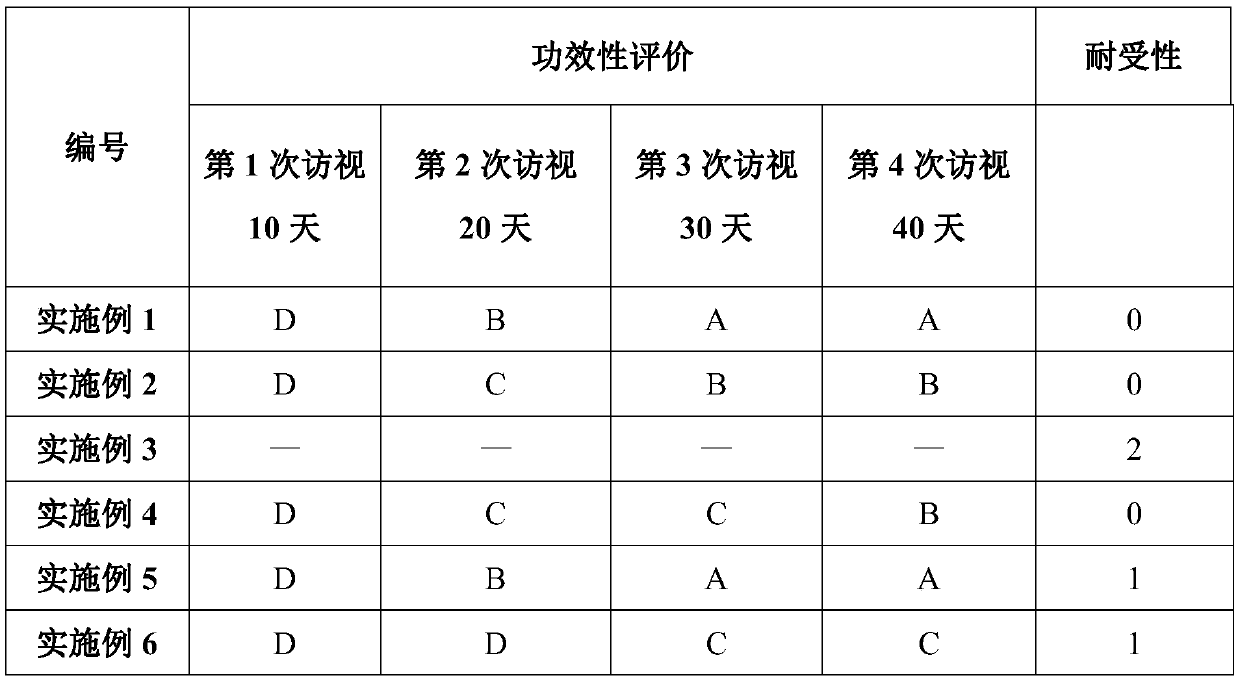
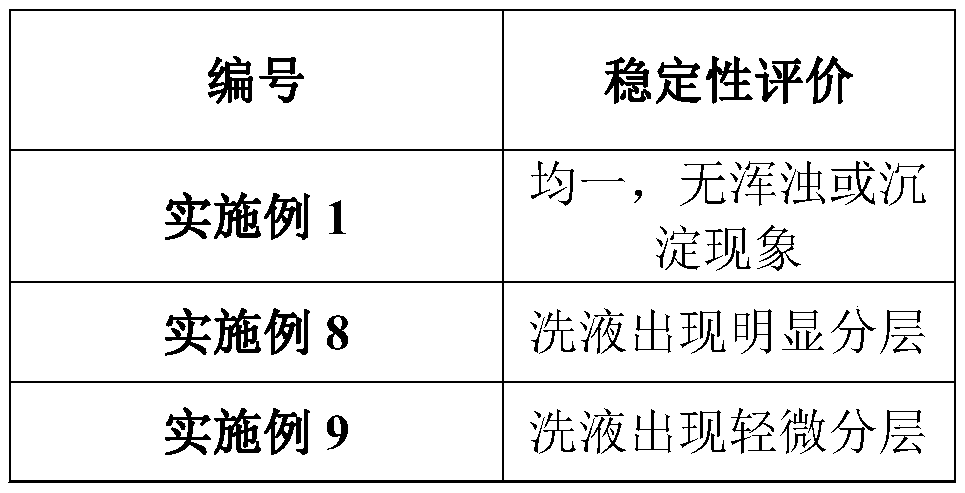
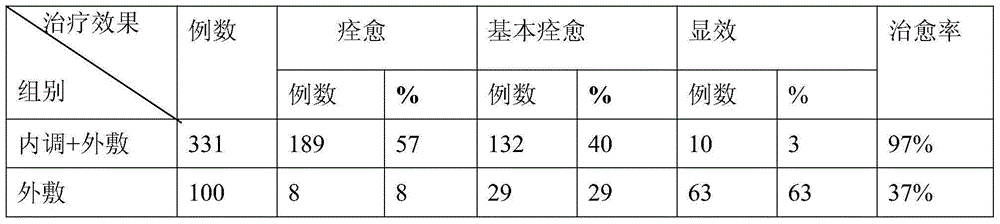
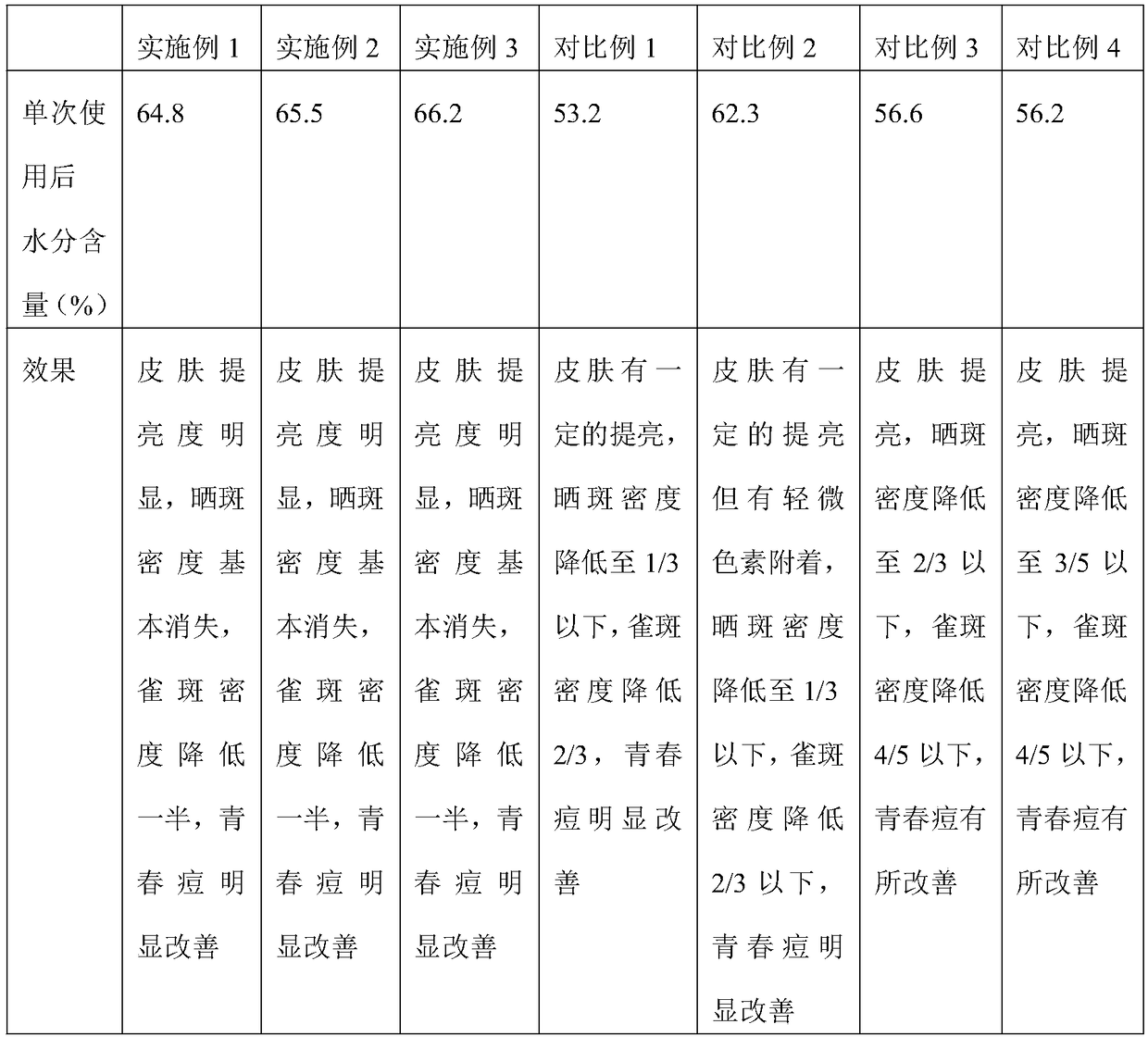
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
51 results about "Vitamin a acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
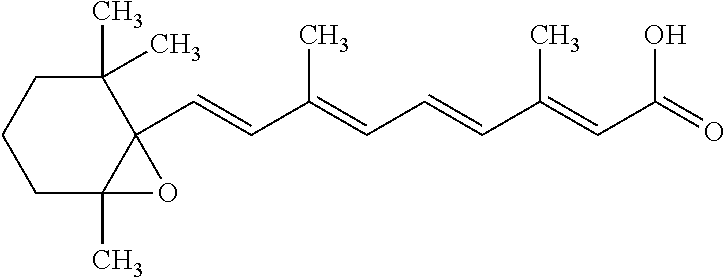
Vitamin A acid (retinoic acid: tretinoin) is a vitamin A derivative used in the topical treatment of acne. It acts by 'unseating' comedones, improvement developing slowly over a period of 2 to 3 or more months, and is also said to prevent the formation of new lesions.
