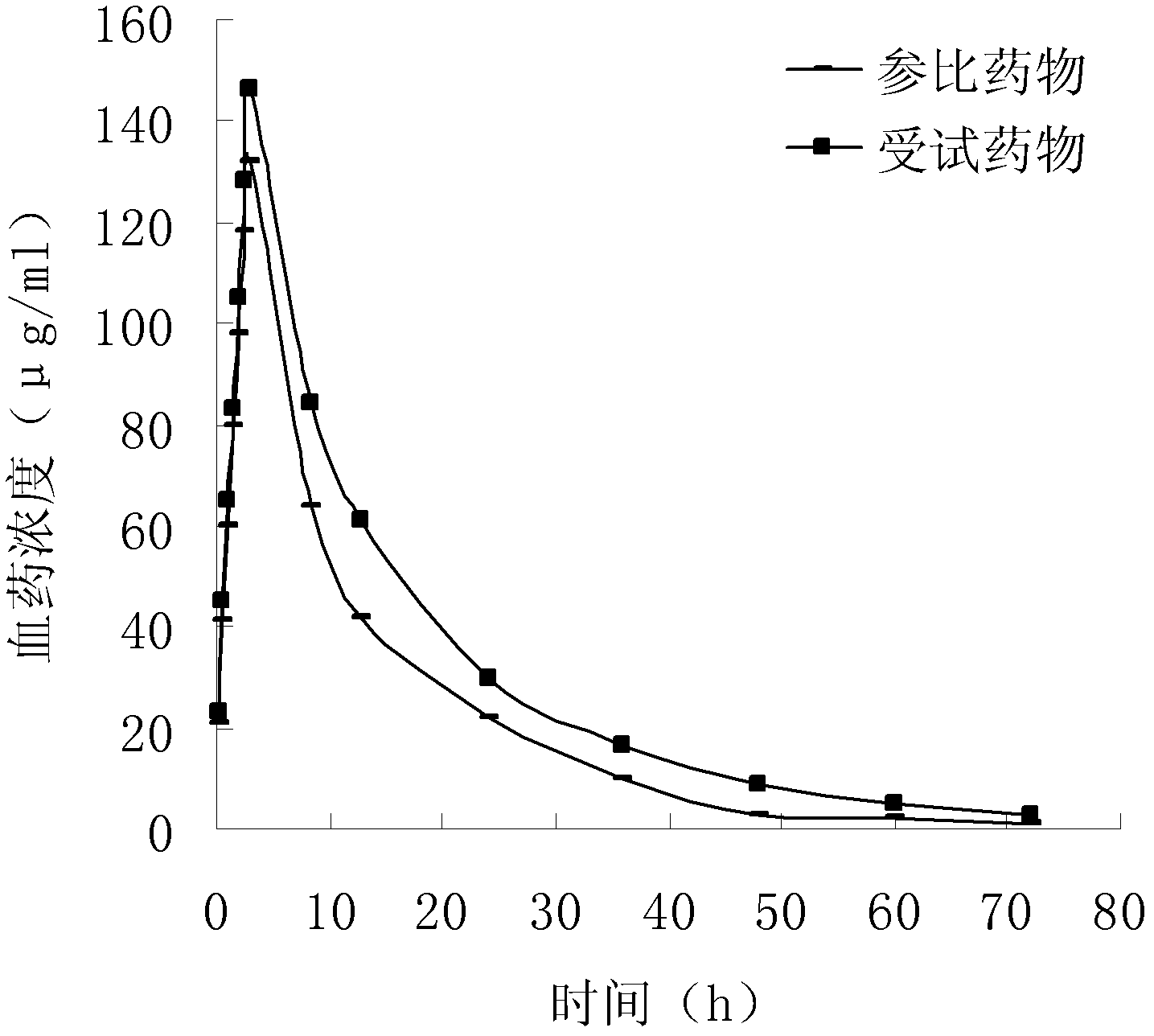
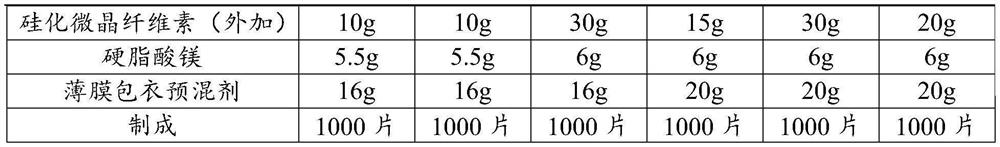
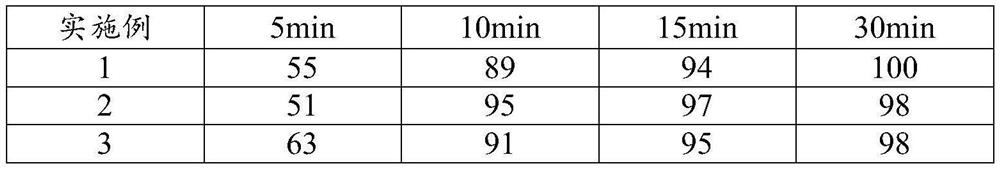
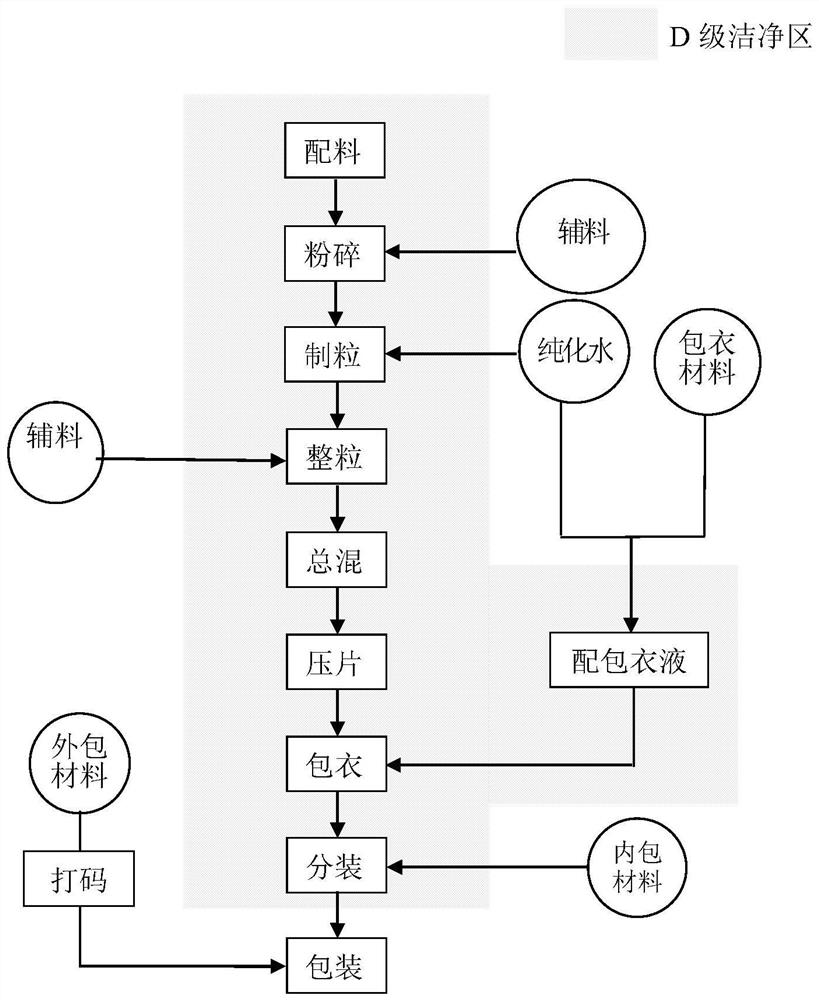
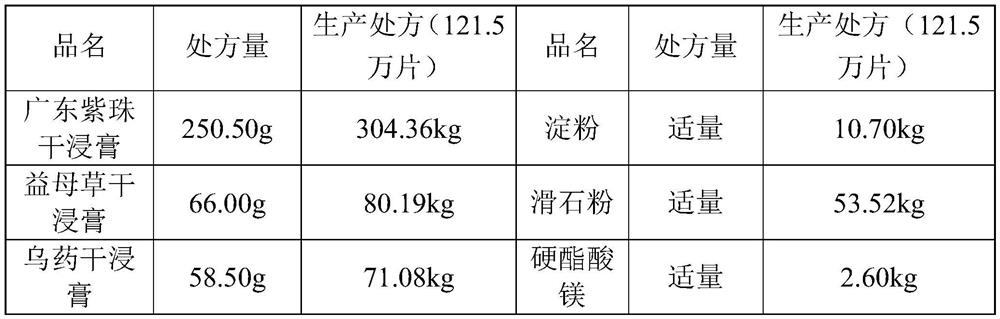
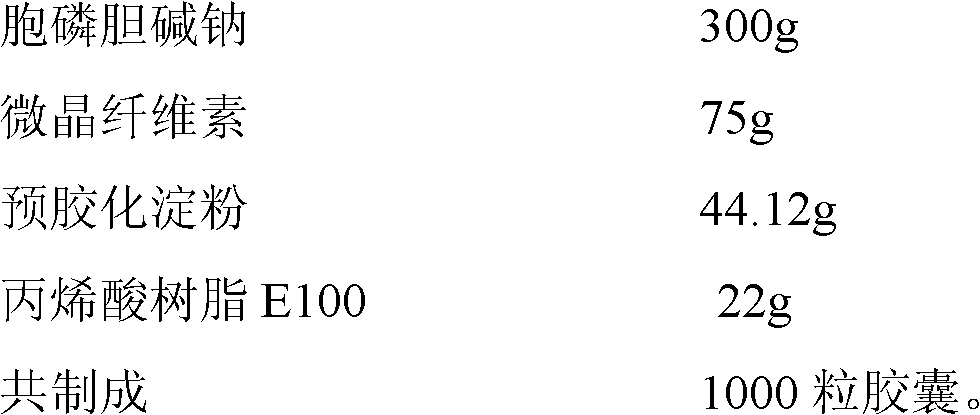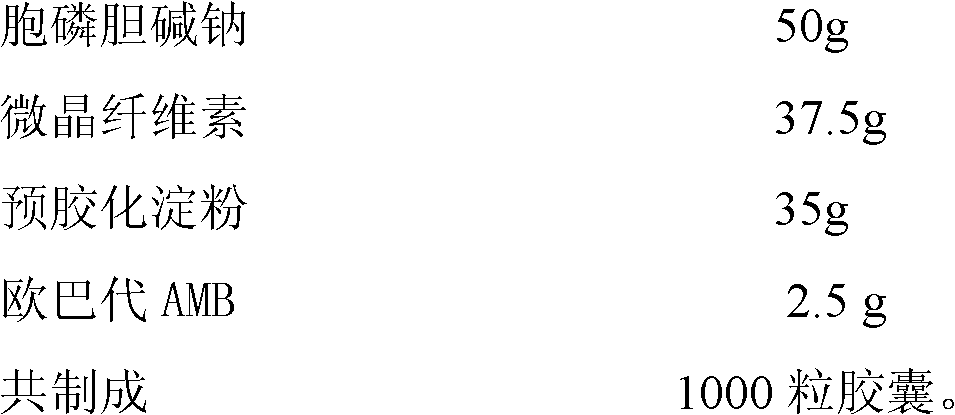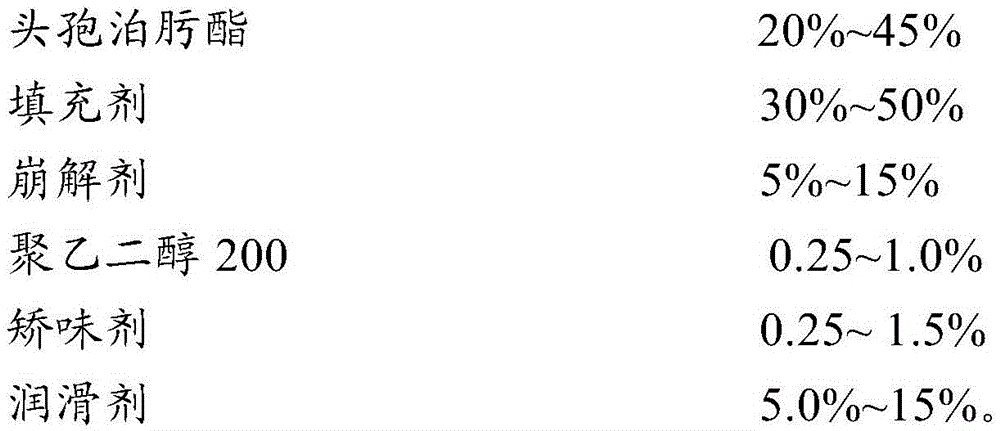Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
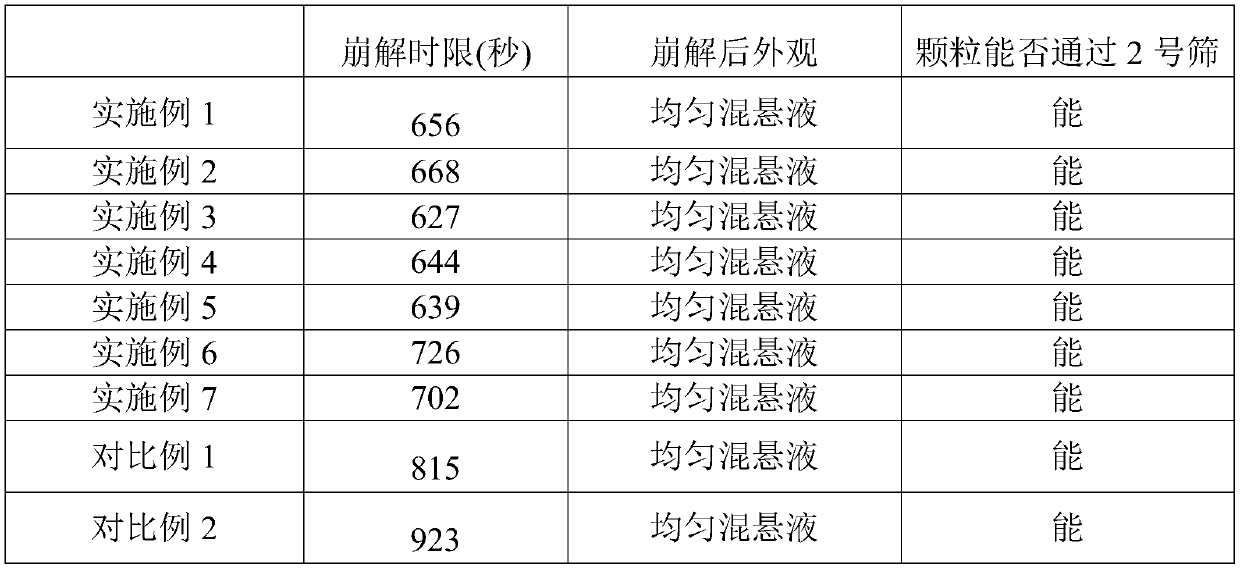
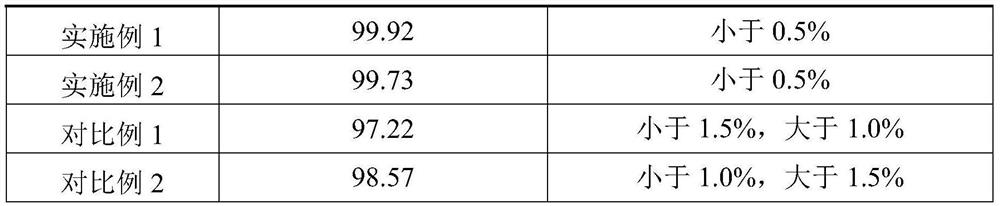
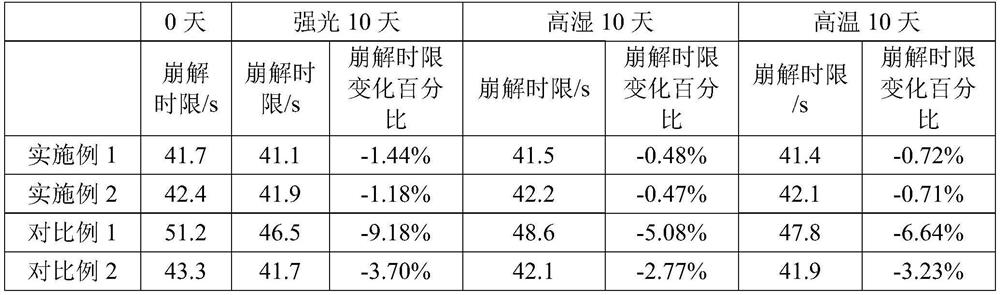
53results about How to "Improved disintegration time" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
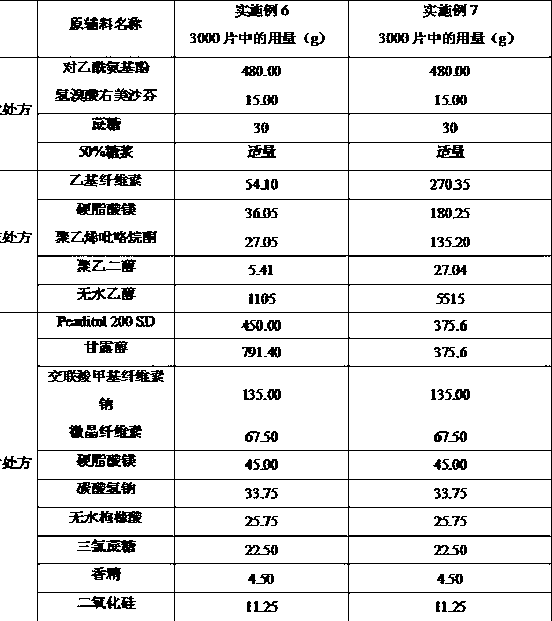
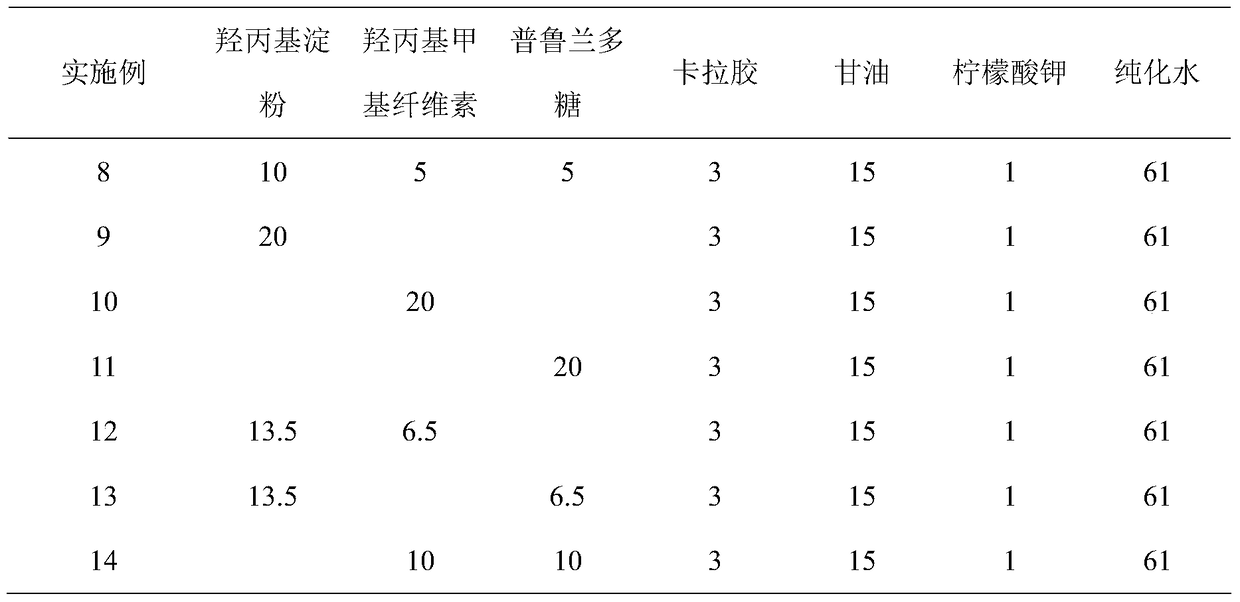
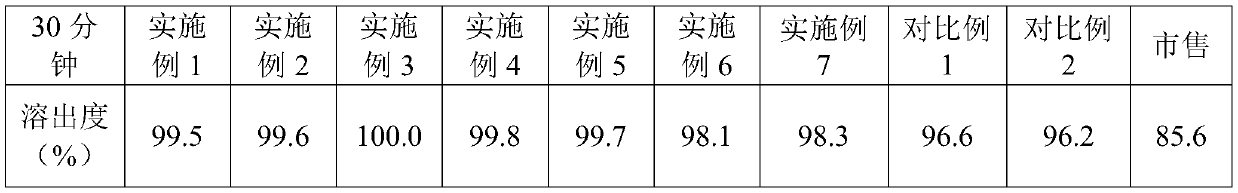
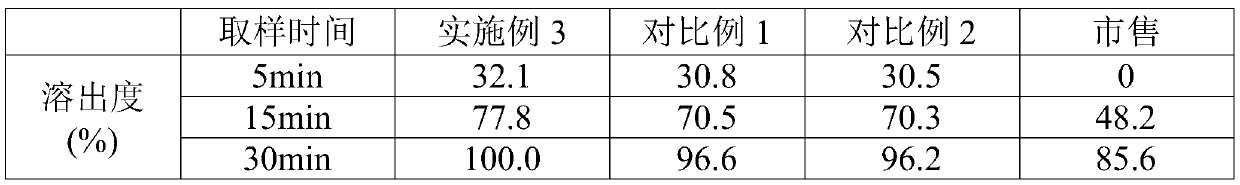
Vonoprazan oral quick-dissolving film agent and method for preparing same
ActiveCN105663096AAvoid difficultiesImprove complianceOrganic active ingredientsDigestive systemVonoprazanPharmaceutical formulation
The invention discloses a Vonoprazan oral quick-dissolving film agent and a method for preparing the same, and belongs to the field of medicine preparations.The Vonoprazan oral quick-dissolving film agent particularly comprises, by weight, 10-15% of Vonoprazan active monomers, 50-55% of hydroxypropyl methylcellulose, 25-30% of maltodextrin, 0.8-1.0% of hyaluronic acid and 5-10% of plasticizers.The Vonoprazan oral quick-dissolving film agent and the method have the advantages that the water-soluble hydroxypropyl methylcellulose, the water-soluble maltodextrin and the water-soluble hyaluronic acid are preferably used as film-forming materials, the appropriate plasticizers with the appropriate weight ratio are screened, and accordingly the film agent with excellent disintegration time and excellent mechanical performance can be prepared; the disintegration time limit of the Vonoprazan oral quick-dissolving film agent can be obviously shortened, accordingly, the shortcoming that water is required when existing most oral solid preparations are about to be administered can be overcome, the medicine administration time cannot be delayed even under the condition of deficiency of water resources, and the medication compliance of patients can be improved.
