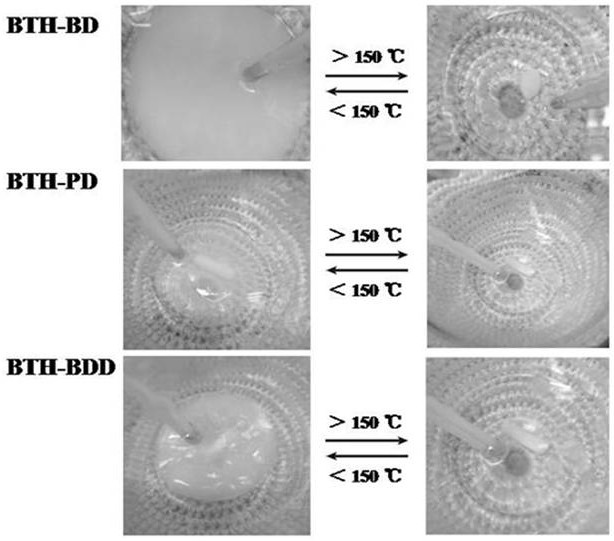
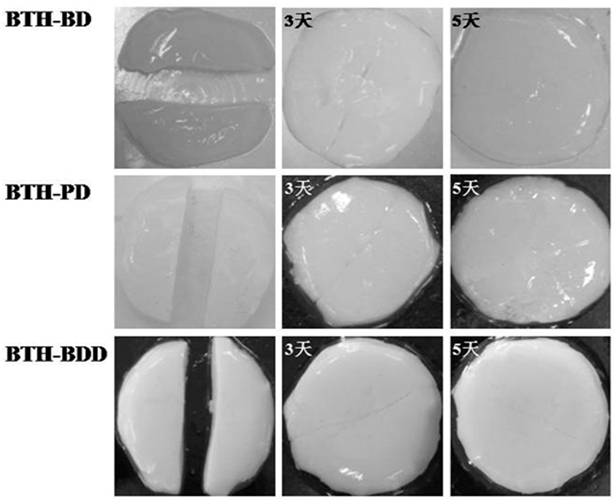
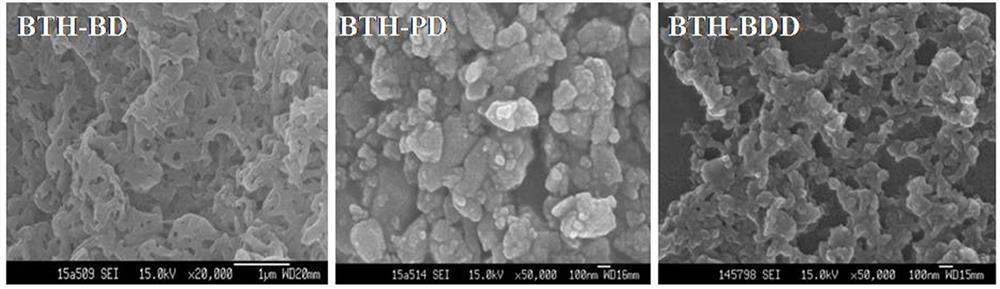
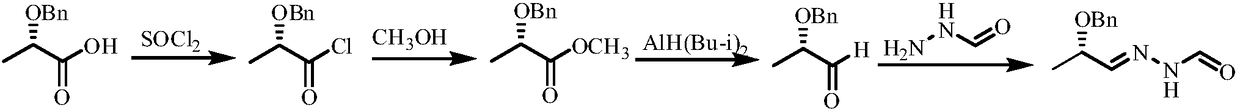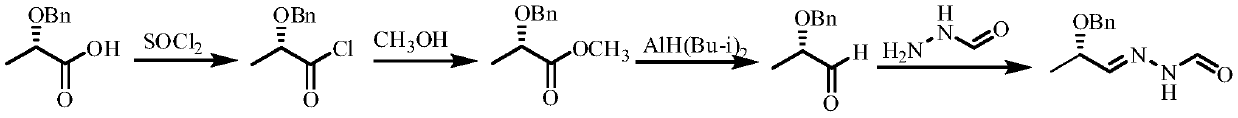Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
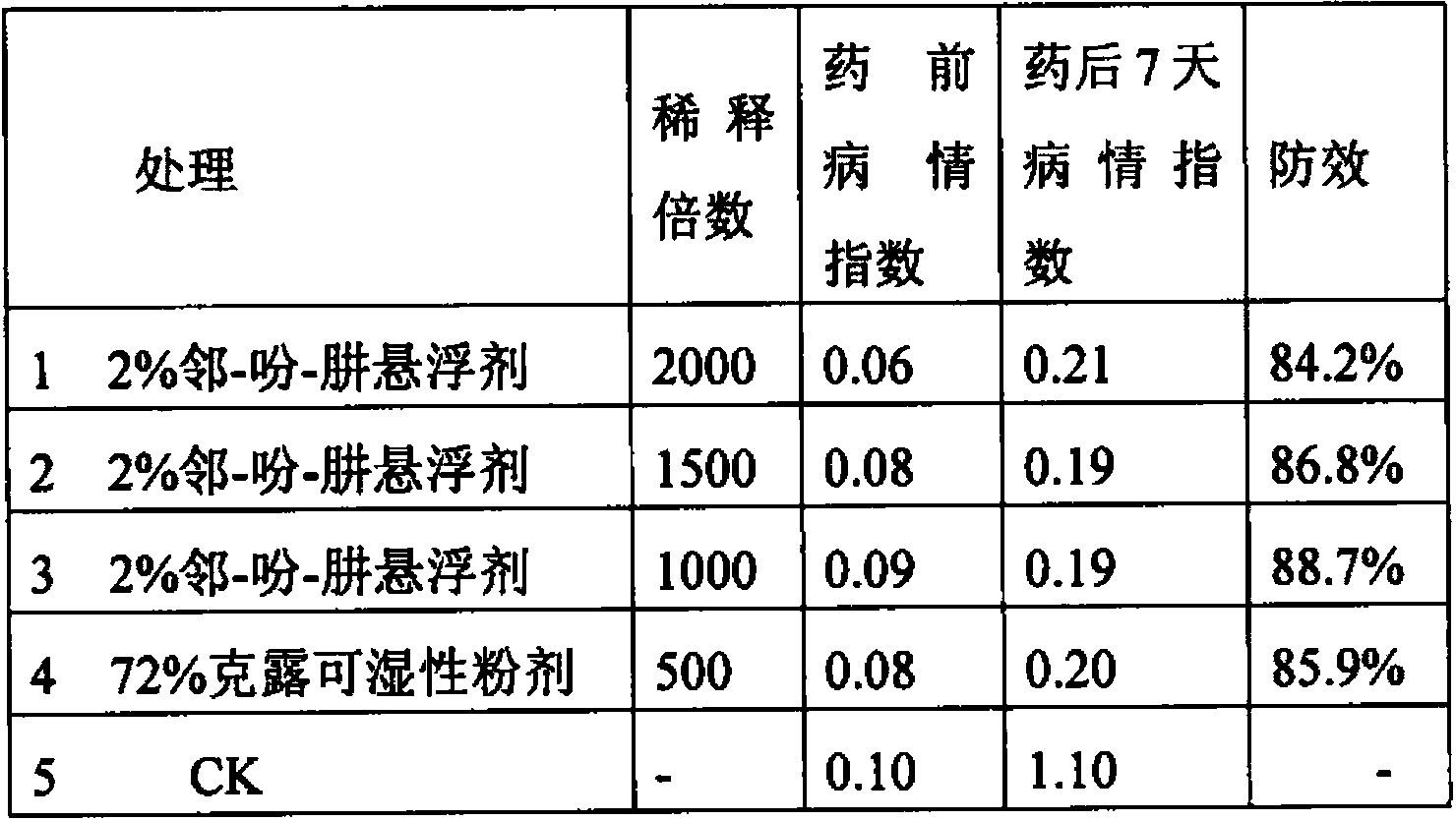
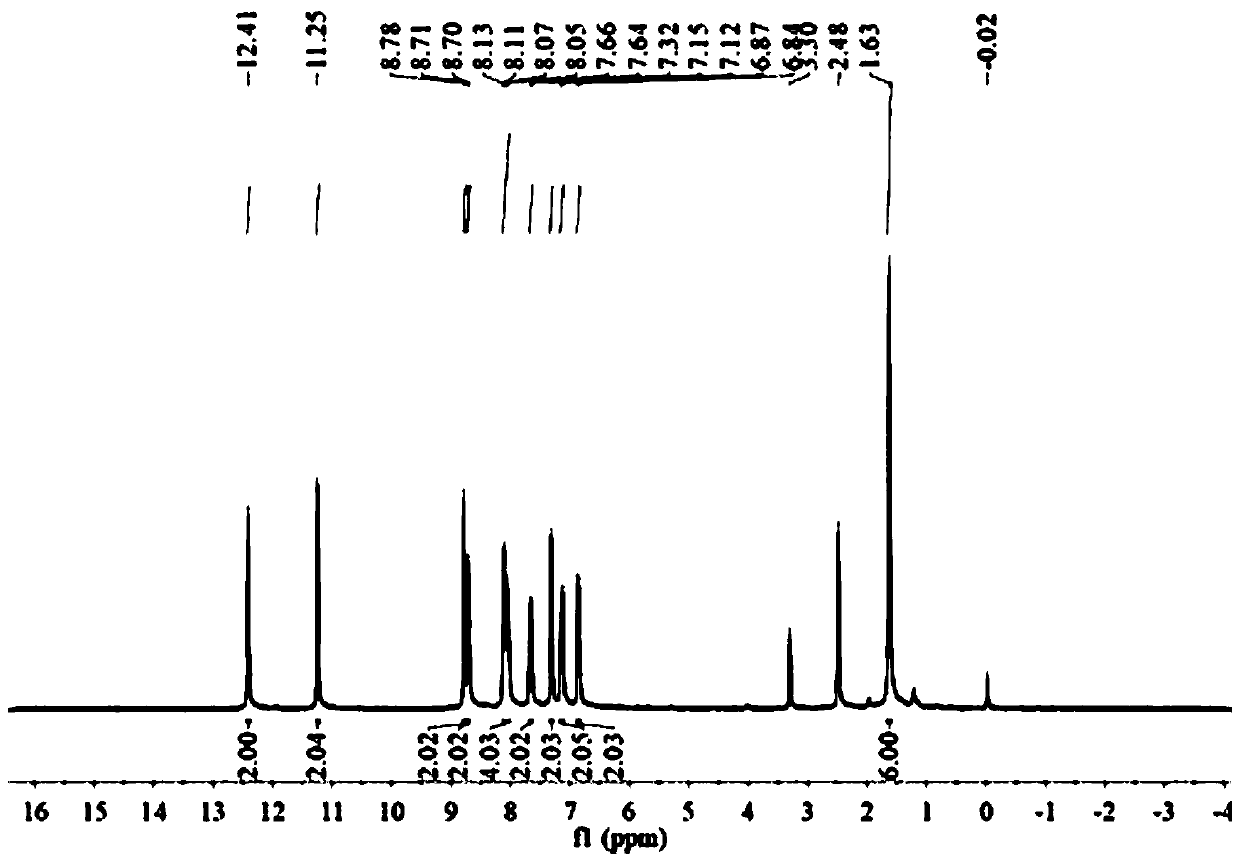
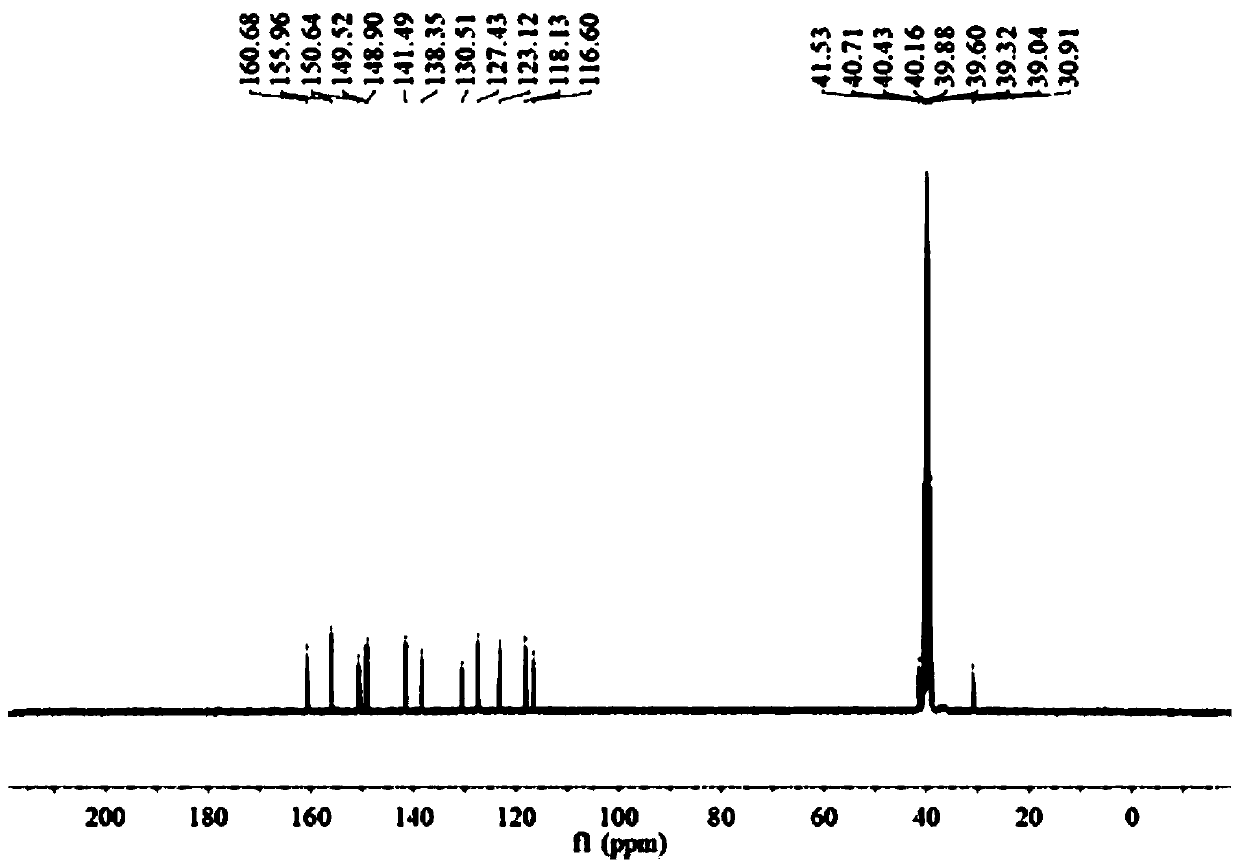
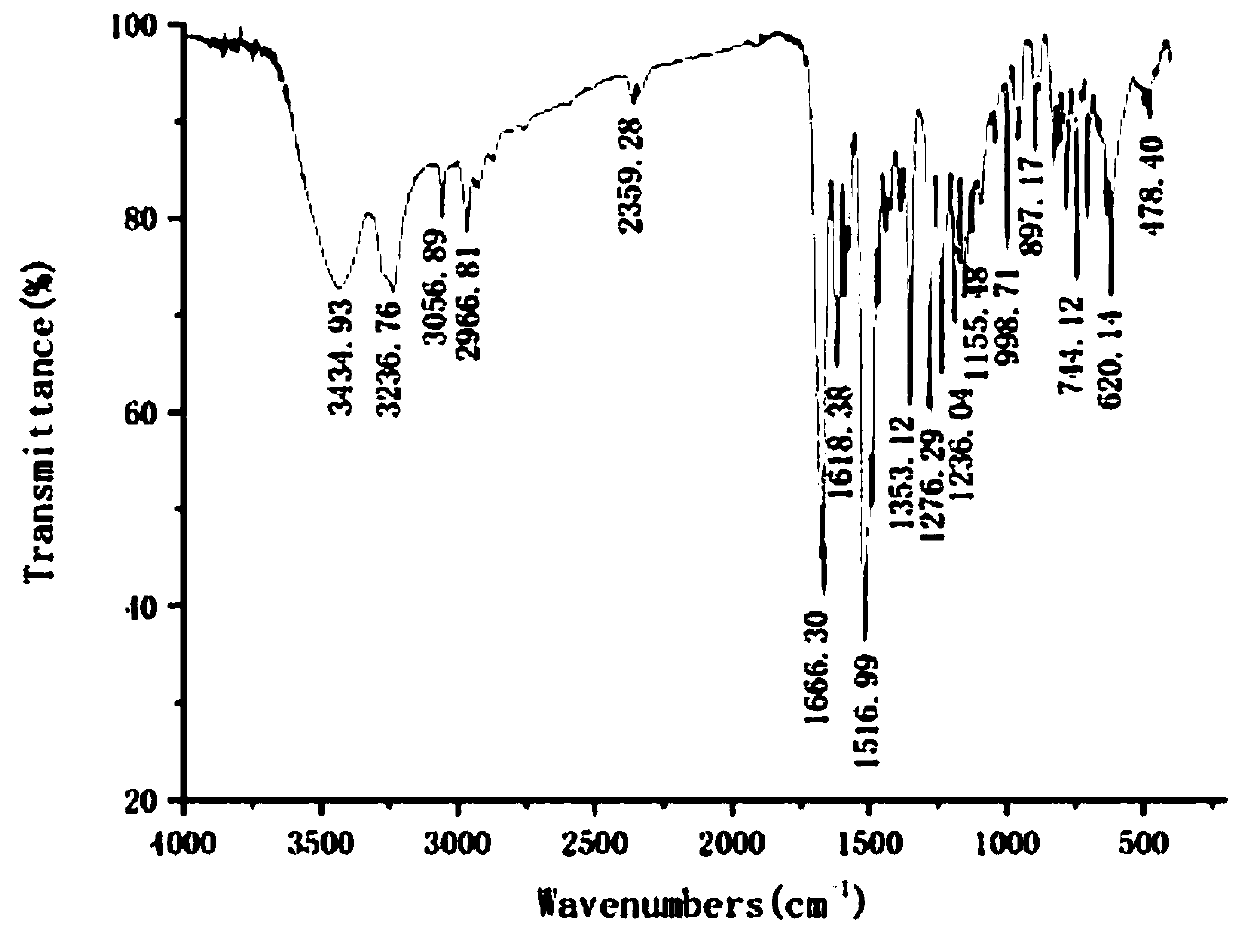
73 results about "Formylhydrazine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Synonym: Formic acid hydrazide, Formylhydrazine CAS Number 624-84-0. Linear Formula HCONHNH 2. Molecular Weight 60.06 . Beilstein Registry Number 635759 . EC Number 210-867-9. MDL number MFCD00007589. PubChem Substance ID 24850161