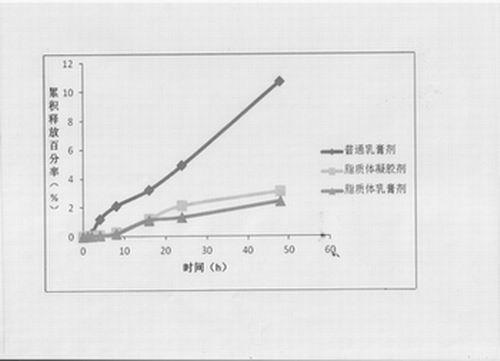
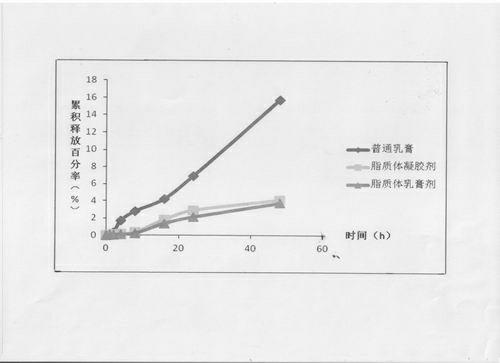
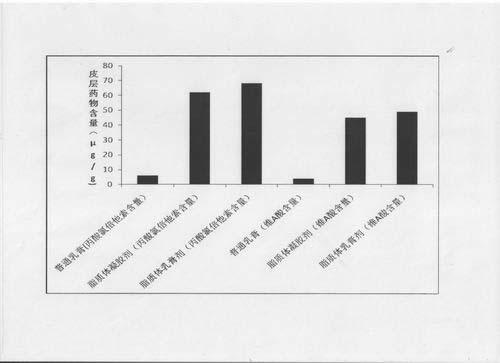
Compound clobetasol propionate liposome and preparation thereof
ActiveCN102429913AEasy to storeIncrease resistanceHydroxy compound active ingredientsEmulsion deliveryLipid formationDisease
The invention provides a compound clobetasol propionate liposome and a preparation thereof, which are mainly used for treating diseases such as psoriasis vulgaris, dermatitis, eczema and the like. The liposome prepared from neutral synthetic phospholipids, lipids with positive charges, and cholesterol can coat clobetasol propionate and vitamin A acid simultaneously, and is added with a cream substrate or gel substrate to be prepared into compound clobetasol propionate liposome cream or gel. Compared with the common cream, the liposome preparation has the advantages that: the amount of the medicine retained in skin is larger, the skin penetration rate is lower, the content of the medicine in local skin can be improved, the treatment index is improved, and transdermal absorption dose is reduced, so that the toxic and side effects of the medicine are reduced.
Owner:JIANGSU SEMPOLL PHARMA
Compound preparation fortreating psoriasis vulgaris and its preparation process
InactiveCN1234365CDoes not affect biological activityReduce irritation responseDermatological disorderAnhydride/acid/halide active ingredientsClinical efficacyTretinoin
A medicine for treating psoriasis vulgaris contains clobetasol propionate and vitamin A acid. Its preparing process is also disclosed. Its advantages are high curativ effect, and low toxic by-effect.
Owner:JIANGSU SEMPOLL PHARMA
Compound preparation fortreating psoriasis vulgaris and its preparation process
InactiveCN1517092ADoes not affect activityDoes not affect anti-inflammatory effectOrganic active ingredientsDermatological disorderTretinoinCurative effect
A medicine for treating psoriasis vulgaris contains clobetasol propionate and vitamin A acid. Its preparing process is also disclosed. Its advantages are high curativ effect, and low toxic by-effect.
Owner:JIANGSU SEMPOLL PHARMA
Self micro emulsifying composition of tretinoin medicines and its preparing method
InactiveCN1943567APromote dissolutionPromote absorptionCapsule deliveryEmulsion deliveryMedicineOil phase
A compound of vitamin A acid medicine and self micro emulsifying and its preparation method belongs to technical field of medicine. In said invention, components of compound and percent weight are : medicine 0.5%-3%, emulsifying agent 30%-70%, emulsifying agent assistant 5%-40 %, oil phase 10%-50% and stabilizer 0%-2%. Preparation method of said compound is : dispersing medicine and stabilizer into one or random multi-components described hereinabove, heating slightly and making components soluble evenly and completely to obtain self micro emulsifying compound , said compound can be contained into soft capsule with opacifier.
Owner:EAST CHINA UNIV OF SCI & TECH
Skin protection cream capable of eliminating striae gravidarum
InactiveCN106344435AFunction and effect are effectiveSmall molecular weightCosmetic preparationsToilet preparationsHydroxyprolineCuticle
The invention provides skin protection cream capable of eliminating striae gravidarum. The skin protection cream is characterized by comprising, by weight, 10-15 parts of epidermal cell growth factors, 10-15 parts of kaempferol, 5-10 parts of methylsilanol hydroxyproline aspartate, 5-10 parts of vitamin A acid and 10-15 parts of collagen. The skin protection cream has the advantages that the striae gravidarum can be lightened and eliminated by the skin protection cream, and the treatment time can be shortened.
Owner:吉安市御美丽健康产业股份有限公司
Preparation method of composite drug-eluting stent and its drug coated layer
The invention discloses a making method of drug coating on the elution rack, which comprises the following steps: dissolving vitamin A acid drug and polymer in the organic solvent to obtain the coating liquid of vitamin A acid; coating the liquid on the predisposed metal rack surface; evaporating solvent; obtaining the buried vitamin A acid coating; dissolving estrogen drug and polymer in the organic solvent to obtain estrogen coating liquid; coating on the surface of estrogen coating liquid; evaporating; obtaining the product; or dissolving vitamin A acid, estrogen drug and polymer in the organic solvent to obtain the composition of drug and polymer; coating on the predisposed metal rack surface; evaporating; burying in the drug coating of vitamin A acid and estrogen.
