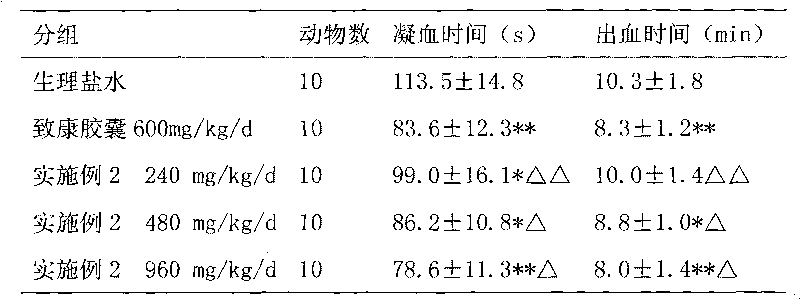
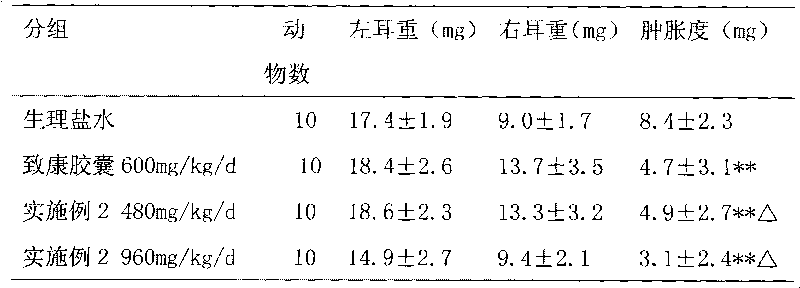
Medicament for treating metrorrhagia and metrostaxis, hematemesis, hematochezia and traumatic hemorrhage and preparation method thereof
A technology for the treatment of metrorrhagia and trauma, applied in the directions of drug combinations, pharmaceutical formulations, and medical preparations containing active ingredients, etc., can solve the problems of unstable quality, delayed disintegration, short validity period, etc., and achieve the effect of reducing the dosage.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The raw material weight component of present embodiment comprises following material:
[0033] 70 parts of rhubarb, 40 parts of coptis, 40 parts of Panax notoginseng, 20 parts of Angelica dahurica, 45 parts of donkey-hide gelatin beads, 35 parts of calcined keel, 35 parts of baiji, 20 parts of myrrh, 35 parts of sea octopus, 40 parts of madder, dragon's blood 20 parts dried fruit, 3 parts pearls, 2 parts borneol, 10 parts licorice, 10 parts microcrystalline cellulose, 1.5 parts croscarmellose sodium, 10 parts hydroxypropyl-β-cyclodextrin, β-cyclodextrin 10 parts of essence, 1.5 parts of micronized silica gel, 5 parts of film coating powder
[0034] The raw material weight component of present embodiment is made up of following:
[0035] 70 parts of rhubarb, 40 parts of coptis, 40 parts of Panax notoginseng, 20 parts of Angelica dahurica, 45 parts of donkey-hide gelatin beads, 35 parts of calcined keel, 35 parts of baiji, 20 parts of myrrh, 35 parts of sea octopus, 40 p...
Embodiment 2
[0050] The raw material weight component of present embodiment comprises following material:
[0051] 80 parts of rhubarb, 45 parts of coptis, 45 parts of Panax notoginseng, 25 parts of Angelica dahurica, 50 parts of donkey-hide gelatin beads, 40 parts of calcined keel, 40 parts of baiji, 30 parts of myrrh, 40 parts of sea octopus, 45 parts of madder, dragon's blood 30 parts of dried fruit, 6 parts of pearl, 3 parts of borneol, 12 parts of licorice, 16 parts of microcrystalline cellulose, 2 parts of croscarmellose sodium, 15 parts of hydroxypropyl-β-cyclodextrin, β-cyclodextrin 14 parts of essence, 2 parts of micronized silica gel, 6 parts of film coating powder.
[0052] The raw material weight component of present embodiment is made up of following:
[0053] 80 parts of rhubarb, 45 parts of coptis, 45 parts of Panax notoginseng, 25 parts of Angelica dahurica, 50 parts of donkey-hide gelatin beads, 40 parts of calcined keel, 40 parts of baiji, 30 parts of myrrh, 40 parts of ...
Embodiment 3
[0068] The raw material weight component of present embodiment comprises following material:
[0069] 90 parts of rhubarb, 50 parts of coptis, 50 parts of Panax notoginseng, 35 parts of Angelica dahurica, 55 parts of donkey-hide gelatin beads, 50 parts of calcined keel, 50 parts of baiji, 35 parts of myrrh, 50 parts of sea octopus, 50 parts of madder, dragon's blood 35 parts of dried fruit, 8 parts of pearl, 4 parts of borneol, 16 parts of licorice, 18 parts of microcrystalline cellulose, 2.5 parts of croscarmellose sodium, 20 parts of hydroxypropyl-β-cyclodextrin, β-cyclodextrin 18 parts of essence, 2.5 parts of micronized silica gel, and 8 parts of film coating powder.
[0070] The raw material weight component of present embodiment is made up of following:
[0071] 90 parts of rhubarb, 50 parts of coptis, 50 parts of Panax notoginseng, 35 parts of Angelica dahurica, 55 parts of donkey-hide gelatin beads, 50 parts of calcined keel, 50 parts of baiji, 35 parts of myrrh, 50 p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
| friability | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com