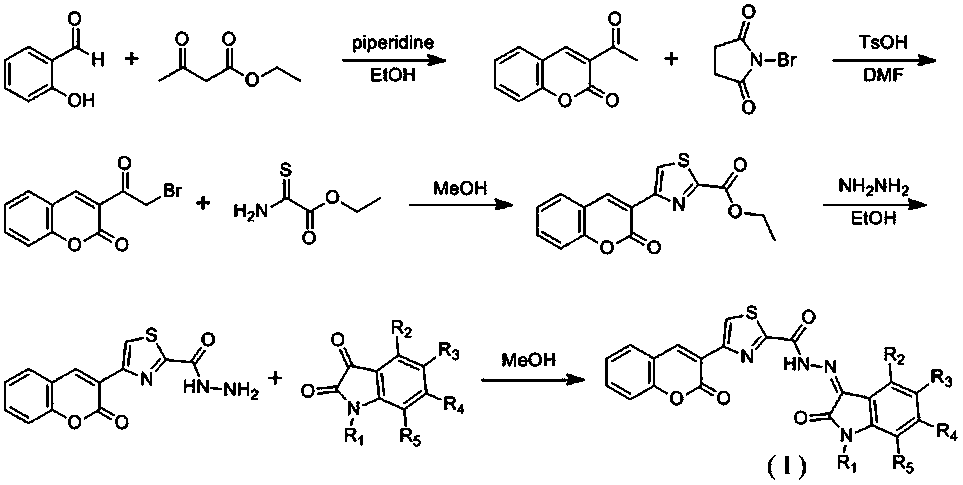
Coumarin-thiazole-indolone compounds, and preparation method and application thereof
InactiveCN104829608AEnhanced inhibitory effectHigh activityAntibacterial agentsOrganic active ingredientsAcetic acidSalicylaldehyde
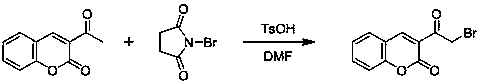
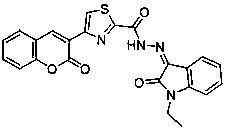
The invention discloses coumarin-thiazole-indolone compounds, and a preparation method and application thereof. The compounds are disclosed as Formula (I). The preparation method comprises the following steps: reacting