Owner:AFFILIATED HUSN HOSPITAL OF FUDAN UNIV +1
Synthetic method of all-trans vitamin A acid medicament
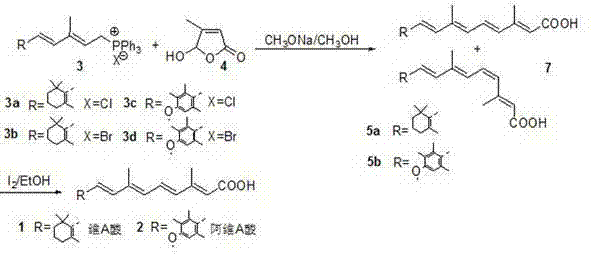
The invention discloses a synthetic method of an all-trans vitamin A acid medicament. The method comprises the following steps of: (1) preparing an intermediate: adding a solvent and 2-5 equivalent weight of alkali into 5-hydroxy-4-methyl-2-furanone, reacting at the temperature of 35-40 DEG C for 1-3 hours, undergoing a ring opening reaction, cooling to between 5 DEG C below zero and 0 DEG C below zero, dropwise adding 0.75-1.0 equivalent weight of a C15-triphenyl phosphine salt solution slowly for reacting for 3-4 hours, heating to the room temperature for reacting for 6-10 hours, adding 40L of ice water, extracting with a mixed solvent consisting of ethyl acetate and petroleum ether in the ratio of 1:4, combining organic phases, washing with water for 2-3 times, and drying with anhydrous sodium sulfate to obtain a mixture of a 11cis,13contra-vitamin A acid and a 11contra,13contra-vitamin A acid compound; and (2) isomerizing: dissolving a 11cis,13contra and 11contra,13contra-vitamin A acid mixture solid obtained in the step (1) with a solvent, adding 0.1-20 percent by mole of iodine, and stirring and reacting at the temperature of 25-35 DEG C for 25-48 hours to obtain the all-trans vitamin A acid medicament. The synthetic method has the advantages of easiness for operating, high yield, low cost, high product content and capability of avoiding pollution of heavy metals.
Owner:HUNAN NORMAL UNIVERSITY
Compound vitamin A acid gel preparation for treating acne and its preparing method
InactiveCN1850101AObvious superiorityEasy to usePharmaceutical delivery mechanismDermatological disorderGel preparationAlcohol
The present invention discloses a compound gel preparation-compound tretinoin gel preparation for curing acnes. It is made up by using carbomer, glycerine, propylene alcohol, Tween, triethanolamine, clindamycin phosphate, tretinoin, EDTA and distilled water as raw material through a certain preparation process. Said invention also provides the concrete steps of its preparation method.
Owner:李海涛
Pharmaceutical composition composed of hydroxamic acid compounds and vitamin A compounds and application thereof
ActiveCN104116728AReduce harmEnhanced inhibitory effectHydroxy compound active ingredientsUnknown materialsHuman cancerCancer cell
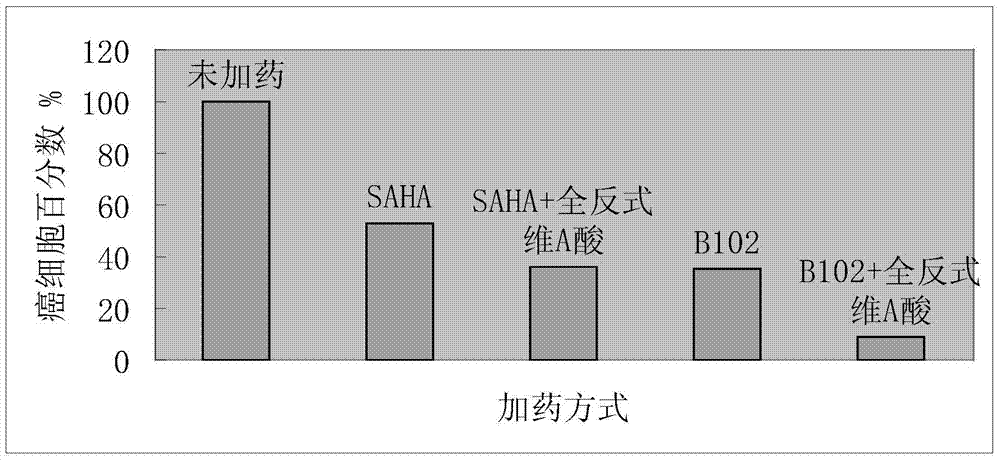
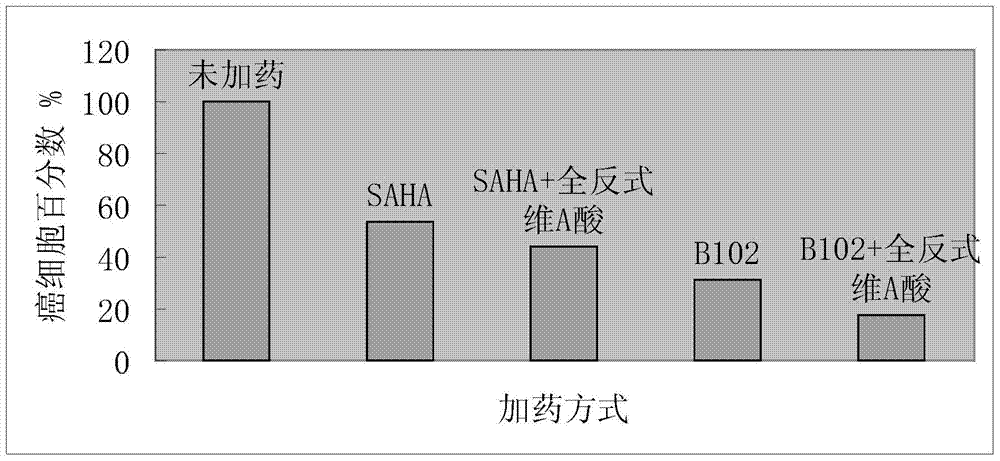
The invention relates to the field of medicinal chemistry and particularly relates to a pharmaceutical composition composed of hydroxamic acid compounds and vitamin A compounds and an application thereof. The pharmaceutical composition provided by the invention has the capacity of inhibiting the proliferation of cancer cells, so as to achieve the purpose of curing cancers. The pharmaceutical composition provided by the invention has an obvious assisted synergistic effect, the capacity of inhibiting the proliferation of cancer cells of the pharmaceutical composition is stronger than that of single use of the hydroxamic acid compounds, and the pharmaceutical composition particularly has excellent anti-proliferation activity on human cancer cells.
Owner:BEIJING UNIV OF CHEM TECH
Composition containing PVA-124 and its application
InactiveCN100379447CTo achieve the purpose of transdermal absorptionGood treatment effectPeptide/protein ingredientsDermatological disorderFiberPolyvinyl alcohol
The invention discloses a composition containing PVA-124 and its application which comprises the following components (by weight parts), polyvinyl alcohol PVA-124, fibroblast growth factor, 0.025% of vitamin A acid, auxiliary material and water.
