Synthetic method of all-trans vitamin A acid medicament
A synthesis method and all-trans technology, applied in the direction of organic chemistry, etc., can solve the problems of high catalyst price, low selectivity, heavy metal residues, etc., and achieve the effects of high product purity, high yield and low cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
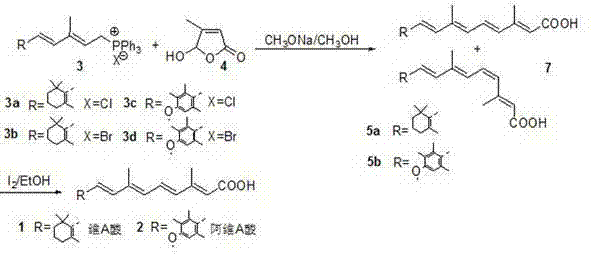
[0019] (1) Preparation of 11 cis, 13 trans-retinoic acid and 11 trans, 13 trans-retinoic acid mixture (5a):
[0020] Mix 1.37Kg (12.00mol) of 5-hydroxy-4-methyl-2-furanone, 2.70Kg (49.98mol) of sodium methoxide with 20L of anhydrous methanol, o Stir at C for 2h, cool to -5~0 o C, 5.01Kg (10.00 mol) [3-methyl-5-(2,6,6-trimethyl-1-cyclohexen-1-yl)-2,4-pentadiene]-triphenyl Dissolve the phosphine chloride salt in 15L of anhydrous methanol, and slowly add it dropwise to the above solution, keeping the temperature at -5~0 o C, after the addition, keep warm for 4 hours, then react at room temperature for 6-10 hours, add 40L of ice water, extract with a mixed solvent of 2×30L ethyl acetate / petroleum ether=1:4, and adjust the pH of the water layer with concentrated hydrochloric acid= 3~4, stirred for 30min, extracted with 3×30L ethyl acetate / petroleum ether=1:3 mixed solvent, combined the organic layer, then washed with 2×20L water, dried over anhydrous sodium sulfate, evaporated th...
Embodiment 2
[0024] (1) Preparation of 11 cis, 13 trans-retinoic acid and 11 trans, 13 trans-retinoic acid mixture (5a):
[0025] Mix 1.37g (12.0mmol) of 5-hydroxy-4-methyl-2-furanone, 1.26g (52.6mmol) of lithium hydroxide and 15ml of N,N-dimethylformamide, o Stir at C for 2h, cool to -5~0 oC, 5.01g (10mmol) [3-methyl-5-(2,6,6-trimethyl-1-cyclohexen-1-yl)-2,4-pentadiene]-triphenyl Dissolve phosphine chloride salt in 15ml N,N-dimethylformamide, slowly add dropwise to the above solution, keep the temperature at -5~0 o C. After the addition, keep the reaction for 4 hours, then react at room temperature for 6-10 hours, add 40ml of ice water, extract with a mixed solvent of 2×30ml ethyl acetate / petroleum ether=1:4, and adjust the pH of the water layer to 3 with concentrated hydrochloric acid ~4, stirred for 30min, extracted with a mixed solvent of 3×30ml ethyl acetate / petroleum ether=1:3, combined the organic layers, then washed with 2×20ml water, dried over anhydrous sodium sulfate, and evap...
Embodiment 3
[0029] (1) Preparation of 11-cis, 13 trans-acitretin and 11 trans, 13 trans-acitretin mixture (5b)
[0030] Mix 0.627Kg (5.49mol) of 5-hydroxy-4-methyl-2-furanone, 1.35Kg (24.99mol) of sodium methoxide with 10L of anhydrous methanol, and o Stir at C for 2h, cool to -5~0 o C, 2.64Kg (5.00mol) [5-(4-methoxy-2,3,6-trimethylphenyl)-3-methyl-2,4-pentadiene]-triphenylphosphine Dissolve the chloride salt (3c) in 7.5L of anhydrous methanol, and slowly add it dropwise to the above solution, keeping the temperature at -5~0 o After adding C, keep it warm for 4 hours, then react at room temperature for 6-10 hours, add 20L of ice water, extract with a mixed solvent of 2×15L ethyl acetate / petroleum ether=1:4, and adjust the pH of the water layer to 3 with concentrated hydrochloric acid ~4, a yellow solid precipitated, stirred for 30min, filtered, washed with water, dried to obtain 1.225Kg yellow solid, HPLC showed 11-cis, 13 trans-acitretin: 11trans, 13trans-acitretin=50: 50.
[0031] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com