salicylaldehyde and ethyl acetoacetate to obtain 3-acetyl-2H-benzopyranyl-2-one, carrying out bromination, cyclization and hydrazinolysis reaction to obtain 4-(2-oxo-2H-benzopyranyl-3-yl)thiazolyl-2-formylhydrazine, and finally, reacting with various substituted isatin to obtain the target compounds. The compounds can be used as a raw material of antibacterial drugs. The preparation method has the advantage of simple and accessible raw materials, and is convenient to operate.
Owner:JISHOU UNIVERSITY
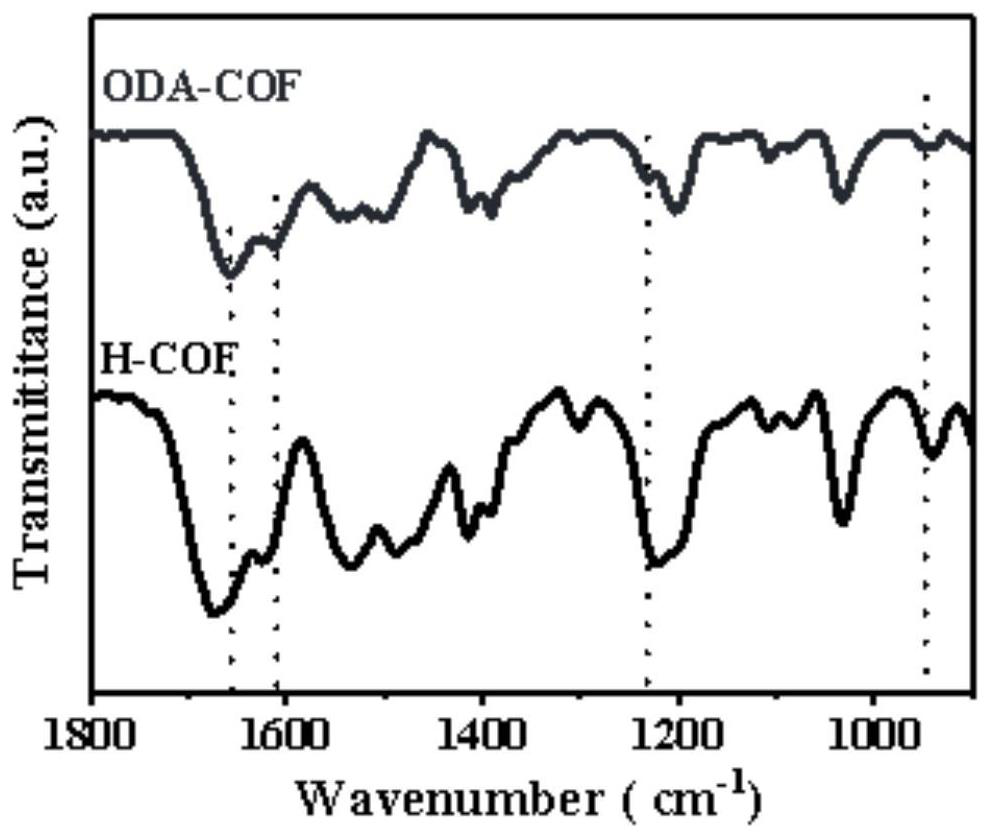
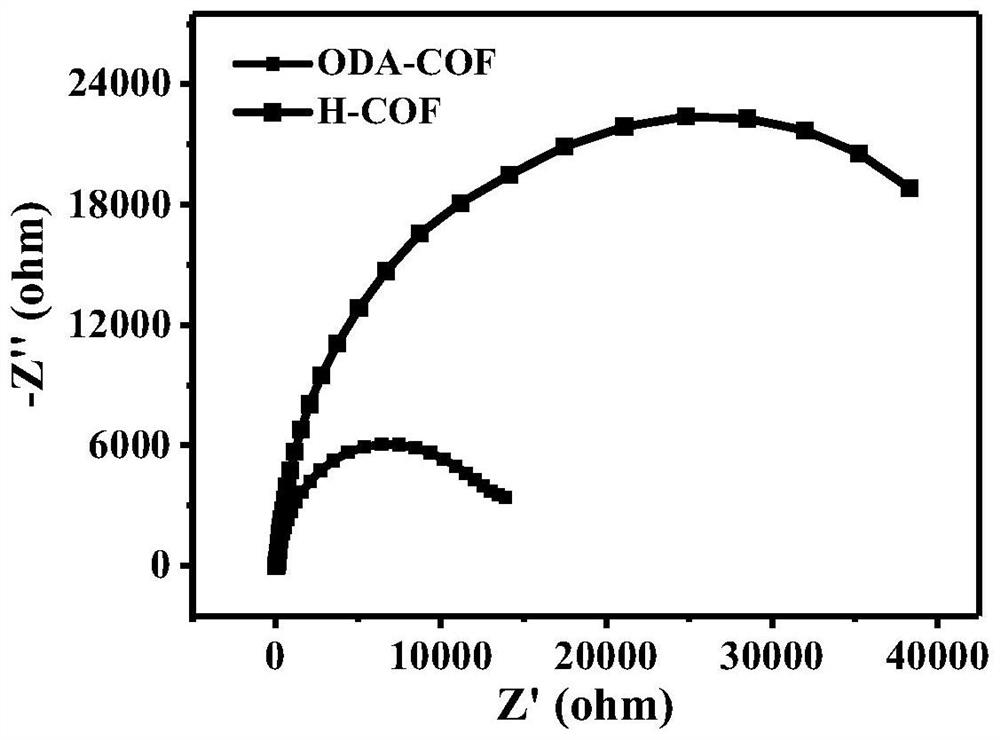
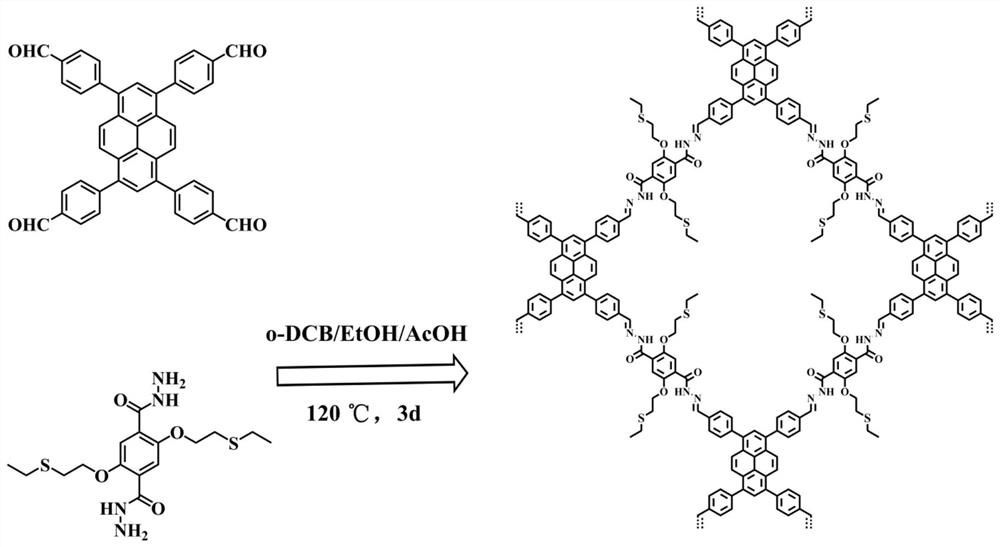
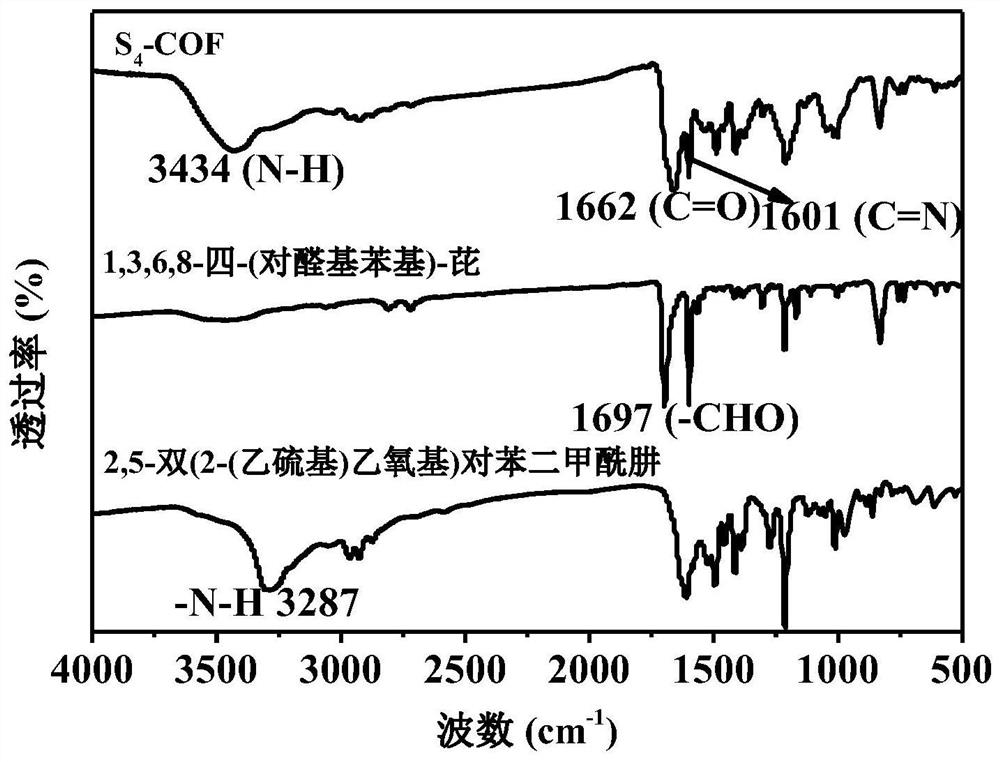
Thioether functionalized pyrenyl covalent organic framework material and preparation method and application thereof
ActiveCN112608490AGood visible light responseHigh synthetic yieldOrganic-compounds/hydrides/coordination-complexes catalystsHydrogen productionPhenyl groupPyrene
The invention belongs to the field of covalent organic framework materials, and particularly relates to a thioether functionalized pyrenyl covalent organic framework material and a preparation method and application thereof. The preparation method comprises the following steps: adding 2, 5-bis (2-(ethylthio) ethoxy) terephthalylhydrazine and 1, 3, 6, 8-4-(p-formylphenyl)-pyrene into a solvent system, and reacting to obtain the thioether functionalized pyrenyl covalent organic framework material. Equipment and chemical reagents used in the synthesis method are easy to obtain, the process operation is simple and convenient, the applicability is high, the industrial application value is high, the synthesis yield is relatively high, and the pyrenyl covalent organic framework material prepared by the method has good response to visible light, has good potential application value in the field of hydrogen production by photocatalytic decomposition of water, and is easy to popularize and utilize.