Owner:上海第二医科大学附属第九人民医院
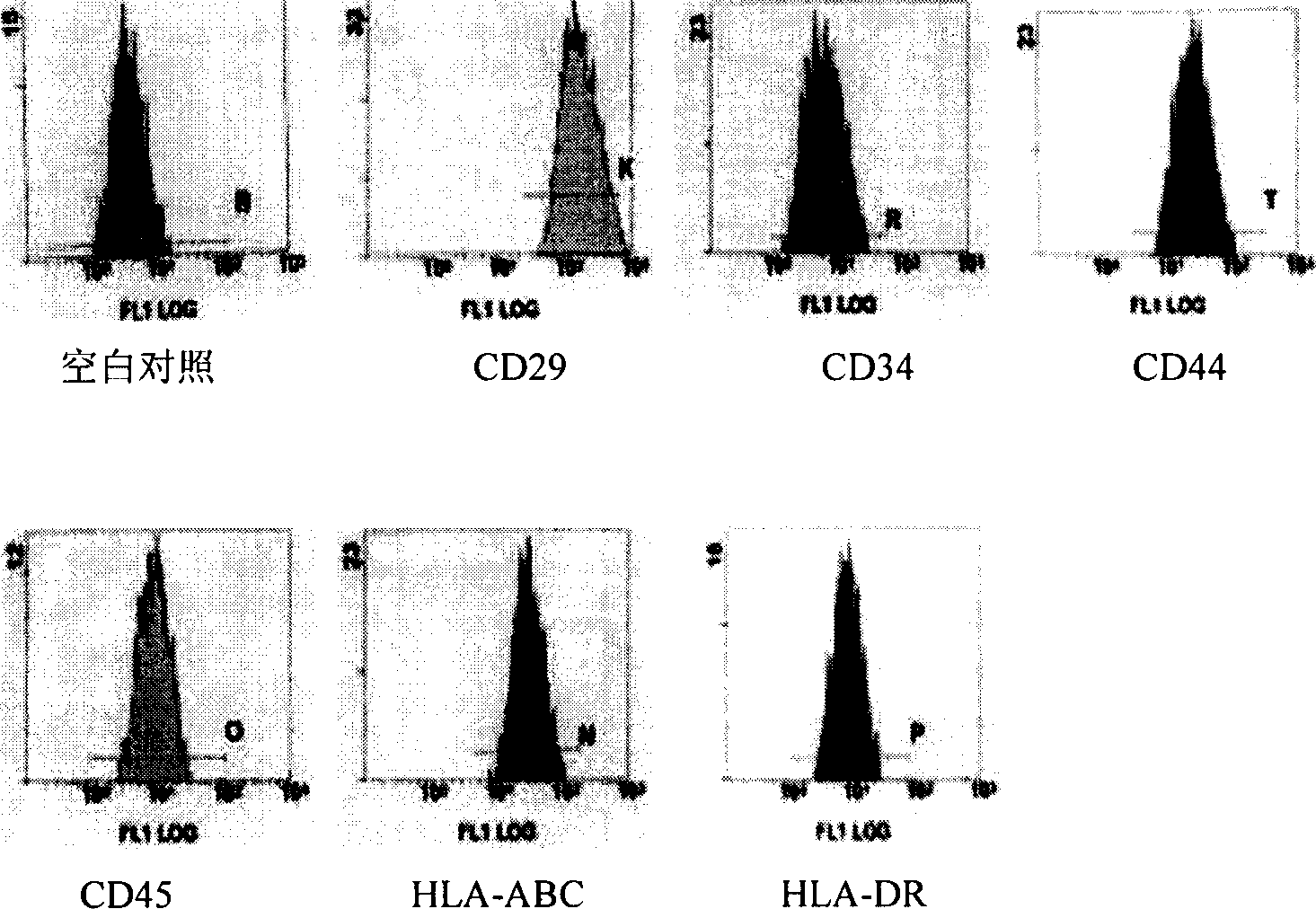
Method of inducing differentiation of human amnion mesenchyme stem cell to nerve cell
The present invention is method of inducing differentiation of human human amnion mesenchyme stem cell to nerve cell. The method is to culture human human amnion mesenchyme stem cell in DMEM / F12 culture medium containing all transcofiguration vitamin A acid, basic fibroblast growth factor and ox embryo blood serum. Through the induction of the method, human human amnion mesenchyme stem cell may be differentiated extracorporeally into neure cell. The nerve cell of the present invention and composition containing the nerve cell may be used widely in treating neurogenic diseases.
Owner:SHENZHEN BEIKE BIOTECH
Complex external medicine for treating acne
InactiveCN1210041CStrong receptor selectivityClearly targetedTetracycline active ingredientsAerosol deliveryTreatment acneTazarotene
The invention relates to a compound external medicine for treating acne. It is characterized in that: the matrix carrier of the compound external drug contains the following two effective active ingredients (weight percentage): 0.01-2% of tazarotene, and 0.25-5% of antibacterial drugs; the effective active ingredient contained in the compound external drug After mixing with a base carrier, it forms a cream or gel or a liniment or film. The advantage of the present invention is that: the present invention contains two main components of retinoic acid and antibacterial for treating acne in the form of a compound preparation, so as to simultaneously exert three (and above) curative effects (dissolving horn plugs, regulating keratinization, Antimicrobial, anti-inflammation, secondary factors to reduce sebum secretion, etc.), played a complementary and synergistic effect in pharmacology.
Owner:CHINA RESOURCES SANJIU MEDICAL & PHARMA
Application of total glycosides of eucommia ulmoides in preparing medicine for treating osteoporosis
InactiveCN101167783AIncrease bone densityDoes not affect weight gainSkeletal disorderPlant ingredientsVegetable oilHard Capsule
The invention relates to application of effective component eucommia total glycoside in the medicament preparation for curing osteoporosis. The medicament for curing osteoporosis which is prepared from effective component eucommia total glycoside can be used in the form of soft capsules and hard capsules, wherein soft capsules are proportioned by one part by weight of eucommia total glycoside and 0.5-8 parts by weight of sea-buckthorn seed oil or edible vegetable oil or polyethylene glycol 400 and are prepared by applying conventional process for preparing soft capsules. Hard capsules are prepared by filling eucommia total glycoside into capsules. Proved by pharmacological test, eucommia total glycoside can not affect weight increasing of animals and no obvious effect is found on organ index. Eucommia total glycoside can obviously increase bone mineral density, greatly strengthen femoral fracture stress and femoral crushing stress, and increase bone length reduce which is caused by vitamin A acid and bone weight reduce. Eucommia total glycoside can not obviously affect serum estrogen level and has good function on osteoporosis of rats which is caused by vitamin A acid resistance.
Owner:孙文基
Compound nicotinamide medicine for antiphlogosis, wet storage and eliminating spot
The compound nicotinamide medicine for antiphlogosis, wet storage and eliminating spot consists of nicotinamide 2-10 wt%, trans- or cis-vitamin A acid 0.025-0.05 wt%, and corticosteroid 0.025-1 wt%. It has functions of moistening skin, diminishing inflammation, inhibiting melanogenesis, and treating eczema, skin pruritus, acne, etc.
Owner:HOSPITAL OF DERMATOLOGY CHINESE ACAD OF MEDICAL SCI
Composition for treating knee-joint cross ligament damage
InactiveCN101147802AInhibition of excessive releaseSignificant effectPeptide/protein ingredientsTetracycline active ingredientsKnee JointLigament structure
The present invention relates to a medicine composition for effectively curing injury of anterior cruciate ligament of knee joint. Said medicine composition contains trans forming growth factor, matrix metal proteinase inhibitor and inflammation bloker, and the optimum components of said medicine composition are transforming growth factor + vitamin A acid + tetracycline + NF -k B inhibitor.