Owner:NANJING GRITPHARMA CO LTD
Naproxen hydrate crystal, preparation method thereof and pharmaceutical composition containing the crystal and sumatriptan
InactiveCN102276447AImprove solubilitySimple processOrganic active ingredientsNervous disorderCross-linkSodium bicarbonate
Owner:HAINAN JINRUI PHARMA
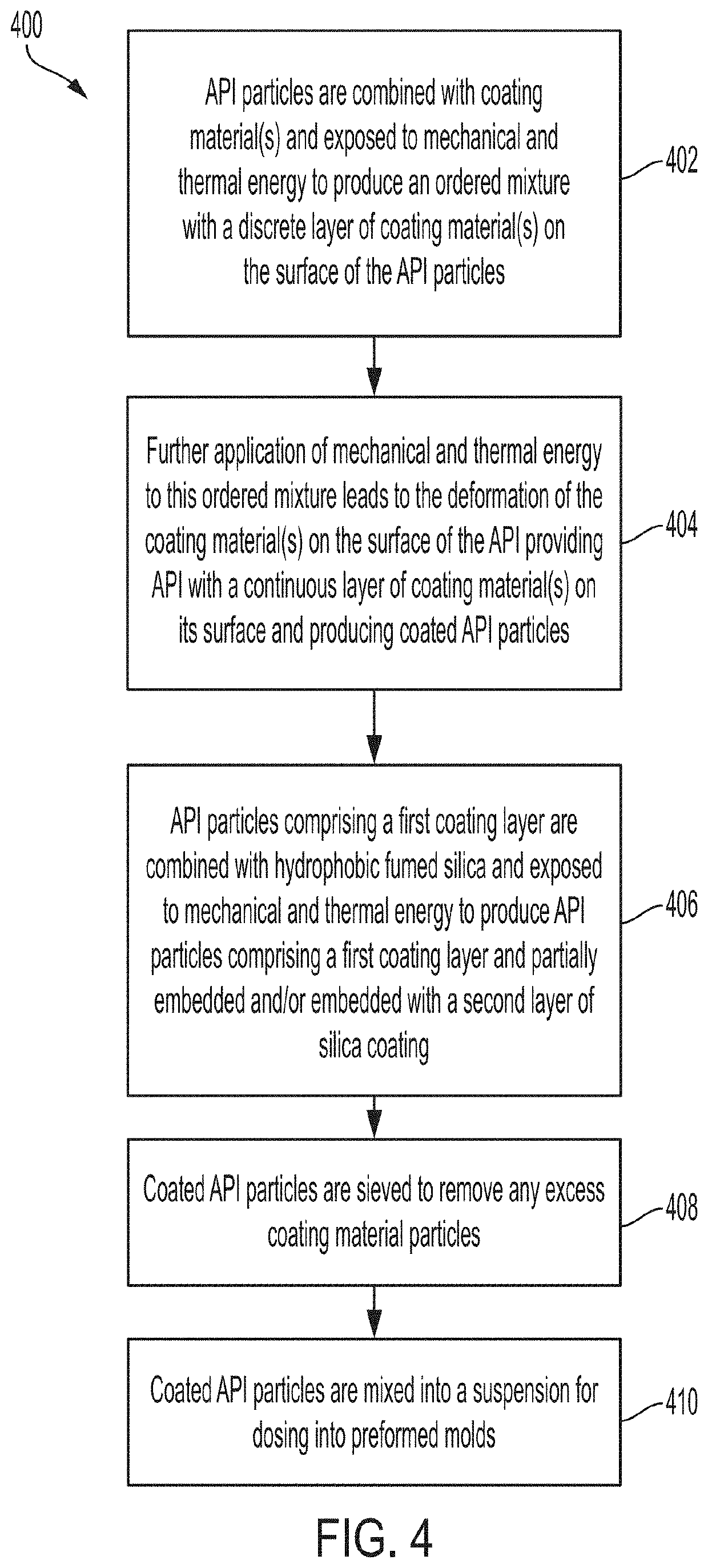
Minimizing agglomeration of drug particle coating material during storage to stabilize disintegration times of pharmaceutical products
ActiveUS20200268667A1Minimize agglomerationReduced stabilityOrganic active ingredientsAntipyreticPhysical chemistrySolvent free
Provided are pharmaceutical compositions and methods for preparing pharmaceutical compositions using solventless mixing methods. Excess coating material that is not bound to a coated API particle may be removed by a sieving process. Coating and dosing ratios can also be optimized to minimize the amount of excess unbound coating material. Specifically, a coating ratio and / or a dosing ratio can be used to minimize the residual amount of excess unbound coating material to minimize agglomeration of coating material during storage. In some embodiments, a pharmaceutical composition is provided, the pharmaceutical composition comprising: 65-85 % w / w API particles; 15-30 % w / w coating material coating the API particles; and 3-15 % w / w matrix surrounding the coated API particles, wherein the pharmaceutical composition comprises a disintegration time rate of less than 10 seconds for at least six months under storage conditions of at least 25° C. and at least 60 % relative humidity.
Owner:CATALENT U K SWINDON ZYDIS LTD
Moisture-proof coating citicoline sodium capsule and preparation method thereof
ActiveCN102525997AGood granularityGood disintegrationOrganic active ingredientsNervous disorderCiticoline sodiumMoisture absorption
The invention provides a moisture-proof coating citicoline sodium capsule and a preparation method thereof. The citicoline sodium capsule is composed of a capsule casing and quick-release moisture-proof micro pills or granules. All components in each capsule containing citicoline sodium quick-release moisture-proof micro pills or granules by percentage are 40% to 70% of citicoline sodium, 10% to 30% of microcrystalline cellulose, 10% to 30% of pregelatinized starch and 2% to 10% of moisture-proof coatings. The moisture-proof micro pills or granules are obtained by being extruded and rounded or pressed and granulated to be coated with moisture-proof coatings. The citicoline sodium capsule is good in moisture-proof function and resolves the problems that the capsule is prone to absorb waterdue to the fact that citicoline sodium is strong in moisture absorption, the capsule casing is prone to be fragile, and the like.
Owner:QILU PHARMA CO LTD
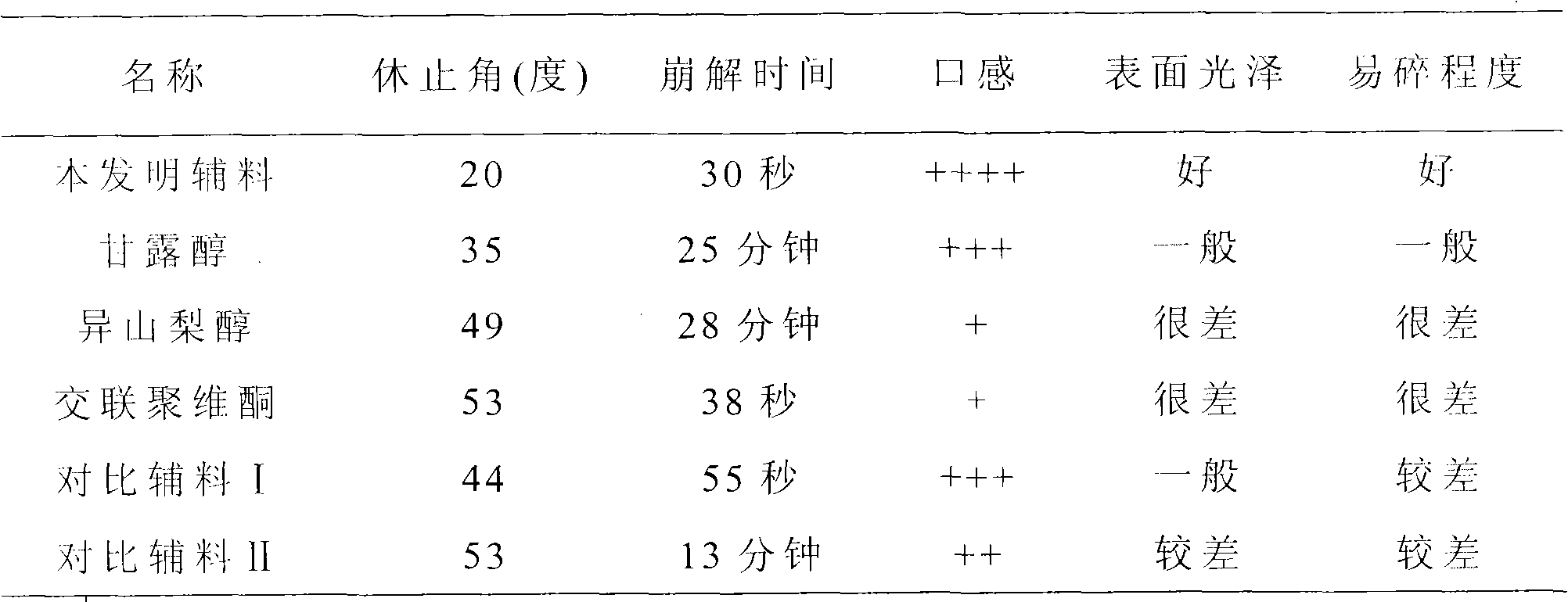
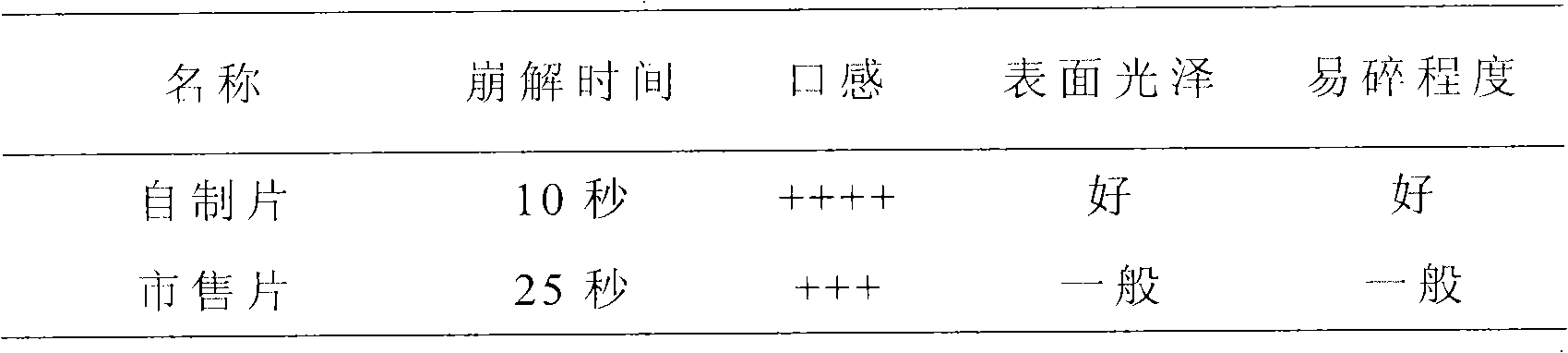
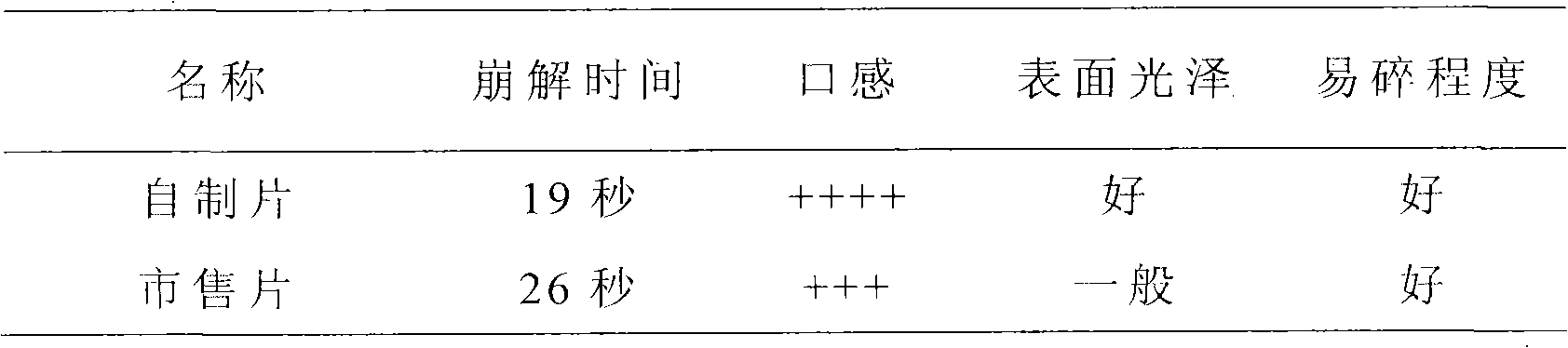
New multi-functional auxiliary material for orally disintegrating tablets and preparation method thereof
InactiveCN101829333AImprove liquidityGood disintegrationPill deliveryPharmaceutical non-active ingredientsMANNITOL/SORBITOLOrally disintegrating tablet
The invention relates to a new multi-functional auxiliary material for orally disintegrating tablets and a preparation method thereof, and belongs to the field of pharmacy. The preparation method comprises the following steps of: weighting and mixing mannitol, isosorbide and crospovidone; under the condition of using water as a solvent, carrying out high-shear dispersion on the obtained mixture so as to obtain solution; and then carrying out spray drying on the solution to obtain the new multi-functional auxiliary material.