Owner:HUAZHONG UNIV OF SCI & TECH
Synthetic method of 5-acylbenzo[a]carbazole compound
The invention discloses a synthetic method of a 5-acylbenzo[a]carbazole compound. According to the synthetic method, the 5-acylbenzo[a]carbazole compound is synthesized through cascade reaction between a 2-aryl-3-formylhydrazine compound and sulfur ylide. The synthetic method has the advantages of simplicity and convenience in operation, mild conditions, wide substrate application range and the like and is suitable for industrial production.
Owner:HENAN NORMAL UNIV
Schiff base fluorescent probe QCS and method for preparing same
ActiveCN107056779AThe synthesis process is simpleHigh yieldOrganic chemistryMaterial analysis by observing effect on chemical indicatorSalicylaldehydeHydrazine compound
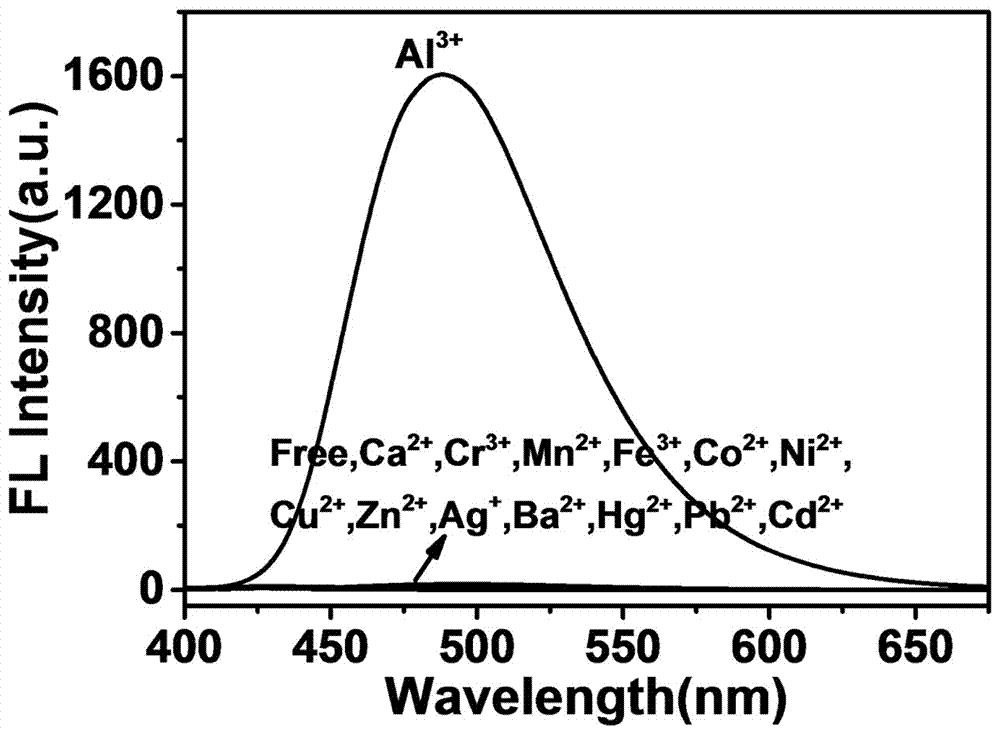
The invention provides a Schiff base fluorescent probe QCS 3-[N-(2-phenol methylene)] formylhydrazine base-1-(quinoline-2-base)-9H-beta-carboline QCS and a method for preparing the same. The method includes preparation steps of carrying out reaction on 3-methyl formate-1-(quinoline-2-base)-9H-beta-carboline and hydrazine hydrate to obtain 3-formylhydrazine-1-(quinoline-2-base)-9H-beta-carboline; carrying out reaction on the 3-formylhydrazine-1-(quinoline-2-base)-9H-beta-carboline and salicylaldehyde to obtain the Schiff base fluorescent probe QCS. The Schiff base fluorescent probe QCS and the method have the advantages that probe QCS solution is bright blue green in ultraviolet lamps (365 nm) in the presence of Al3+, new emission peaks occur at 490 nm locations of fluorescence emission spectra, and the fluorescence intensity is gradually improved along with increase of the concentration of the Al3+; the Schiff base fluorescent probe QCS can be used for quickly and quantitatively detecting micro / trace amounts of Al3+ from different sources in real time.