Owner:CHONGQING UNIV
Acne removing skin cleaning cream
InactiveCN102397221AEfficient removalImprove softening effectCosmetic preparationsToilet preparationsAlkylphenolLinear alkylbenzene
The invention provides acne removing skin cleaning cream, which consists of the following components in parts by weight: 8 parts of pearl powder, 25 parts of kaolin, 8 parts of bone meal, 15 parts of pulse flour, 5 parts of milk, 8 parts of Vaseline, 15 parts of starch, 8 parts of vitamin A acid, 10 parts of urea, 0.8 part of alkylphenol polyoxyethylene, 0.8 part of linear alkylbenzene sulphonic acid, 0.1 part of sodium benzoate and 150 parts of pure water. The cleaning cream is prepared by the following steps of: dissolving sodium benzoate into water; adding other raw materials except milk under uniform stirring; adding the milk; and fully mixing uniformly. The invention aims to provide acne removing skin cleaning cream. The cleaning cream contains fine particles which can be used for effectively removing aged horny layers and dirt by grinding on face; and meanwhile, the cleaning cream contains acne removing active ingredients, so that cleaning and acne removing functions are integrated.
Owner:吴桂标
High efficiency siRNA molecule to target gene Rig-G
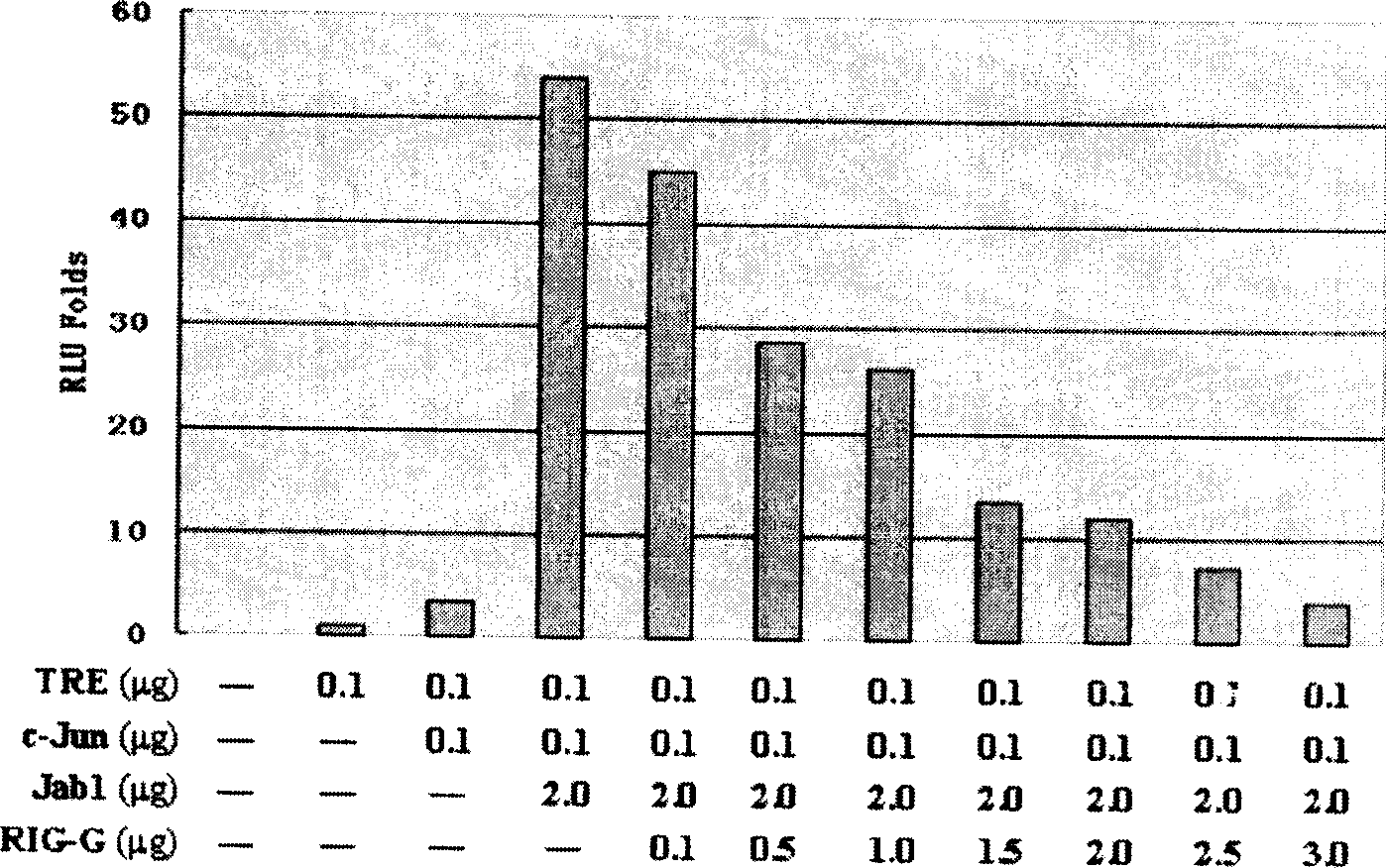
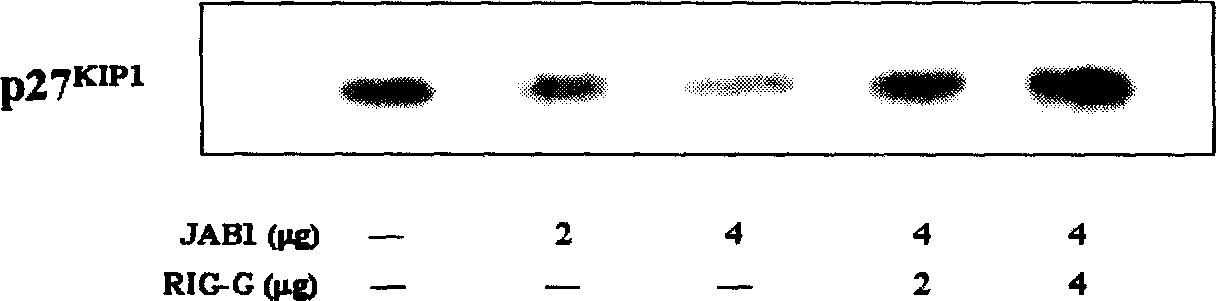
The present invention relates to one kind of small segment double stranded RNA molecule (siRNA) with high efficiency interference on the expression of target gene Rig-G. The high efficiency siRNA is prepared through the following steps: 1. selecting site in the cDNA sequence of target gene to synthesize DNA template; 2. extracorporeal transcription to synthesize RNA segment; or constructing siRNA expression plasmid. Using these siRNA can inhibit the expression of Rig-G gene effectively and lay foundation for establishing Rig-G deficiency type cell strain mold, and is favorable to understanding the effect of Rig-G in vitamin A acid signal conduction network and revealing the molecular mechanism for vitamin A acid to induce APL cell differentiation.
Owner:RUIJIN HOSPITAL ATTACHED TO SHANGHAI NO 2 MEDICALUNIV
Skin protection cream for eliminating stretch marks
InactiveCN107375030APreparation Technology ScienceSimple preparation processCosmetic preparationsToilet preparationsWrinkle skinVitamin C
The invention relates to the field of daily chemical products and beauty products, and particularly relates to a skin protection cream for eliminating stretch marks. The skin protection cream for eliminating stretch marks is prepared from clostridium botulinum wall-broken protein liquid, vitamin A acid, vitamin C, collagen and lavender essential oil. The skin protection cream for eliminating stretch marks has the following beneficial technical effects: (1) the small molecular protein, natural vitamins, collagen and plant essential oil are adopted as main components, the preparation technology is scientific, and the stretch marks can be effectively eliminated and reduced; (2) the collagen can directly permeate into the bottom layer of the skin and has high affinity with the tissues around so as to promote normal growth of the skin cells and improve the stretch marks; (3) the skin protection cream has the effects of moistening, whitening, wrinkle resisting and the like on the skin and has high safety; (4) the preparation technology of the stretch-marks cream is simple and suitable for industrial production.