Owner:SHANGHAI HUAMAO PHARMA
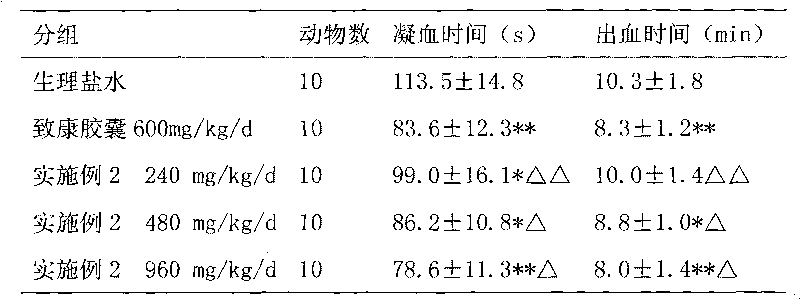
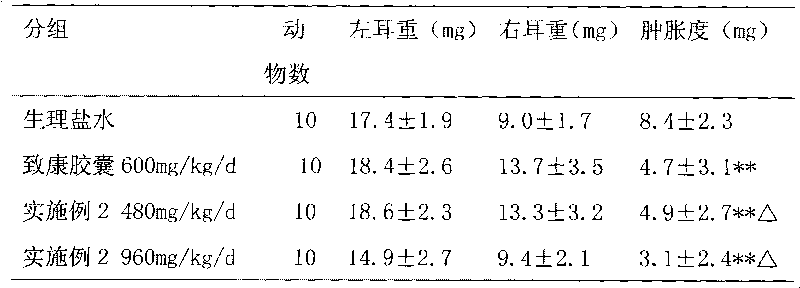
Medicament for treating metrorrhagia and metrostaxis, hematemesis, hematochezia and traumatic hemorrhage and preparation method thereof
ActiveCN101703690AEnhance anti-inflammatoryGood hemostatic effectHydroxy compound active ingredientsInanimate material medical ingredientsMetrorrhagiaTrauma hemorrhage
The invention relates to a medicament for treating metrorrhagia and metrostaxis, hematemesis, hematochezia and traumatic hemorrhage and a preparation method thereof. The preparation method comprises the following steps of: 1, mixing rhubarb, golden thread and dahurian angelica root, adding an ethanol, merging ethanol extract, and concentrating the ethanol extract; 2, adding sanchi into the ethanol, merging ethanol extract and concentrating the ethanol extract; 3, taking common bletilla pseudobulb decocting liquid and concentrating the liquid into a thick paste with a relative density between 1.15 and 1.30 at the temperature of 60 DEG C; 4, decocting dregs in the three steps, India madder root and liquoric root twice, merging decoction, concentrating the decoction, mixing the concentrated decoction with the thick paste obtained in step 3, reducing pressure and drying the mixture to obtain a dry paste and crushing the dry paste; 5, evenly mixing products obtained by step 1 and step 2, carrying out high-speed centrifugal spraying drying, and simultaneously spraying aqueous solution of hydroxypropyl-beta-cyclodextrin to prepare dispersed fine powder; and 6, mixing and crushing medicaments such as cuttlebone, calcined dragon bone and the like, evenly mixing the mixture with two fine powder obtained by the step 4 and step 5, adding medicaments such as microcrystalline cellulose and the like, and evenly mixing, drying and palletizing the mixture. The medicament simplifies production process, saves production cost and has obvious curative effect.
Owner:XIAN CHIHO PHARMA
Minimizing agglomeration, aeration, and preserving the coating of pharmaceutical compositions comprising ibuprofen
ActiveUS20200268676A1Minimize agglomerationReduced stabilityOrganic active ingredientsAntipyreticSolvent freePharmaceutical Substances
Provided are pharmaceutical compositions and methods for preparing pharmaceutical compositions comprising Ibuprofen using solventless mixing methods. Excess coating material that is not bound to coated Ibuprofen may be removed by a sieving process. Coating and dosing ratios can also be optimized to minimize the amount of excess unbound coating material. Additionally, the compositions can be formulated to preserve the functional coating of coated Ibuprofen and to minimize aeration of Ibuprofen when mixed into suspension.
Owner:CATALENT U K SWINDON ZYDIS LTD
Voglibose tablet and preparation method thereof
ActiveCN108309946AHigh dissolution rateImprove efficacyOrganic active ingredientsMetabolism disorderFiberTablet dissolution
The invention provides a voglibose tablet and a preparation method thereof. The voglibose tablet is prepared from 1 part by weight of voglibose, 450-550 parts by weight of lactose, 130-170 parts by weight of starch, 20-30 parts by weight of high substituted hydroxypropyl fibers, 3-4 parts by weight of magnesium stearate, 0.1-0.5 parts by weight of propolis, 5-10 parts by weight of sodium lauryl sulfate, 4-6 parts by weight of polysorbate 80, 30-40 parts by weight of a co-solvent and 40-50 parts by weight of a disintegrant. The voglibose is used as the main drug, and high-substituted hydroxypropyl fiber, propolis, polysorbate 80 synergistic voglibose, lactose, starch, magnesium stearate and sodium lauryl sulfate are used in a good ratio so that the tablet dissolution degree is greatly improved, the disintegration time limit is optimized and the drug efficacy is improved. The voglibose tablet has a good dissolution degree and good disintegration effects, can well exert the efficacy of the voglibose tablet and can improve the drug efficiency. Through combination of the initial mixing process and low / high-speed agitation, the tablet dissolution degree is further improved and the quality of the tablet is improved.
Owner:HAINAN HUALON PHARM
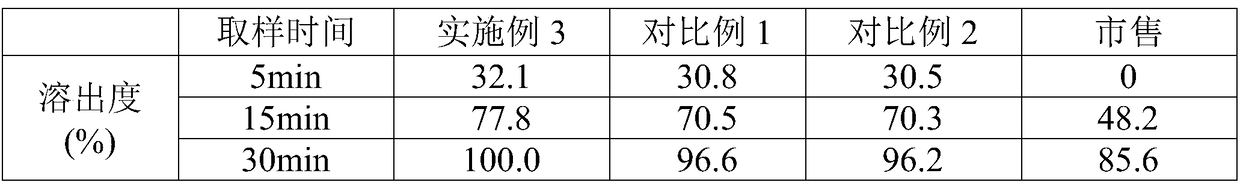
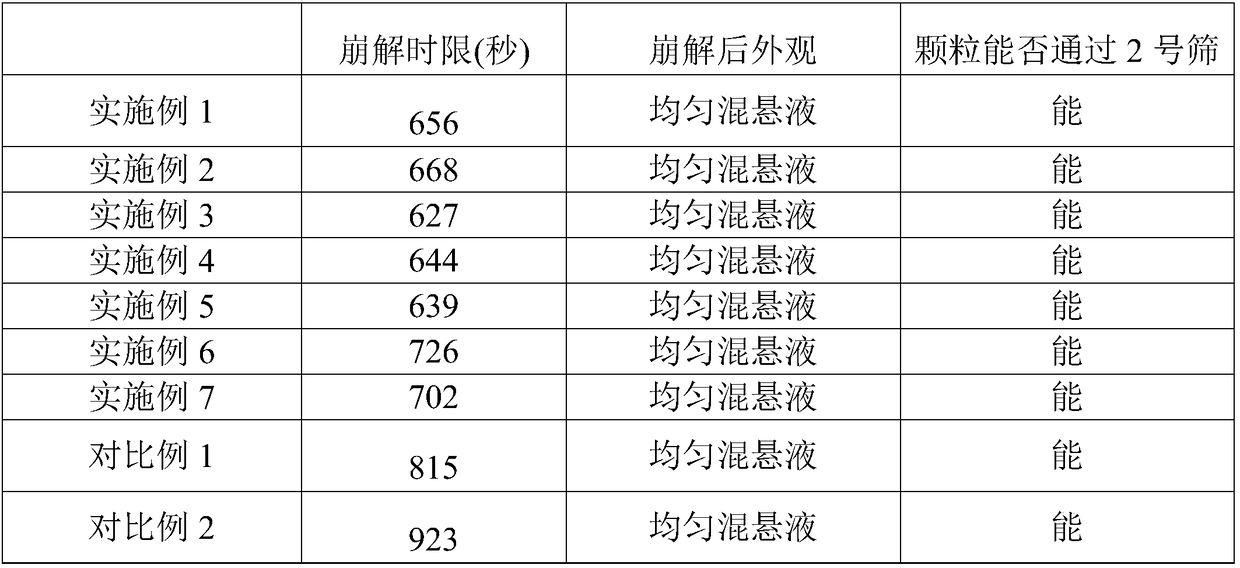
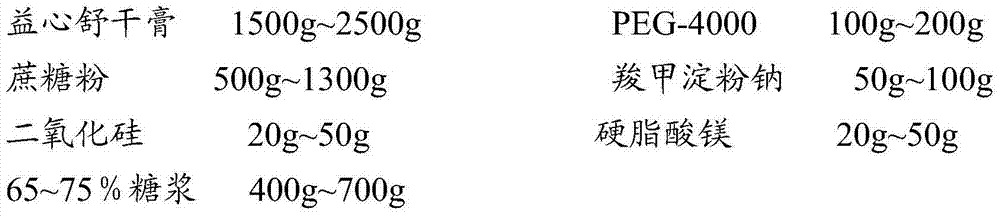
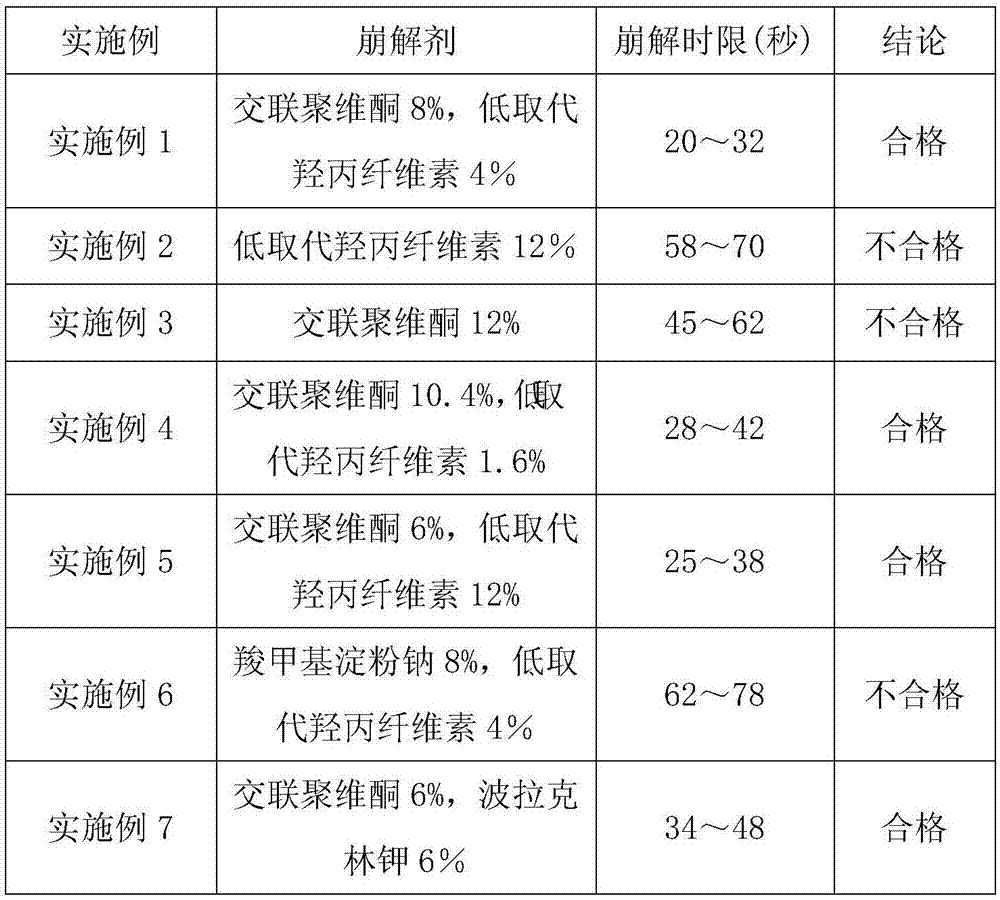
Heart relaxing tablet and preparation method thereof
ActiveCN103784726AUniform particle sizeGreat tastePharmaceutical non-active ingredientsPill deliveryCarboxymethyl starchSucrose
The invention discloses a heart relaxing tablet which is prepared from heart relaxing dry paste, PEG (Polyethylene Glycol)-4000, sucrose powder, carboxymethyl starch sodium, silica, magnesium stearate and a sirup agent, wherein the sirup agent is an adhesive which is relatively high in viscosity and suitable for a loose botanical drug with high elasticity, the prepared tablet is uniform in particle size, and the hardness and release rate of the tablet are both better than those of particles prepared by a traditional process. The invention also discloses a preparation method of the heart relaxing tablet; during preparation, the heart relaxing dry paste is added internally and externally so that the tablet is uniform in color after a coating is removed, the characters are improved and the medicinal absorption speed is increased as well. Through elaborate selection of auxiliary materials, the heart relaxing tablet is good-looking in appearance, good in color and gloss, good in taste, long in storage period, convenient to use, transport and carry, low in cost and high in yield.