Owner:CHINA THREE GORGES UNIV
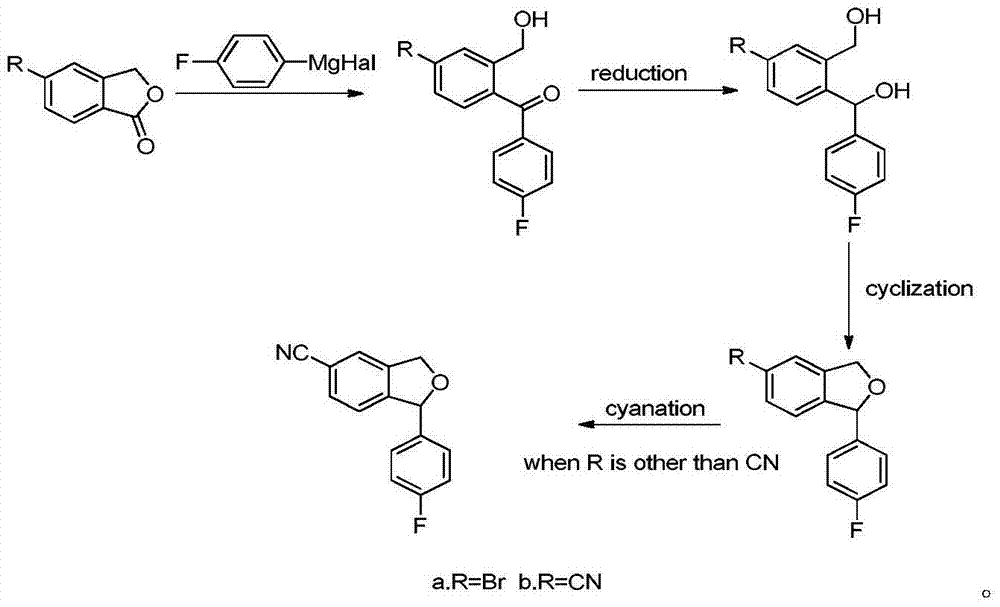
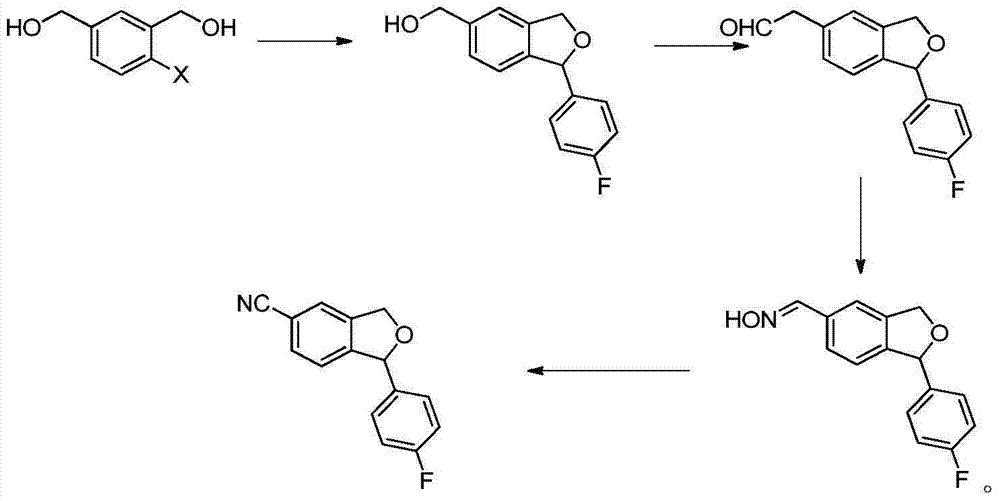
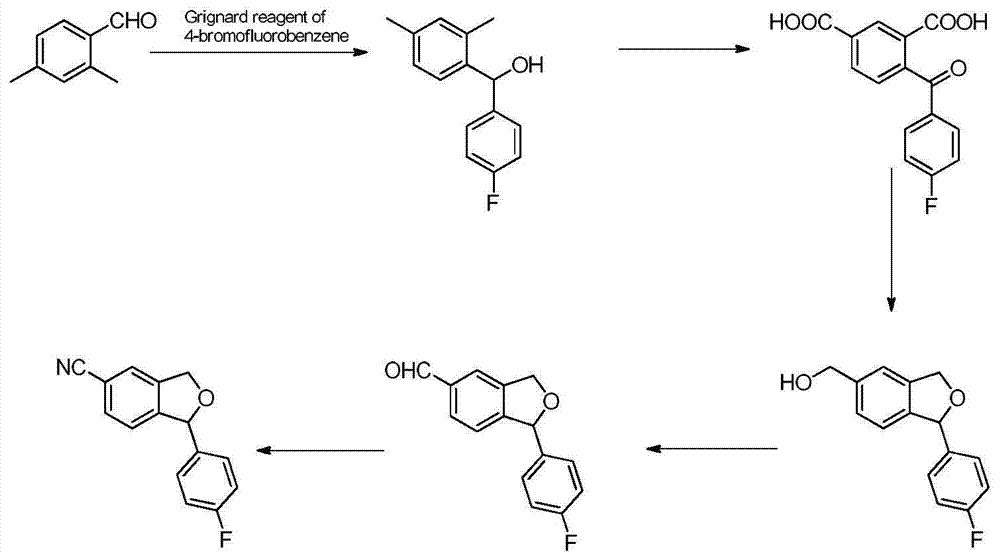
Preparation method of 5-cyanogen-1-(4-fluobenzene)-1,3-dihydrogenated-isobenzofuranone
The invention provides a preparation method of 5-cyanogen-1-(4-fluobenzene)-1,3-dihydrogenated-isobenzofuranone. The preparation method comprises the following steps: reducing fluorobenzoic acid ester through hydrazine hydrate to obtain fluorobenzene formylhydrazine; enabling the fluorobenzene formylhydrazine to react with 5-bromine-2-hydroxybenzaldehyde under acidic conditions to obtain 4-fluorine-[(5-bromine-2- hydroxy phenyl) methene] hydrazide benzoic acid; oxidizing the 4-fluorine-[(5-bromine-2- hydroxy phenyl) methene] hydrazide benzoic acid through an oxidizing agent to obtain 5-bromine-2-(4-fluorine benzoyl)-benzaldehyde; reducing the 5-bromine-2-(4-fluorine benzoyl)-benzaldehyde through a reducing agent to obtain 4-bromine-Alpha 1-(4-fluorine phenyl)-1,2-xylyl alcohol; performing condensation reaction on the 4-bromine-Alpha 1-(4-fluorine phenyl)-1,2-xylyl alcohol by using toluenesulfonic acid as a catalyst to obtain 1-(4-fluobenzene)-5-bromine-1,3-dihydro- isobenzofuranone; enabling the 1-(4-fluobenzene)-5-bromine-1,3-dihydro- isobenzofuranone to react with copper cyanide to obtain the product. The method is low in cost, simple in technology, easy-operated in reaction, high in yield and suitable for industrialized production.
