Compound clobetasol propionate liposome and preparation thereof
A technology of liposome preparation and compound propionate chloride, which is applied in the field of diseases, can solve the problems that unfavorable drugs can penetrate the stratum corneum, can not be prepared at the same time, and have a large particle size range, so as to achieve small particle size, improve stability, and large The effect of the envelope volume
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
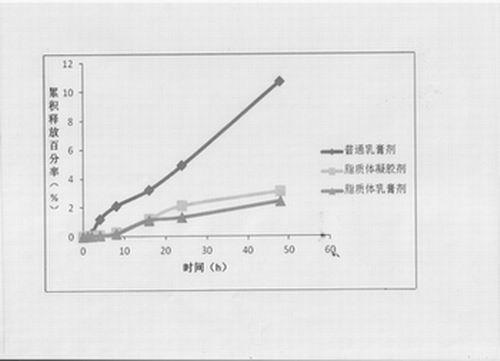
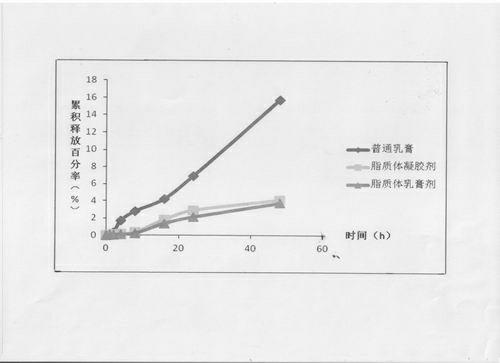
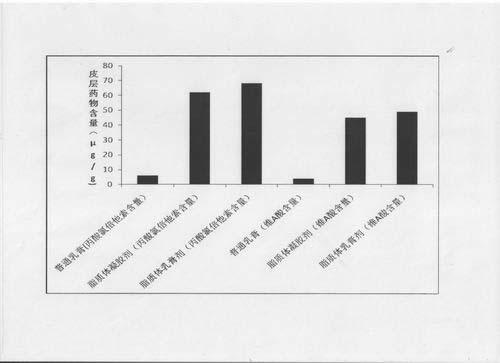
Image
Examples
Embodiment 1
[0029] Embodiment 1: the preparation of drug liposome solution
[0030] Table 1 The prescription table of compound clobetasovitrin A acid liposome solution
[0031] name Quantity, g / 100g Clobetasol Propionate 0.10 Tretinoin 0.05 HSPCs 1.00 Dimyristoylphosphatidylcholine 1.00 Stearamide 0.40 cholesterol 1.00 pH7.4 Phosphate Buffer 96.35
[0032] Preparation process: Dissolve clobetasol propionate, retinoic acid, HSPC, dimyristoylphosphatidylcholine, stearylamide, and cholesterol in 6 times the weight of ethanol, and then slowly inject the drug solution through a syringe Heated to 50°C (and stirred by magnetic force) in the pH7.4 phosphate buffer, after the addition, kept stirring until the ethanol was completely removed, then the liposome suspension was passed through high-pressure milk twice, and finally passed through 100nm polymer Carbonate membrane to obtain liposome solution.
[0033] The encapsulation efficiency...
Embodiment 2
[0034] Embodiment 2: the preparation of drug liposome solution
[0035] Table 2 The prescription table of compound clobetasol propionate liposome solution
[0036] name Quantity, g / 100g Clobetasol Propionate 0.50 Tretinoin 0.50 Dipalmitoylphosphatidylcholine 1.50 dilauroylphosphatidylcholine 0.50 Stearamide 0.20 cholesterol 0.70 pH7.4 Phosphate Buffer 96.10
[0037] Preparation process: Dissolve clobetasol propionate, tretinoin, dipalmitoylphosphatidylcholine, dilauroylphosphatidylcholine, stearylamide, and cholesterol in 6 times the weight of ethanol, and then dissolve the liquid Slowly inject the pH 7.4 phosphate buffer solution heated to 50°C (with magnetic stirring) through the syringe. After the addition, keep stirring until the ethanol is completely removed, and then pass the liposome suspension through high-pressure milk for a second time. , and finally pass through a 100nm polycarbonate membrane to obtain a li...
Embodiment 3
[0040] Embodiment 3: the preparation of drug liposome solution
[0041] Table 3 The prescription table of compound clobetasovitrin A acid liposome solution
[0042] name Quantity, g / 100g Clobetasol Propionate 0.10 Tretinoin 0.05 HSPCs 0.50 dilauroylphosphatidylcholine 0.50 Stearamide 0.50 cholesterol 1.00 pH7.4 Phosphate Buffer 97.35
[0043] Preparation process: Dissolve clobetasol propionate, retinoic acid, HSPC, dilauroylphosphatidylcholine, stearylamide, and cholesterol in 6 times the weight of ethanol, and then slowly inject the drug solution through a syringe to heat Put it into the pH7.4 phosphate buffer at 50°C (with magnetic stirring). After the addition, keep stirring until the ethanol is completely removed, then pass the liposome suspension through high-pressure milk twice, and finally pass through 100nm polycarbonate Ester film, namely liposome solution.
[0044] The encapsulation efficiency of clobetasol ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| Average particle size | aaaaa | aaaaa |
| Average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com