Owner:苏州爱喜邦物联网科技有限公司
Retinoic acid release control nanomicrosphere and its preparation method
InactiveCN100462070CProlong the action timeImprove stabilityGranular deliveryDermatological disorderControlled releaseMicrosphere
The invention relates to a medicinal control release nanometer and method for making same, in particular a retinoic acid release control nanomicrosphere and its preparation method, the constituents of the composition are polylactic acid (PLA), vitamin A acid (RA) and gelatin aqueous solution.
Owner:于美丽 +1
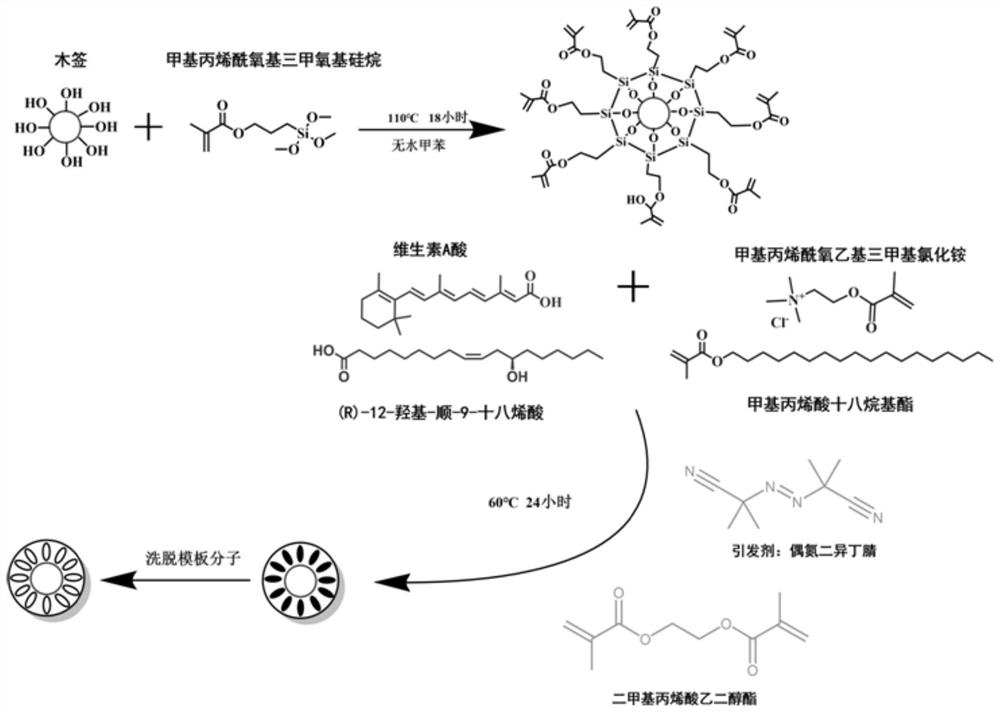
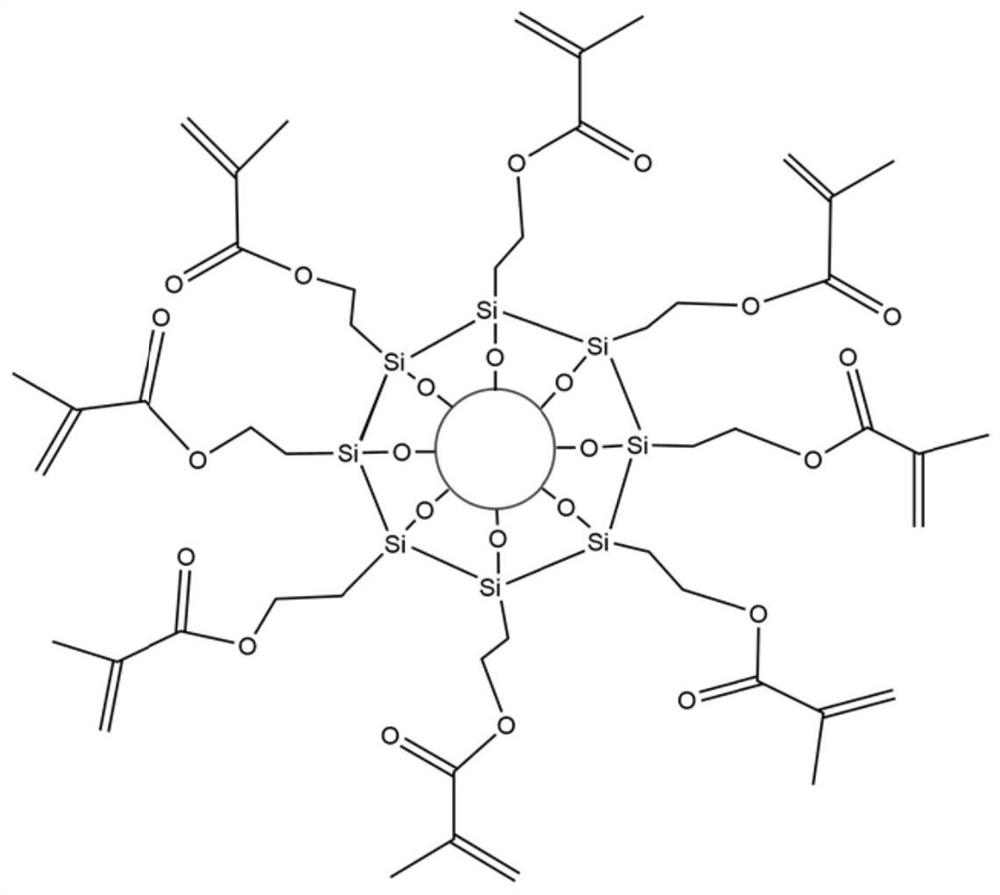
Bongkrekic acid high-selectivity enrichment solid-phase micro-extraction probe as well as preparation method and application thereof
ActiveCN113713781AEasy to makeLow priceOther chemical processesMaterial analysis by electric/magnetic meansFunctional monomerBongkrek acid
The invention discloses a preparation method of a bongkrekic acid high-selectivity enrichment solid-phase micro-extraction probe. The preparation method comprises the following steps of: providing a solid substrate; carrying out surface modification on the solid substrate to enable the surface of the solid substrate to be rich in methacryloyloxy so as to obtain a surface modified solid substrate; and carrying out a molecular imprinting polymerization reaction on the surface modified solid substrate through a molecular imprinting technology by taking methacryloyloxyethyl trimethyl ammonium chloride and octadecyl methacrylate as functional monomers and vitamin A acid and (R)-12-hydroxy-cis-9-octadecenoic acid as template molecules to enable a molecularly imprinted coating with high selective adsorption capacity on bongkrekic acid to be attached to the surface of the solid substrate. The solid-phase micro-extraction probe disclosed by the invention can be used for efficiently extracting and enriching bongkrekic acid in complex food, the enrichment coefficient can reach thousands of times, and the detection limit can reach the level of [mu]g / L. The probe is simple to manufacture, low in price, disposable, capable of effectively preventing cross contamination and memory effect, and especially suitable for rapid analysis and detection of food safety.
Owner:INST OF ANALYSIS GUANGDONG ACAD OF SCI (CHINA NAT ANALYTICAL
Retinoic acid release control nanomicrosphere and its preparation method
InactiveCN1565436AProlong the action timeImprove stabilityGranular deliveryDermatological disorderControlled releaseMicrosphere
The invention relates to a medicinal control release nanometer and method for making same, in particular a retinoic acid release control nanomicrosphere and its preparation method, the constituents of the composition are polylactic acid (PLA), vitamin A acid (RA) and gelatin aqueous solution.