Owner:HAINAN HULUWA PHARMA GRP CO LTD
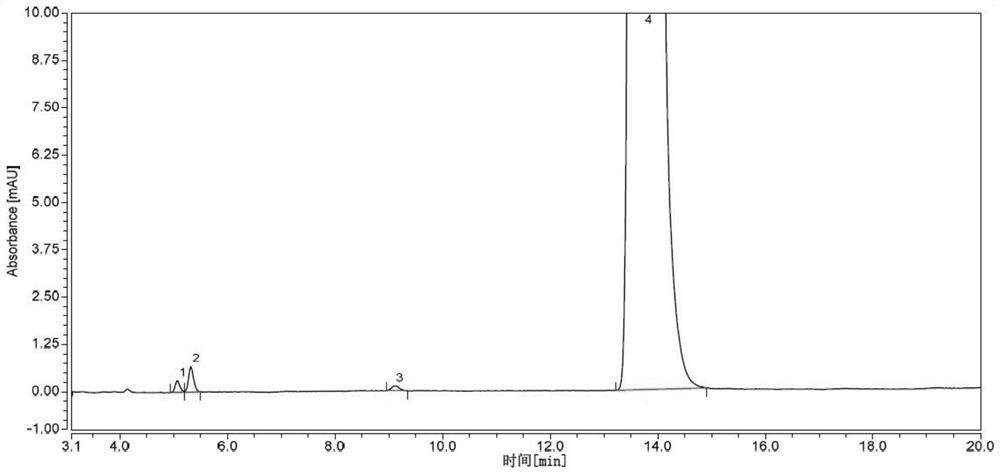
Cefpodoxime proxetil dispersible tablet and preparation method thereof
ActiveCN103479589AShort disintegration timeModerate hardnessAntibacterial agentsOrganic active ingredientsMedicinePolyethylene glycol
The invention discloses a cefpodoxime proxetil dispersible tablet which comprises the following raw materials in percentage by weight: 20-45 percent of cefpodoxime proxetil, 30-50 percent of filler, 5-15 percent of a disintegrating agent, 0.25-1.0 percent of a wetting agent, 0.25-1.5 percent of a flavoring agent and 5.0-15 percent of polyethylene glycol 200. The preparation method adopts a specific wetting agent, so as to improve mobility, dissolubility and uniformity in mixing of the product and facilitate the storage of the product, and after the product is stored for a period of time, no obvious changes occur. According to the invention, the cefpodoxime proxetil dispersible tablet is obtained through direct powder mixing, granulation and tabletting, and the preparation method is simple without any special equipment, and is suitable for industrial production.
Owner:HAINAN HULUWA PHARMA GRP CO LTD
Gastrodine tablets disintegrating in oral cavity and process for producing same
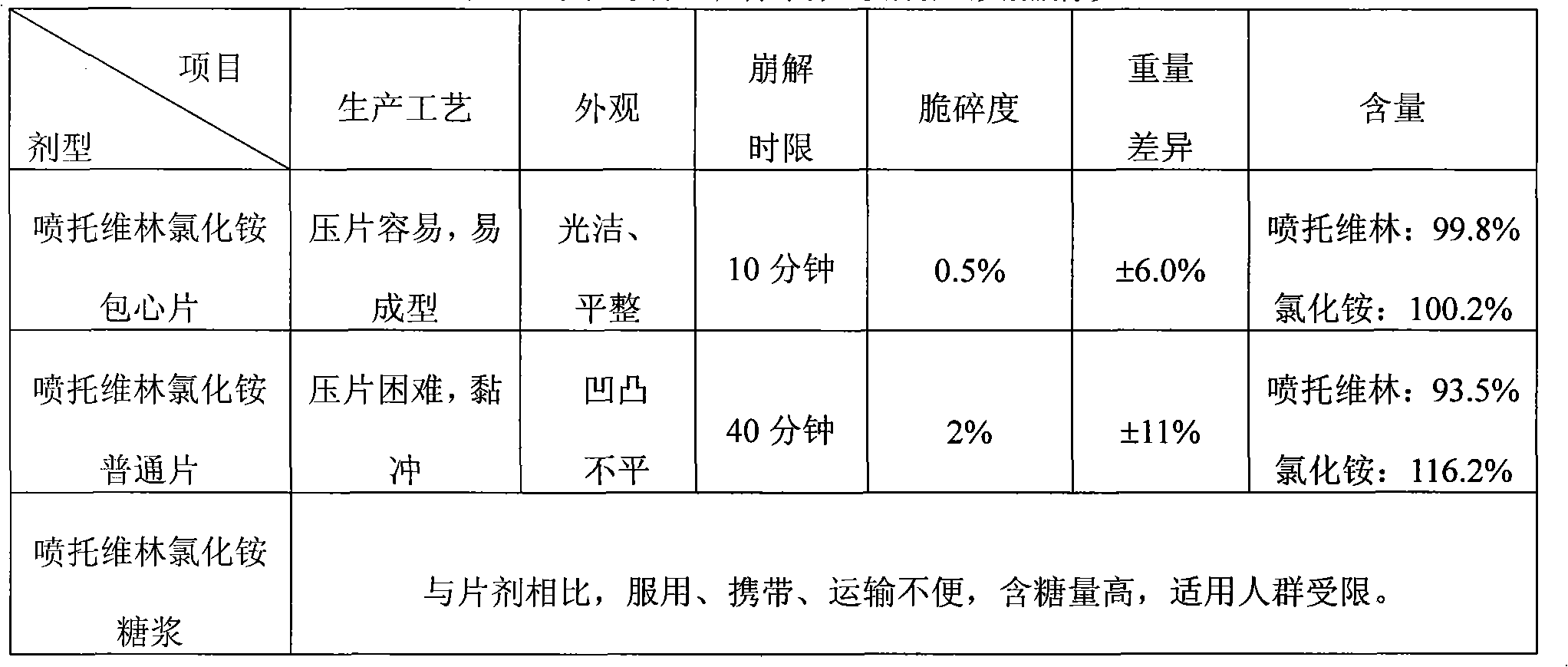
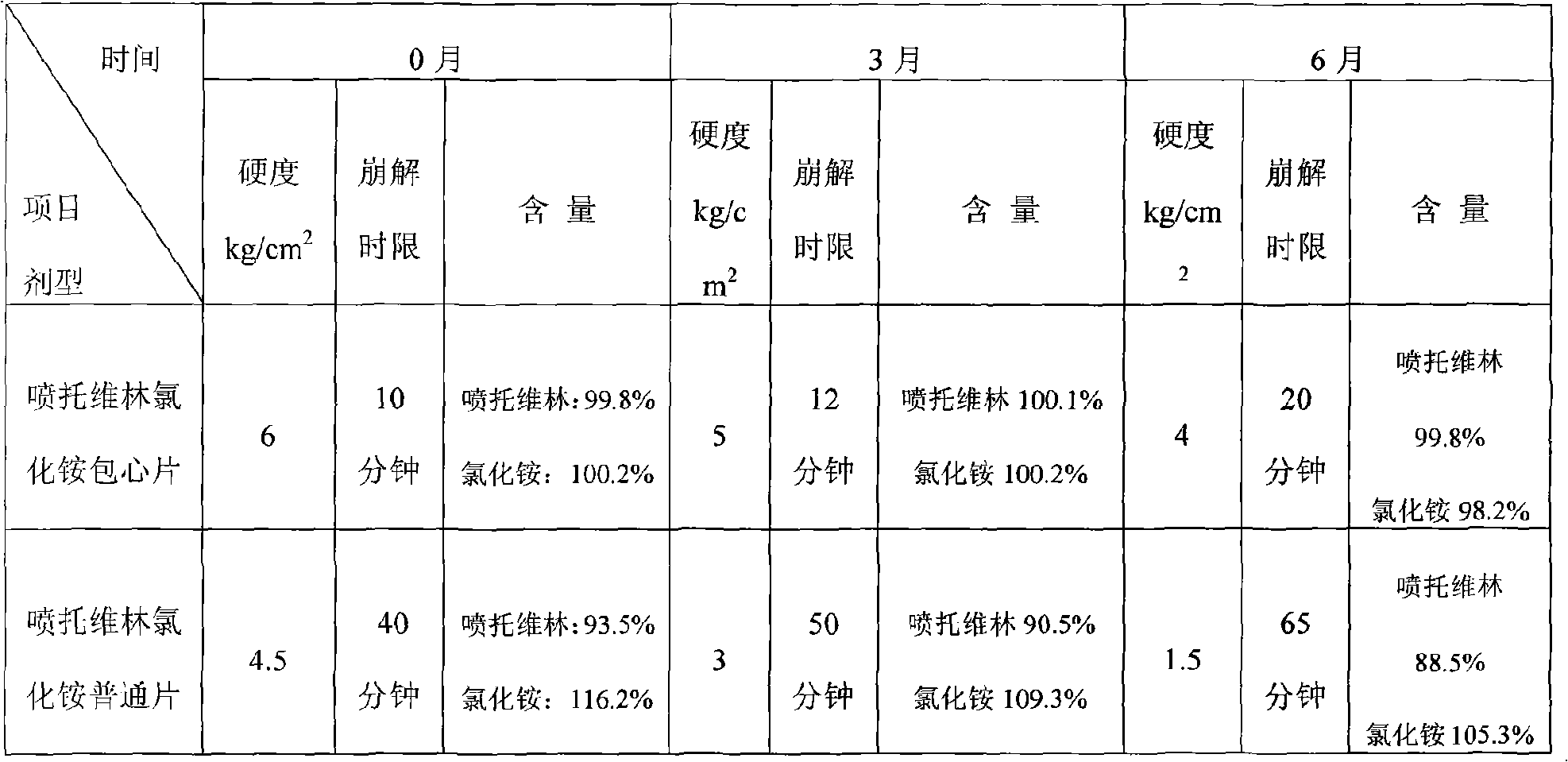
ActiveCN1923185AImproved disintegration timeMeet technical requirementsOrganic active ingredientsNervous disorderOrally disintegrating tabletLow-substituted hydroxypropylcellulose
The invention relates to a gastrodine mouse mouse disintegration tablet, which uses gastrodine as main drug, with gastrodine coat capsule, diluter, and disintegrator. Wherein, the invention is characterized in that: said capsule is made from gastrodine, coated by acrylic acid resin and acrylic acid; and the disintegrator is low-replace hydroxypropyl cellulose and crosslink polyvinyl pyrrolidon. The invention has better disintegration time limit, with uniform solution.
