Compound oral disintegrating tablet containing acetaminophen and dextromethorphan
A technology of acetaminophen and orally disintegrating tablets, which is applied to medical preparations containing active ingredients, medical preparations with non-active ingredients, and pharmaceutical formulas to achieve long-lasting, definite and powerful effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
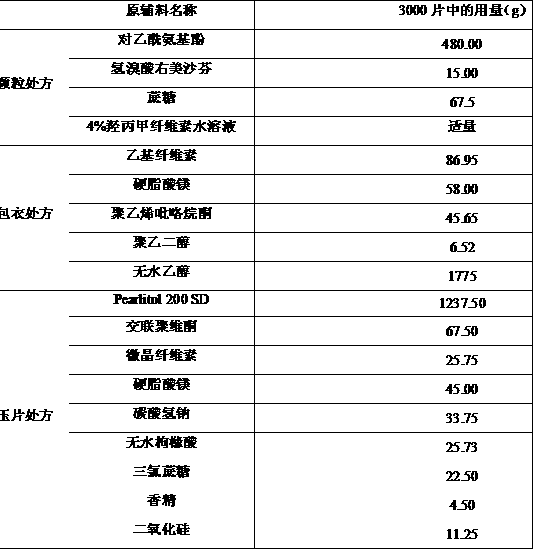
[0033] Example 1: Preparation of coated granules with ethyl cellulose as coating material and tableting
[0034] prescription:
[0035]
[0036] Preparation Process:
[0037] Preparation of drug-containing granules:
[0038] 1. Preparation of adhesive: Weigh an appropriate amount of hypromellose and add it to purified water, stir to dissolve, and prepare a 4% (w / v) hypromellose aqueous adhesive.
[0039] 2. Crush acetaminophen, dextromethorphan hydrobromide, and sucrose separately, pass through an 80-mesh sieve, and set aside.
[0040] 3. Weigh the prescribed amount of acetaminophen, dextromethorphan hydrobromide fine powder, and sucrose respectively, mix well, and set aside.
[0041] 4. Granulation: Add the mixture of raw and auxiliary materials under item 3 into the centrifugal pelletizer, and set the equipment parameters as follows: fan frequency 10Hz, turntable speed 250rpm, spray speed 10rpm, spray gun pressure 0.02MPa, during the granulation process according to A...
Embodiment 2
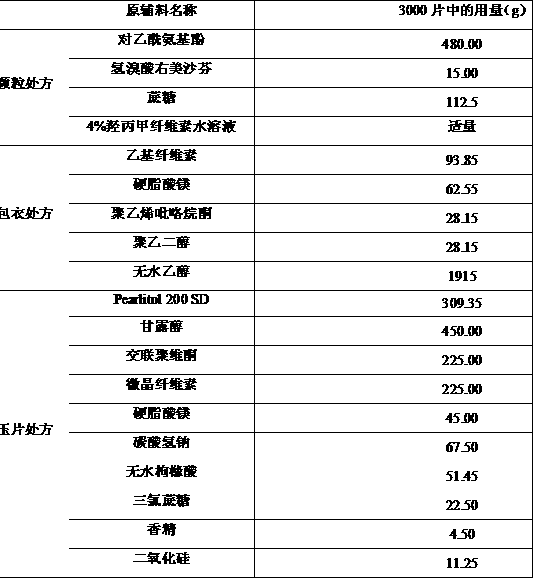
[0056] Example 2: Preparation of coated granules with ethyl cellulose as coating material and tableting
[0057] prescription:
[0058]
[0059] The preparation process of drug-containing granules, coated granules and compressed tablets is the same as in Example 1. The average tablet weight of the prepared orally disintegrating tablets is 750 mg, the hardness is 3-4 kg, and the disintegration time limit is less than 60 s.
[0060]
Embodiment 3
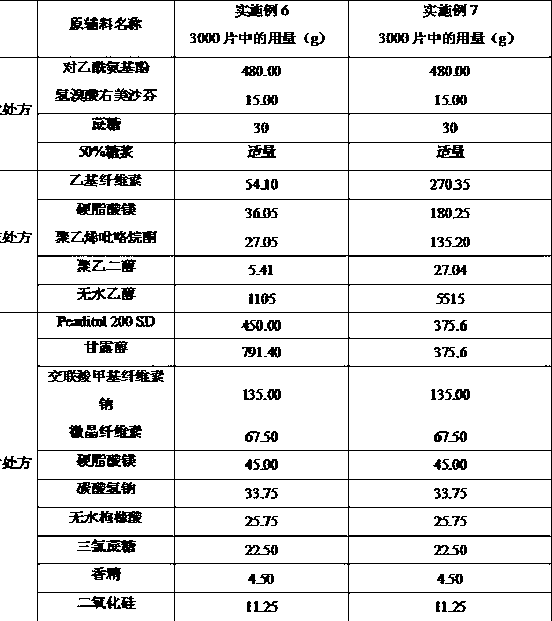
[0061] Example 3: Preparation of coated granules with ethyl cellulose as coating material and tableting
[0062] prescription:
[0063]
[0064] The preparation process of drug-containing granules, coated granules and compressed tablets is the same as in Example 1. The average tablet weight of the prepared orally disintegrating tablets is 750 mg, the hardness is 3-4 kg, and the disintegration time limit is less than 60 s.
[0065]
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com