Owner:于美丽 +1
Liposome vitamin A acid aerosol for treating chronic obstructive pulmonary disease
InactiveCN100441187CGood curative effectLong lastingAerosol deliveryRespiratory disorderAdditive ingredientCholesterol
Owner:THE FIRST AFFILIATED HOSPITAL OF THIRD MILITARY MEDICAL UNIVERSITY OF PLA
Freckle-removing and skin-brightening lotion and preparation method thereof
InactiveCN111329772AFreckle removal effect is goodGood color appearanceCosmetic preparationsToilet preparationsVitamin CSalicylic acid
The present invention relates to the field of cosmetics, and particularly relates to a freckle-removing and skin-brightening lotion and a preparation method thereof. The raw materials for preparing the freckle-removing and skin-brightening lotion include hydroquinone, hydroxyacetic acid, vitamin C, vitamin A acid, an antioxidant and a solvent, and also include salicylic acid and pigment; the massratio of the salicylic acid and the hydroxyacetic acid is 1 : (1.5-3); the pigment comprises beta-carotene and carmine; and the antioxidant is a phenolic antioxidant, preferably propyl gallate. The hydroquinone is compounded with the specific proportion of the hydroxyacetic acid and the salicylic acid, and the specific amount of the beta-carotene is added as the pigment, so that the prepared freckle-removing and skin-brightening lotion is mild and non-irritant, has double effects of rapid freckle removing and skin lightening, and has relatively high storage stability.
Owner:齐振乾
Heat-preservation energy-saving material and preparation method thereof
The invention discloses a heat-preservation energy-saving material and a preparation method thereof. The heat-preservation energy-saving material is prepared from the following raw materials: construction waste, vitamin B, gypsum powder, crab shell powder, wormcast, vinasse, ultrafine ceramic powder, cement, tributyl citrate, polybutene rubber, zinc oxide, aluminium oxide, graphite, sepiolite, quartz powder, kelp powder, sodium silicate, ethyecellulose, liquid paraffin, isopropyl palmitate, tea polyphenol, vitamin a acid, pine oil and epoxy resin. The heat-preservation energy-saving material disclosed by the invention has the advantages of high compactness, high anti-pressure ability, good heat insulation and heat preservation effect and long service life, the construction waste, the vinasse and the like are used as raw materials, and wastes are made profitable so as to be more environmentally-friendly.
Owner:苏州格瑞格登新材料科技有限公司
Medicine for treating acne and application method thereof
ActiveCN105055484ASignificant effectNot easy to relapseHeavy metal active ingredientsHydroxy compound active ingredientsGlycerolPotassium hydroxide
The invention relates to a medicine for treating acne and an application method thereof. The medicine comprises an internal adjustment preparation and an external application medicine, wherein the internal adjustment preparation comprises angelica, zinc sulfate, balloon flower and vitamin B; the external application medicine comprises a white medicine and a black medicine, wherein the white medicine comprises stearic acid, potassium hydroxide, glycerol, ethylparaben, water, vitamin A acid and metronidazole as effective ingredients; the black medicine comprises rhubarb, sulfur, almond, ginkgo and litharge as effective ingredients. The medicine comprising the internal adjustment preparation and the external application medicine, provided by the invention, is obvious in curative effect and convenient to use, has a beautifying effect and has market popularization values.
Owner:陈静
Whitening mask and preparation method thereof
InactiveCN109394674AReduce inflammationImprove wrinkle resistanceCosmetic preparationsToilet preparationsGellan gumCottonseed oil