Owner:北京科信聚润医药科技有限公司
Ambroxol hydrochloride oally disintegrating tablet and preparation method thereof
InactiveCN107137366AEvenly dispersedImprove or eliminate stickinessOrganic active ingredientsPill deliveryCross-linkProcess equipment
The invention discloses an ambroxol hydrochloride oally disintegrating tablet and a preparation method thereof. The orally disintegrating tablet consists of effective dose of ambroxol hydrochloride and pharmaceutical adjuvants, wherein the pharmaceutical adjuvants include a taste masking agent, a filler, a flavoring agent, a disintegrant, an adhesive and a lubricant; and the disintegrant is a combination of cross-linked povidone and other disintegrants. The ambroxol hydrochloride oally disintegrating tablet prepared by the invention has the characteristics of being convenient to take without the use of water, rapid in disintegrating, rapid in absorption, high in bioavailability and the like. The preparation method of the finished product (the ambroxol hydrochloride oally disintegrating tablet) provided by the invention is low in cost, simple in processing equipment, easy and controllable in implementation, low in operating cost, economic and convenient, and applicable to large-scale production.
Owner:CHONGQING CONQUER PHARML
PH dependent type colon positioning hard capsule
ActiveCN103520226ASimple preparation processNot easy to dehydration agingDigestive systemPharmaceutical delivery mechanismAcrylic resinHard Capsule
The invention belongs to the field of pharmaceutical preparation, relates to an alimentary canal drug preparation, and particularly relates to a pH dependent type colon positioning capsule. The pH dependent colon positioning hard capsule includes a hard capsule injected with drug ingredients and a coating film; a coating liquid for forming the coating film has the use amount which is 1-N times that of the following prescription: 2 g of a coating material comprising acrylic resin No.III, EudragitRL100 and / or EudragitRS100; the weight of EudragitRL100 and / or EudragitRS100 accounts for 18-25% of the total weight of the coating material; the coating liquid also comprises 2-3 ml of a plasticizer and 92-98 ml of a solvent of the coating material. An outermost layer of the hard capsule is coated with the polymer coating film, so that injected drugs are ensured not to leak, and the colon positioning drug release requirements can be met.
Owner:惠州市九惠药业有限公司
Compound oral disintegrating tablet containing acetaminophen and dextromethorphan
ActiveCN103169706ASimple preparation processGreat tasteOrganic active ingredientsNervous disorderPyrrolidinonesMannitol
The invention relates to the field of pharmaceutical preparation, and in particular relates to a compound oral disintegrating tablet containing acetaminophen and dextromethorphan hydrobromide. The compound oral disintegrating tablet contains the following components of: a. acetaminophen and dextromethorphan hydrobromide coating particles, wherein the particle diameter of the coating particles is 60-100 meshes, the coating particles contain particles including acetaminophen and dextromethorphan hydrobromide, a coating material coated on the particle surface, and the coating material comprises ethylcellulose and at least one pore-foaming agent; b. a filler which is directly compressible mannitol, mannitol, microcrystalline cellulose or a mixture thereof; c. a disintegrating agent selected from crospolyvinylpyrrolidone or croscarmellose sodium; d. a lubricant which is talcum powder or magnesium stearate; and e. an effervescing agent.
Owner:CHONGQING PHARMA RES INST
A kind of vegetable soft capsule rubber composition and preparation method thereof
ActiveCN105434397BReduce usageObvious cost advantagePharmaceutical non-active ingredientsCapsule deliveryPullulanPlasticizer
The invention relates to a vegetable soft capsule shell composition and a preparation method thereof. The composition comprises the following ingredients by content: 0-30% of hydroxypropyl starch, 0-20% of hydroxypropyl methyl cellulose, 0-20% of pullulan, 0.1-10% of one or more gels, 0.5-30% of one or more plasticizers, 0-5% of one or more soluble salts, 0-4% of an opacifier, 0-4% of one or more colorants and 10-90% of water, wherein the contents of hydroxypropyl starch, hydroxypropyl methyl cellulose and pullulan are not zero, and the total content is 100%. The preparation method comprises the following steps: adding hydroxypropyl starch into water for dispersion, so as to obtain a solution A; adding other ingredients except hydroxypropyl methyl cellulose into water for dispersion, so as to obtain a solution B; mixing the solution A with the solution B, heating to 90-95 DEG C, carrying out stirring and heat preservation for 0.5-2 hours, cooling to 75-80 DEG C, and carrying out heat preservation, still standing and defoaming to obtain a glue solution; measuring the viscosity of the glue solution after defoaming, wherein the composition is obtained when the viscosity reaches 20,000-30,000 mPa.s.
Owner:XIAMEN KINGDOMWAY BIOTECH CO LTD +1
A kind of voglibose tablet and preparation method thereof
ActiveCN108309946BHigh dissolution rateImprove efficacyOrganic active ingredientsMetabolism disorderTablet dissolutionMagnesium stearate
The invention provides a voglibose tablet and a preparation method thereof. The voglibose tablet is prepared from 1 part by weight of voglibose, 450-550 parts by weight of lactose, 130-170 parts by weight of starch, 20-30 parts by weight of high substituted hydroxypropyl fibers, 3-4 parts by weight of magnesium stearate, 0.1-0.5 parts by weight of propolis, 5-10 parts by weight of sodium lauryl sulfate, 4-6 parts by weight of polysorbate 80, 30-40 parts by weight of a co-solvent and 40-50 parts by weight of a disintegrant. The voglibose is used as the main drug, and high-substituted hydroxypropyl fiber, propolis, polysorbate 80 synergistic voglibose, lactose, starch, magnesium stearate and sodium lauryl sulfate are used in a good ratio so that the tablet dissolution degree is greatly improved, the disintegration time limit is optimized and the drug efficacy is improved. The voglibose tablet has a good dissolution degree and good disintegration effects, can well exert the efficacy of the voglibose tablet and can improve the drug efficiency. Through combination of the initial mixing process and low / high-speed agitation, the tablet dissolution degree is further improved and the quality of the tablet is improved.
Owner:HAINAN HUALON PHARM
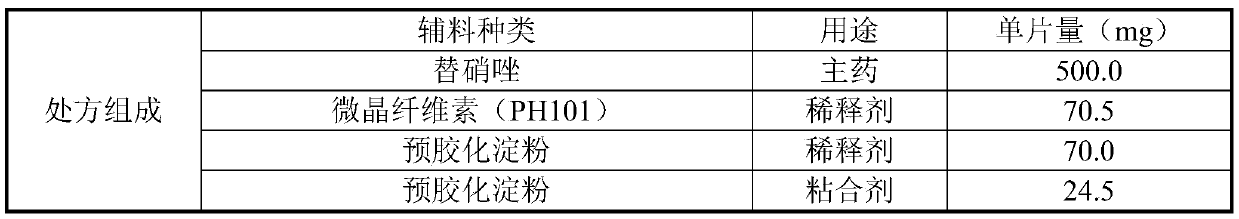
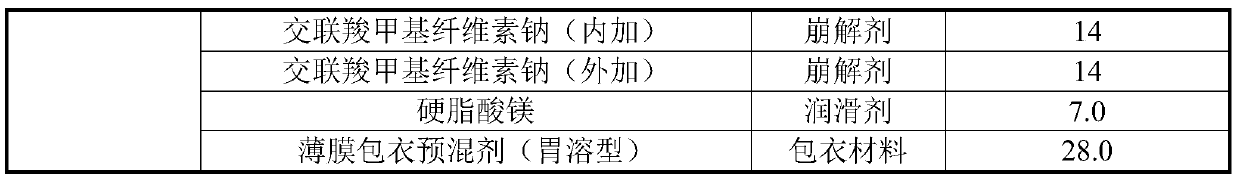
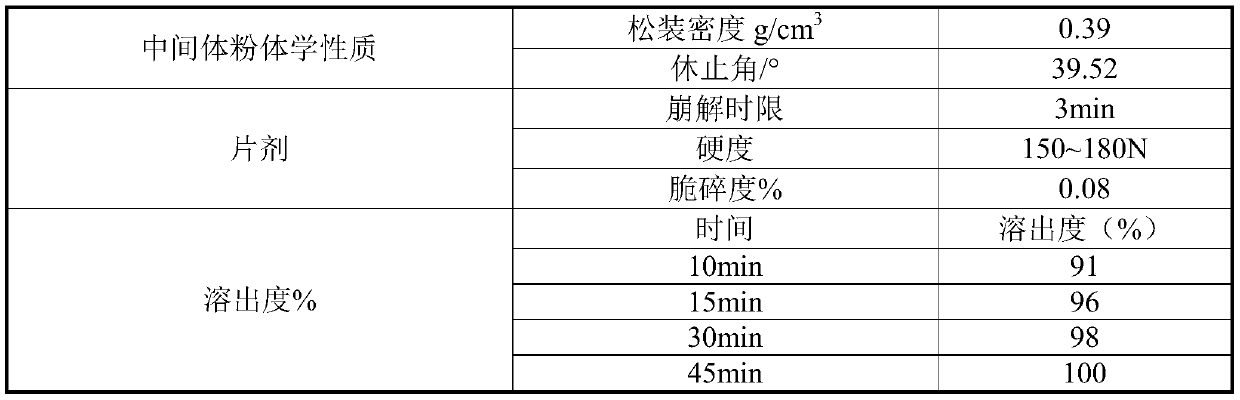
Tinidazole tablet and preparation method thereof
ActiveCN110711181AHigh dissolution rateComplete appearanceAntibacterial agentsOrganic active ingredientsTinidazoleAdhesive
The invention discloses a tinidazole tablet and a preparation method thereof. The tinidazole tablet comprises a tablet core and a coating, wherein the tablet core comprises tinidazole, a diluent, an adhesive, a disintegrating agent and a lubricant. The preparation method disclosed by the invention comprises a step of preparing the tinidazole tablet by using a wet-method one-step pelletizing process. The disintegrating agent is added in different steps by external and external addition modes respectively, so that the time can be shortened, and the production efficiency can be improved; and witha prescription, the dissolution and hardness of the product can be improved, the powder properties of raw materials are improved, the smoothness of a tablet pressing process can be improved, the appearance can be improved, and the product quality can be effectively improved.
Owner:SUZHOU KELUN PHARMA RES CO LTD
Yixinshu tablet and preparation method
InactiveCN109288959AUniform particle sizeGreat tastePharmaceutical non-active ingredientsPill deliverySalvia miltiorrhizaMedicine
The invention relates to a Yixinshu tablet and a preparation method. Specifically, the Yixinshu tablet is prepared by extraction of ginseng, radix ophiopogonis, Schisandra chinensis, radix astragali,Salvia miltiorrhiza, Ligusticum wallichii and hawthorn as the raw materials. During preparation of the tablet, 75% ethanol is employed for granulation, has strong viscosity, and is suitable for botanical drugs with loose texture and strong elasticity, and the obtained particles are subjected to size stabilization, the hardness and taste are good, and after tabletting, the obtained tablet has smooth and flat surface, and has good application value.
Owner:GUIZHOU XINBANG PHARMACEUTICAL CO LTD
Moisture-proof coating citicoline sodium capsule and preparation method thereof
ActiveCN102525997BGood granularityGood disintegrationOrganic active ingredientsNervous disorderCiticoline sodiumMoisture absorption
The invention provides a moisture-proof coating citicoline sodium capsule and a preparation method thereof. The citicoline sodium capsule is composed of a capsule casing and quick-release moisture-proof micro pills or granules. All components in each capsule containing citicoline sodium quick-release moisture-proof micro pills or granules by percentage are 40% to 70% of citicoline sodium, 10% to 30% of microcrystalline cellulose, 10% to 30% of pregelatinized starch and 2% to 10% of moisture-proof coatings. The moisture-proof micro pills or granules are obtained by being extruded and rounded or pressed and granulated to be coated with moisture-proof coatings. The citicoline sodium capsule is good in moisture-proof function and resolves the problems that the capsule is prone to absorb waterdue to the fact that citicoline sodium is strong in moisture absorption, the capsule casing is prone to be fragile, and the like.