The invention discloses a whitening mask and a preparation method thereof. The whitening mask comprises an agent I directly applied to the face, an agent II and an agent III applied to the face aftermixed with the agent II. The agent I is composed of sea buckthorn oil and aloe juice. The agent II is obtained by mixing the sea buckthorn oil, vitamin A acid, almond oil and the aloe juice. The agentIII is obtained by mixing allantoin, gellan gum, sodium alginate, pearl powder and cottonseed oil. The vitamin A acid is wrapped by the sea buckthorn oil to form the three states of the sea buckthornoil wrapped vitamin A acid- aloe juice- almond oil, so that reliable concentration and stable morphology can also be achieved when the agent II and the agent III are mixed and then applied to the face.
Owner:李金国
Culture medium for inducing mesenchymal stem cells to be differentiated into nerve cells
InactiveCN106497876AReduce the number of aberrant deathsCulture processNervous system cells2-MercaptoethanolMicrobiology
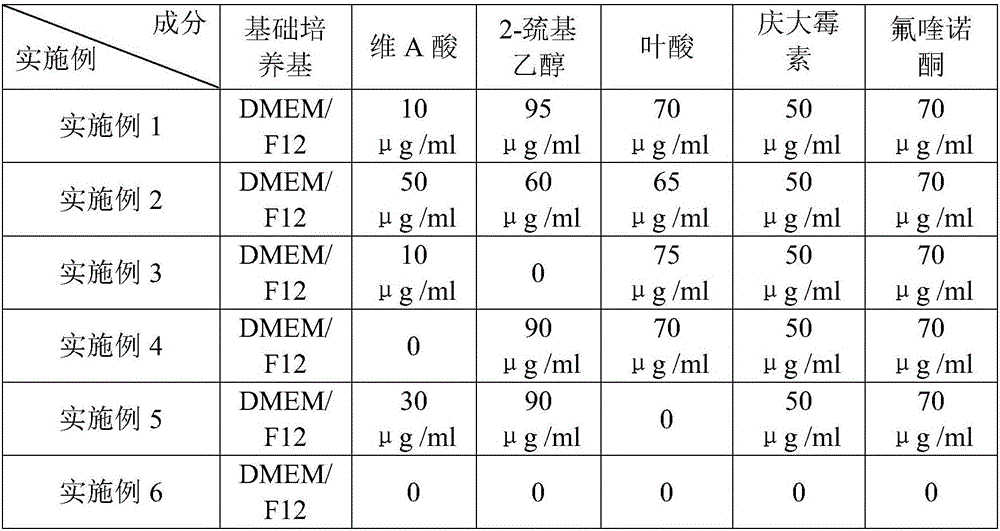
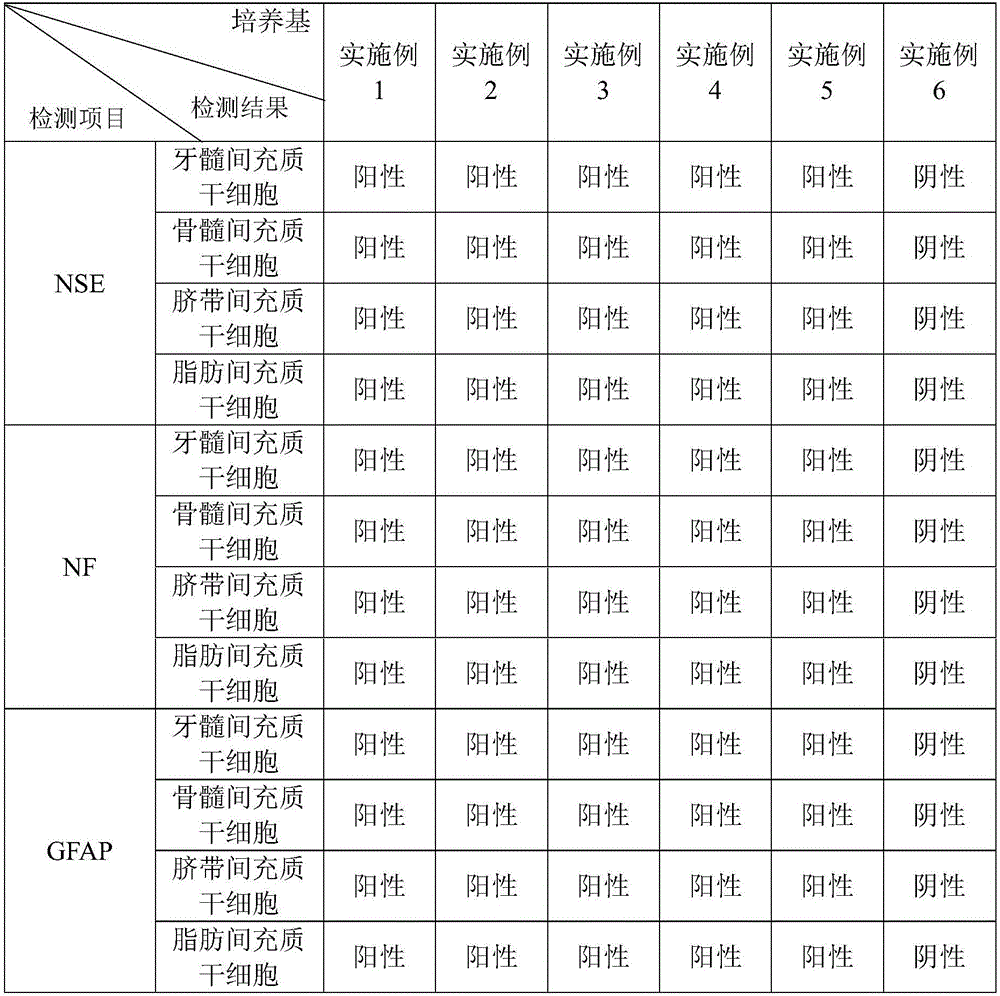
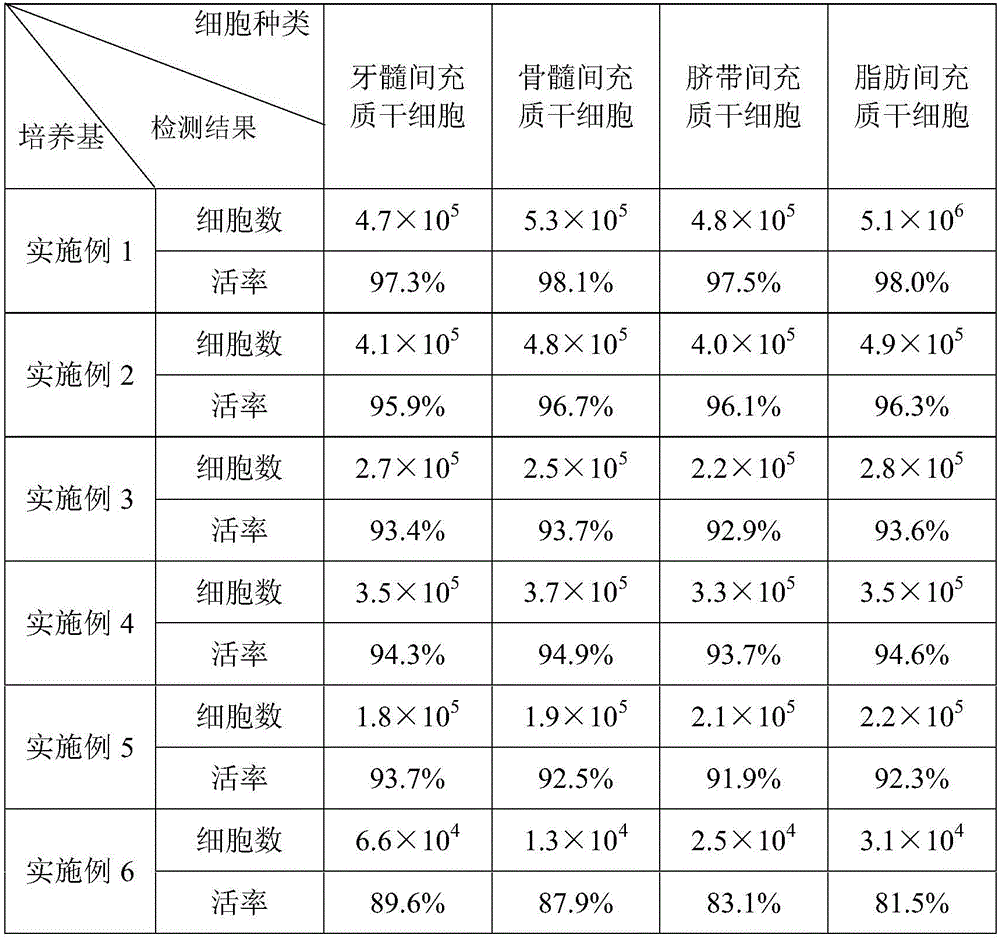
The invention discloses a culture medium for inducing mesenchymal stem cells to be differentiated into nerve cells. The culture medium comprises a basal culture medium and an inductive agent added in the basal culture medium, wherein the inductive agent is prepared from vitamin A acid, 2-mercaptoethanol and folic acid; in addition, in the culture medium for inducing the mesenchymal stem cells to be differentiated into the nerve cells, the concentration of the vitamin A acid is 10 to 50mu g / ml, the concentration of the 2-mercaptoethanol is 60 to 95mu g / ml, and the concentration of the folic acid is 65 to 75mu g / ml. According to the culture medium, the distortion and death number of cells in an inducing process can be effectively reduced, and higher surviving rate of the nerve cells formed by inducing is guaranteed.
Owner:浙江译美生物科技有限公司
Liposome vitamin A acid aerosol for treating chronic obstructive pulmonary disease
InactiveCN1927207AImprove stabilityProlong the action timeAerosol deliveryRespiratory disorderAdditive ingredientCholesterol
The invention relates to a bangosome retinoic acid aerosol which can prevent chronic obstructive pulmonary. The ingredients of the aerosol include: retinoic acid bangosome, cefepime or ceftazidime, fluormone or cetacort and distilled water. The mentioned retinoic acid bangosome is made of the following materials: 1-2 share (by weight) retinoic acid, 10-15 shares (by weight) cholesterol, 10-15 shares (by weight) lecithin and 0.01-0.2 VE. The producing procedures go as follows: mix 2-3 shares (by weight) retinoic acid bangosome with 0.1-0.2 cefepime or ceftazidime, 0.0005-0.0025 fluormone or 0.01-0.05 cetacort; add distilled water to the ingredients at the ratio of 1:15-1:20; fill the material into clean jars to get the aerosol.
Owner:THE FIRST AFFILIATED HOSPITAL OF THIRD MILITARY MEDICAL UNIVERSITY OF PLA
Cream for treating psoriasis and its preparing process
InactiveCN100522137CQuality is easy to controlFast onset of treatmentHydroxy compound active ingredientsAerosol deliverySide effectDistilled oil
The invention provides a kind of cream for treating psoriasis and its preparation method. It is supplemented with zedoary volatile oil and small dose of retinoic acid, and the two medicines are respectively packaged and treated with stearic acid by modern pharmacy method. , glyceryl monostearate, white petrolatum, liquid paraffin, glycerin, triethanolamine, sodium lauryl sulfate, ethylparaben, and distilled water to make an oil-in-water (O / W) compound cream. Compared with the prior art, the medicine of the present invention not only has high safety, good curative effect, no toxic side effect and low recurrence rate, but also has the advantages of stable property, controllable quality, low cost and the like.
Owner:AFFILIATED HOSPITAL OF ZUNYI MEDICAL COLLEGE
Compositions comprising encapsulated tretinoin
PendingUS20190015366A1Low dissolution rateOrganic active ingredientsAerosol deliveryTretinoinCombinatorial chemistry
The present application is directed to compositions comprising microcapsules comprising encapsulated tretinoin, wherein the microcapsule size is less than 50 μm; and to methods of use thereof.
Owner:SOL GEL TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com