Owner:QILU PHARMA CO LTD
Pentoxyverine voltoide and preparation method thereof
InactiveCN101536991AAccurate dosingRapid drug dissolutionOrganic active ingredientsInorganic active ingredientsPharmaceutical preservativesAmmonium chloride mixture
The invention discloses a pentoxyverine voltoide and a preparation method thereof. The tablet uses citric acid pentoxyverine dipping pill as a tablet core, and adopts a dry pressure coating technology to press ammonium chloride, the citric acid pentoxyverine dipping pill and an excipient to a coated tablet. The weight ratio of the ammonium chloride to the citric acid pentoxyverine in the tablet is 12:1, and the balance is the excipient. The invention solves the technical problem of sticky punch in tabletting and leads the whole tabletting process to be easier to realize, and the tabletted tablet has reliable quality and is beautiful. The pentoxyverine voltoide can be clinically used for treating coughing and sputum including acute and chronic bronchitis and pneumonia, and the like caused by various reasons.
Owner:XINAN PHARMA
Allicin multilayer enteric-coated tablet and preparation method thereof
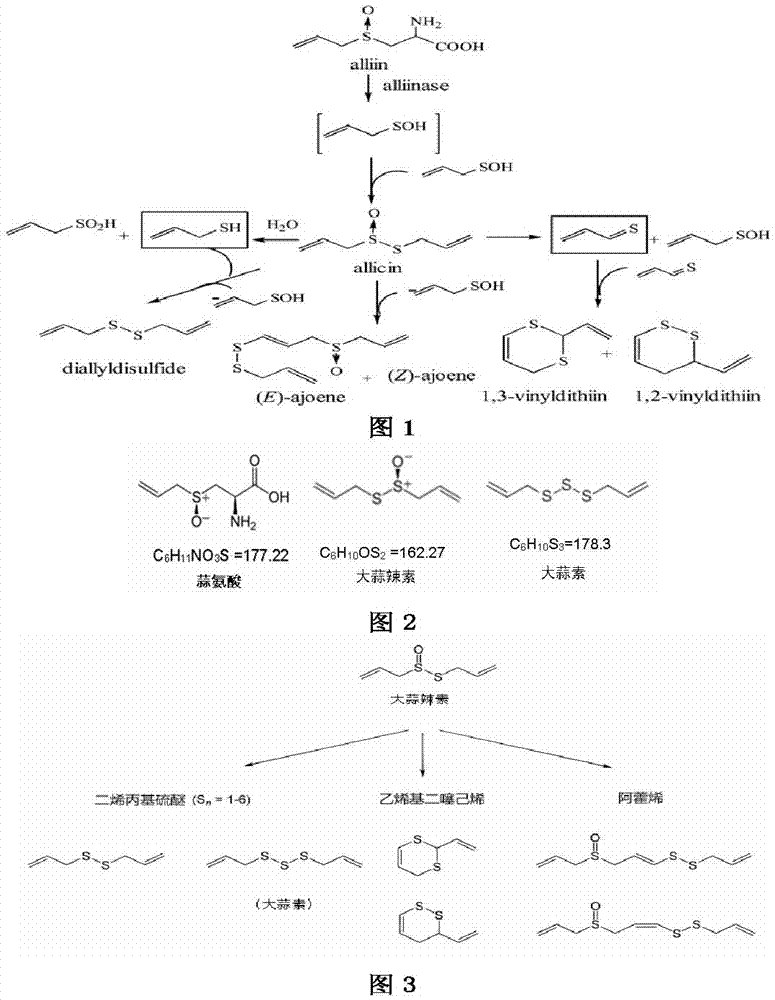
ActiveCN104523637BStable traitsHigh hardnessAntibacterial agentsOrganic active ingredientsFiller ExcipientHardness
The invention relates to the technical field of medicine and pharmacy, and relates to an allicin multilayer enteric-coated tablet and a preparation method thereof. The allicin multilayer enteric-coated tablet is obtained according to the following preparation method: the first step, preparation of an alliin layer : Weigh 55.8 to 68.2 parts of filler I, 3 to 5 parts of binder I, 1.8 to 3.3 parts of internal disintegrant I, and 1.8 to 3.3 parts of external disintegrant I in parts by weight. 3.3 parts, 1.8 to 2.2 parts of glidant I, 0.9 to 1.1 parts of lubricant I and 112.5 to 137.5 parts of alliin were crushed separately. Beneficial effects of the present invention: the allicin multi-layer enteric-coated tablet obtained according to the method of the present invention is stable in properties, and its hardness, disintegration time limit and dissolution rate are all better than the existing alliin / alliinase binary release multi-layer tablet , the difference in tablet weight is not large and stable, and it is suitable for industrial production, and is a method with high economic benefits.
Owner:新疆苏克天牧农业科技有限公司
Pure-plant starch capsule and preparation method thereof
ActiveCN108904464AImproved disintegration timeGuaranteed safe and reliable usePharmaceutical non-active ingredientsCapsule deliverySucrosePlasticizer
The invention discloses a pure-plant starch capsule. The pure-plant starch capsule comprises the following raw materials by weight: 32 to 47 parts of soluble starch, 12-23 parts of honeysuckle flowerextract, 4-12 parts of Herba Lopatheri, 5-11 parts of sucrose, 0.7-1.6 parts of a gelata and 0.7-1.6 parts of a plasticizer. The pure-plant starch capsule of the invention is prepared according to a pure plant recipe and is safe and reliable to use; and the pure-plant starch capsule has high mechanical strength under the synergistic action of the synergistic components consisting of the honeysuckle flower extract and Herba Lopatheri; and a reasonable preparation method is used so as to allow the starch capsule to be excellent in disintegration time.
Owner:上海国创医药股份有限公司
Heart boosting pulse restoring tablet and its preparation method
InactiveCN1824159AThe composition of the prescription is simpleLess excipientsPill deliveryCardiovascular disorderSolventPalpitations
A Chinese medicine in the form of tablet for nourishing qi and Yin, promoting blood circulation and pulse, heart blood stagnation, cardialgia, palpitation, etc is prepared from 6 Chinese-medicinal materials including ginseng, astragalus root, red sage root, Chuan-xiong rhizome, etc, lubricant and diluent (microcrystalline cellulose) through extracting in water, removing solvent to obtain powder and other conventional steps.
Owner:BEIJING SHENKELIANHUA TECH
Nitroglycerin micro-tablet as well as preparation method and preparation thereof
ActiveCN114404374ADisintegrates quicklyDisintegrates and dissolves rapidlyPill deliveryPharmaceutical non-active ingredientsNitroglycerolBioavailability
The invention belongs to the technical field of medicines, and particularly relates to a nitroglycerin micro-tablet as well as a preparation method and a preparation thereof. The diameter of the micro-tablet is 1-4 mm, the weight of the micro-tablet is 2-50 mg, and each micro-tablet contains 0.1-2.5 mg of nitroglycerin. The invention also provides a preparation method of the micro-tablet. The compound used by the micro-tablet can obviously improve the stability of the preparation and accelerate the disintegration time limit; and the micro-tablet is rapidly disintegrated and dissolved and is absorbed by sublingual mucosa at a higher speed, so that the first-pass effect of the liver on the medicine can be avoided, and the onset time of first aid is accelerated. And the problem that the bioavailability is reduced due to the fact that the conventional sublingual tablet is directly swallowed after being taken due to overlong disintegration and dissolution time can also be avoided.
Owner:BEIJING VAGARY PHARM TECH LTD
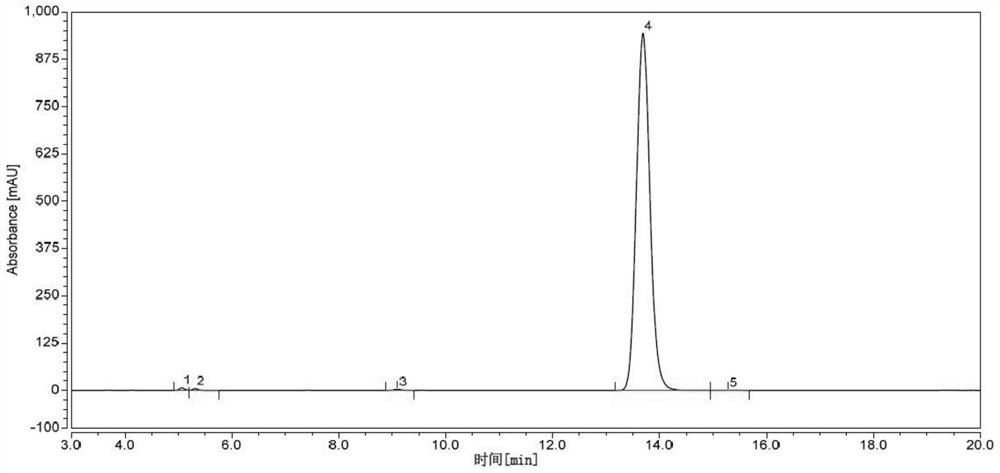
Cefpodoxime axetil dispersible tablet and preparation method thereof
ActiveCN103479589BShort disintegration timeModerate hardnessAntibacterial agentsOrganic active ingredientsMedicinePolyethylene glycol
The invention discloses a cefpodoxime proxetil dispersible tablet which comprises the following raw materials in percentage by weight: 20-45 percent of cefpodoxime proxetil, 30-50 percent of filler, 5-15 percent of a disintegrating agent, 0.25-1.0 percent of a wetting agent, 0.25-1.5 percent of a flavoring agent and 5.0-15 percent of polyethylene glycol 200. The preparation method adopts a specific wetting agent, so as to improve mobility, dissolubility and uniformity in mixing of the product and facilitate the storage of the product, and after the product is stored for a period of time, no obvious changes occur. According to the invention, the cefpodoxime proxetil dispersible tablet is obtained through direct powder mixing, granulation and tabletting, and the preparation method is simple without any special equipment, and is suitable for industrial production.
Owner:HAINAN HULUWA PHARMA GRP CO LTD
Tinidazole tablet and preparation method thereof
PendingCN113230226AHigh yieldReduce energy consumption and production costsAntibacterial agentsOrganic active ingredientsTinidazoleChemistry
The invention discloses a tinidazole tablet and a preparation method thereof, and belongs to the technical field of pharmaceutical preparations. The tinidazole tablet disclosed by the invention is prepared by controlling the dosage and the particle size of the raw materials and the auxiliary materials and adopting a dry granulation process. The tinidazole tablet prepared by the preparation method can effectively improve the dissolution rate, disintegration time limit, hardness and friability of the medicine tablet, effectively overcomes the problem that the nitrite content is increased due to high temperature and high humidity in the existing wet granulation process, and is good in production compliance and high in production efficiency.
Owner:LIVZON GROUP LIVZON PHARMA FACTORY
Kanggongyan tablet and its preparation process
ActiveCN109432036BReduce moisture contentNot easy to absorb moistureInorganic non-active ingredientsCoatingsMotherwortCallicarpa kwangtungensis
The invention relates to an anti-cervicitis tablet and a preparation technology thereof. The preparation technology comprises the following steps: providing raw materials: 300 to 310 parts by weight of callicarpae caulis et folium dry extract, 80 to 85 parts by weight of herba leonuri dry extract, 70 to 75 parts by weight of radix linderae dry extract, 60 to 65 parts by weight of a filler, 2 to 3parts by weight of magnesium stearate and proper amount of a wetting agent; crushing, uniformly mixing and sieving the callicarpae caulis et folium dry extract, the radix linderae dry extract, the herba leonuri dry extract and the filler to obtain a first mixture; mixing the first mixture with the wetting agent, carrying out spraying granulation and drying to obtain dry particles; granulating thedry particles, adding the magnesium stearate while granulating and tabletting to obtain the anti-cervicitis tablet. The preparation technology can obviously improve the humidity resistance of a product.
Owner:YAOSHENGTANG HUNAN PHARMA
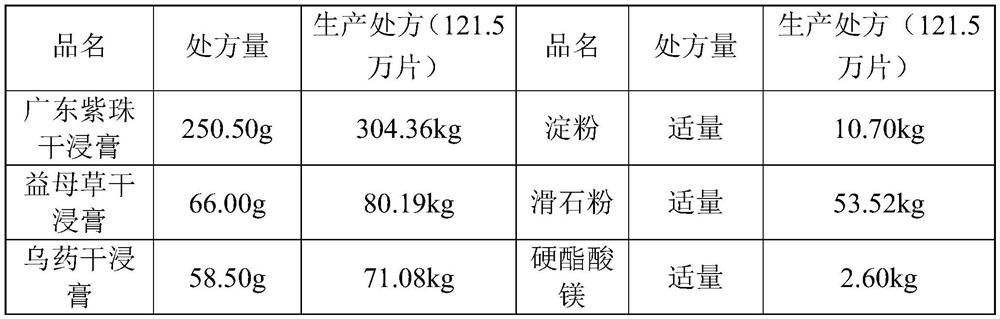
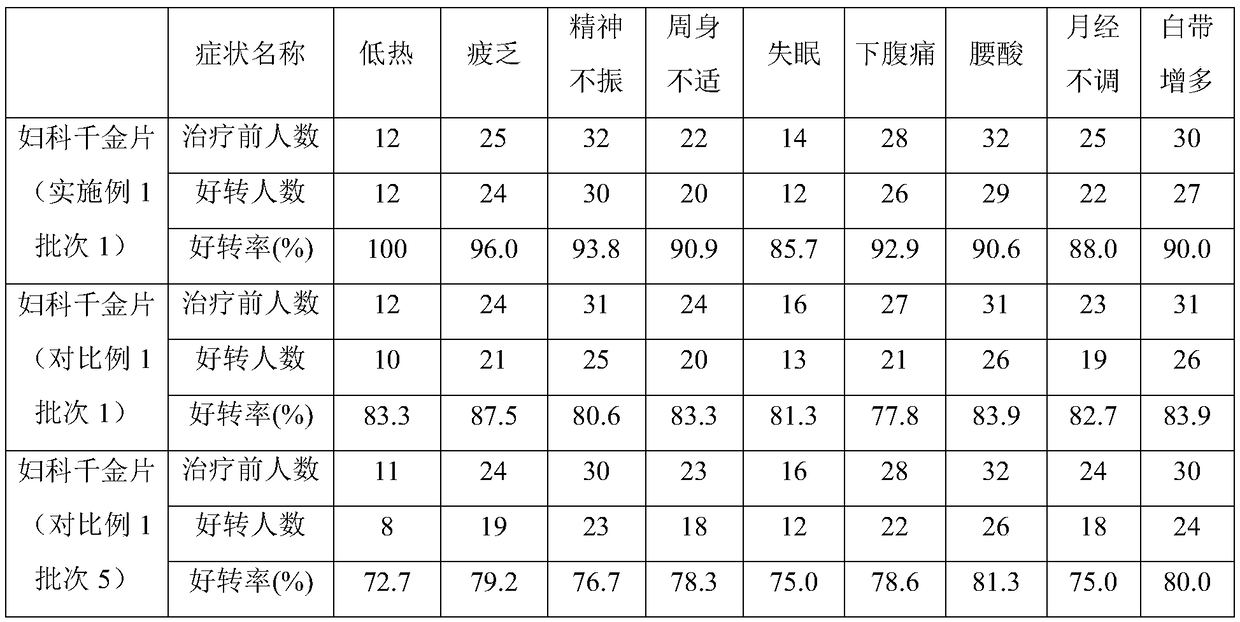
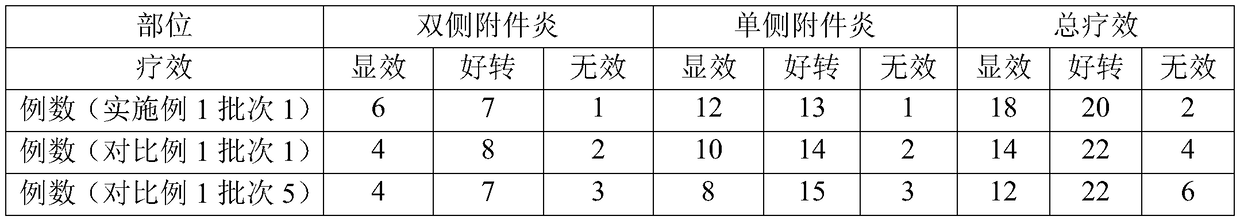
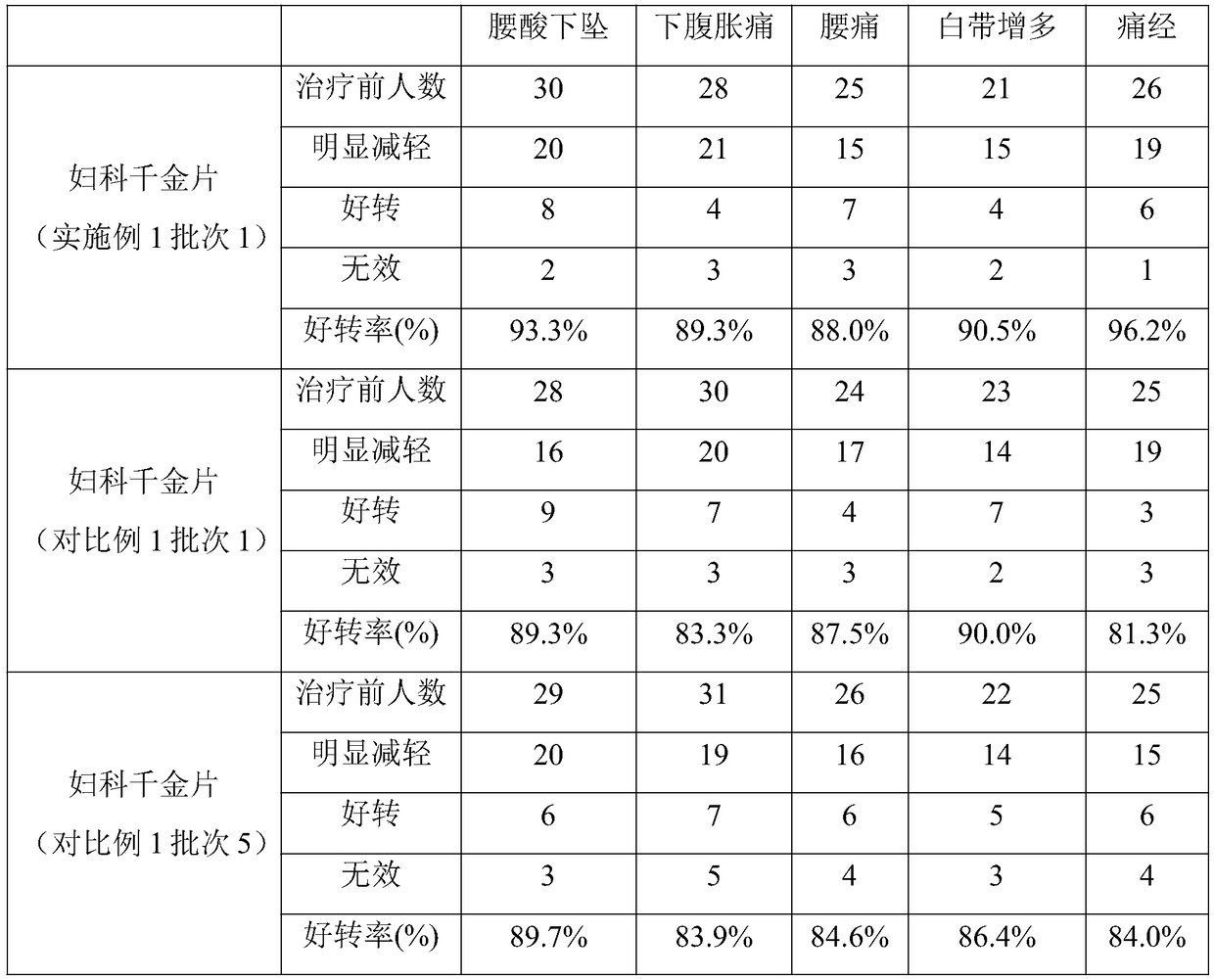
Method for improving disintegration time limit of fuke qianjin tablet
InactiveCN109106786AQuick releaseQuick effectComponent separationPill deliveryCodonopsisClinical efficacy
The invention discloses a method for improving disintegration time limit of fuke qianjin tablet. The fuke qianjin tablet is prepared from the following raw materials: philippine flemingia root, Chinese mahonia stem, herba andrographitis, folium zanthoxyli dissiti, caulis spatholobi, radix angelicae sinensis, radix codonopsis pilosulae and Cherokee rose root. During the preparation process of the fuke qianjin tablet, the content of Z-3-butylidene phthalide and the total volume of andrographolide and dehydrated andrographolide are regulated; in each fuke qianjin tablet, the content of Z-3-butylidene phthalide is not less than 0.015mg and the total volume of andrographolide and dehydrated andrographolide is not less than 1.1mg; HPLC method is optimally adopted for detecting the content. According to the invention, the important influences of the content of Z-3-butylidene phthalide and the total volume of andrographolide and dehydrated andrographolide in the fuke qianjin tablet on the product performances are confirmed and the accurate limitation is performed. Compared with the present fuke qianjin tablet, the fuke qianjin tablet prepared according to the invention has the advantages that the disintegration time limit is improved, the active ingredients are quickly released and can quickly take effect, and the clinical efficacy is further promoted.
Owner:ZHUZHOU QIANJIN PHARMA
A pH-dependent colon-localized hard capsule
ActiveCN103520226BSimple preparation processNot easy to dehydration agingDigestive systemPharmaceutical delivery mechanismAcrylic resinHard Capsule
The invention belongs to the field of pharmaceutical preparations, and relates to a medicinal preparation for the digestive tract, in particular to a pH-dependent colon-localized capsule. A pH-dependent colon-localized hard capsule, comprising a hard capsule injected with pharmaceutical ingredients and a coating film thereof, the coating liquid used to form the coating film and its dosage are 1 to N times that of the following prescription: coating Material 2g, the coating material is, acrylic resin No. Ⅲ, Eudragit RL100 and / or Eudragit RS 100; the Eudragit RL100 and / or Eudragit RS 100 account for 18-25% of the total weight of the coating material; 2~3ml of agent, 92~98ml of solvent for coating material. The outermost layer of the hard capsule of the invention is covered with a polymer coating film, which ensures that the injected medicine does not leak and can meet the requirement of colon-specific drug release.
Owner:惠州市九惠药业有限公司
Atorvastatin calcium tablet and preparation process thereof
PendingCN114392242AIncrease coating rateLittle difference in tablet weightMetabolism disorderInorganic non-active ingredientsCelluloseHigh humidity
The invention provides an atorvastatin calcium tablet and a preparation process thereof, the atorvastatin calcium tablet is composed of a core material and a coating, the core material is prepared from the following raw materials by weight: 10 parts of atorvastatin calcium, 15-20 parts of a filler, 4-6 parts of an adhesive, 8-12 parts of a disintegrating agent, and 3-5 parts of a lubricant; the coating film is prepared from hydroxypropyl methyl cellulose, nano titanium dioxide and polyvinylpyrrolidone according to the mass ratio of (2 to 3): (16 to 18): 1. The atorvastatin calcium tablet is composed of the core material and the coating film, the coating film is prepared from hydroxypropyl methyl cellulose, nano titanium dioxide and polyvinylpyrrolidone according to a certain proportion, the coating uniformity is good, the coating effect is stable, and the prepared atorvastatin calcium tablet is high in coating rate and small in tablet weight difference. The atorvastatin calcium tablet prepared by the invention is good in disintegration time limit, high in stability and good in strong light resistance, high temperature resistance and high humidity resistance.
Owner:HAINAN HAILING CHEMIPHARMA CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com