Owner:SOUTHEAST UNIV
Covalent organic framework material as well as synthesis method and application thereof
InactiveCN111363145ASensitive fluorescence response behaviorFluorescence/phosphorescenceLuminescent compositionsFluoProbesFluorescence response
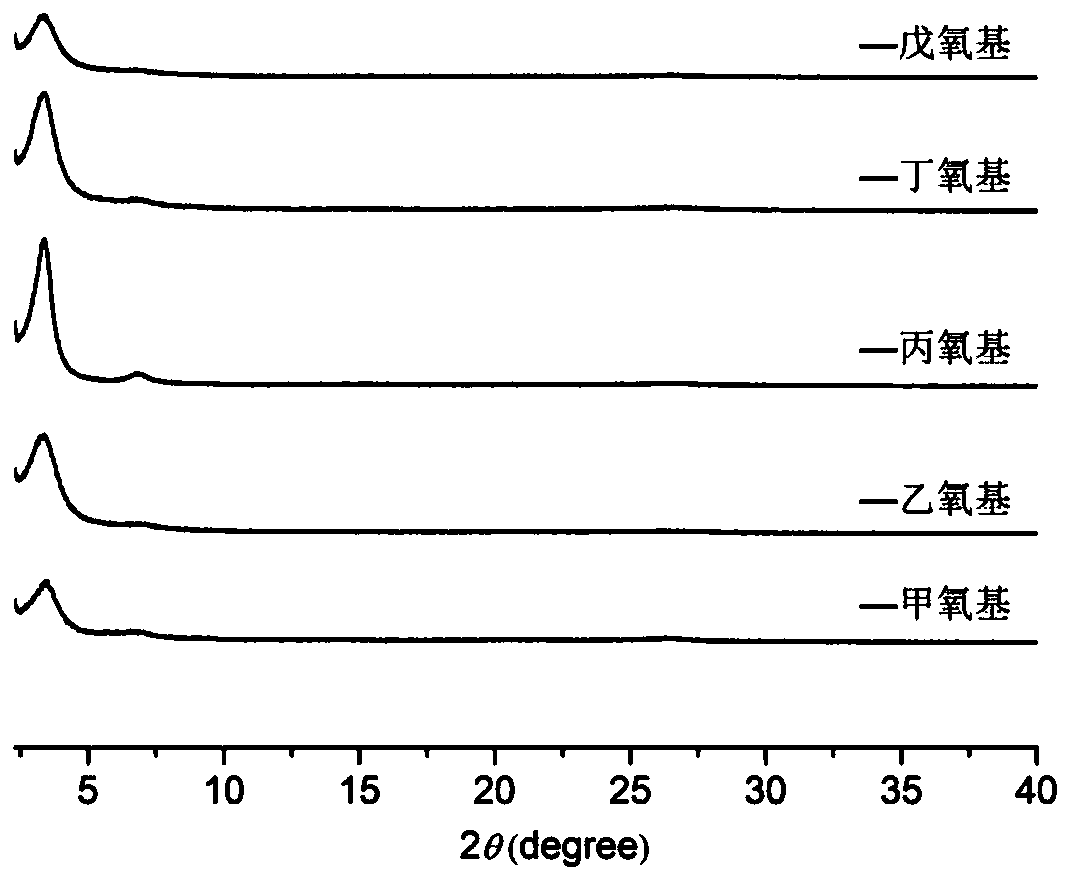
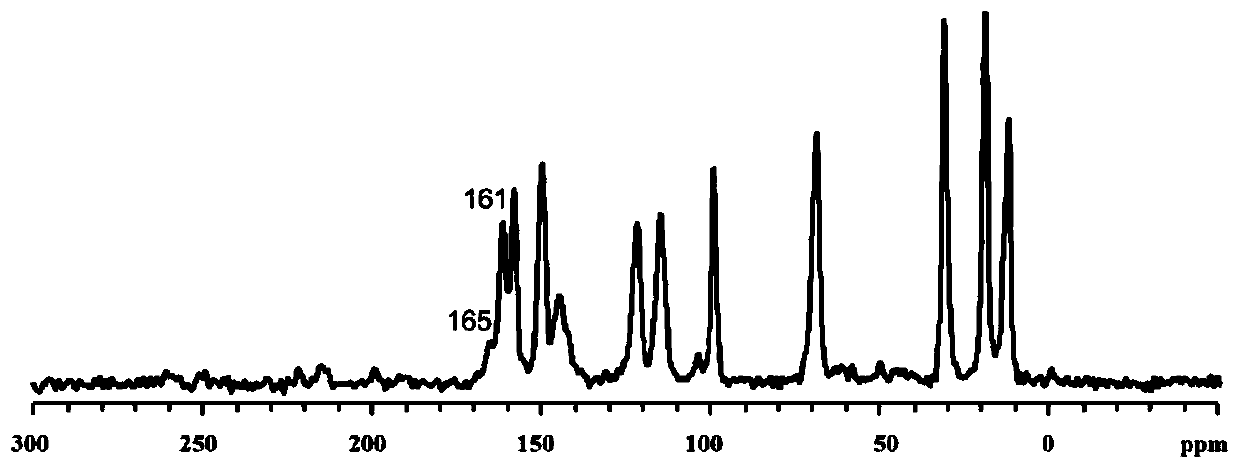
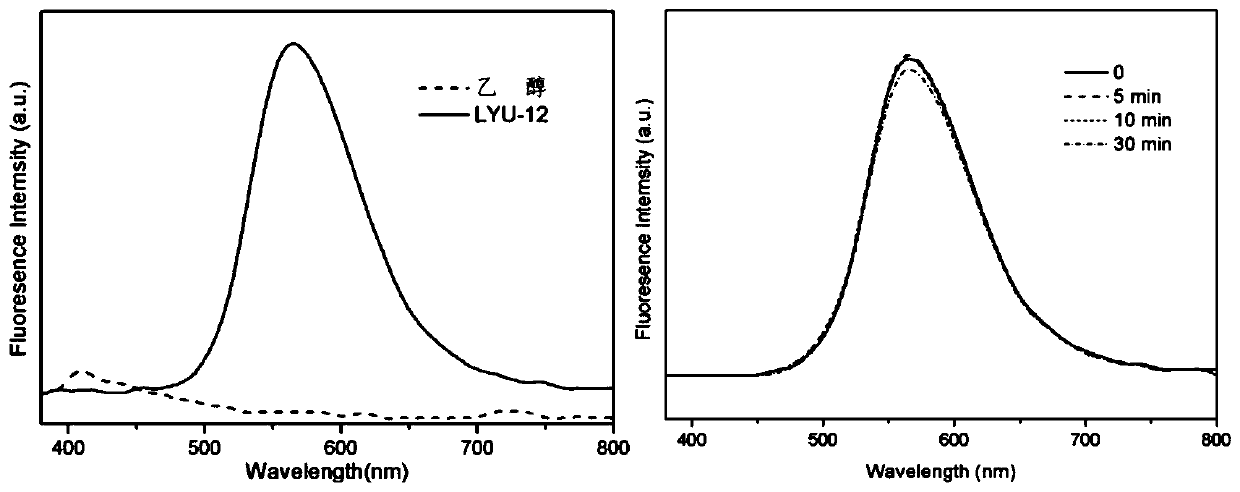
The invention discloses a synthesis method of a covalent organic framework material. The preparation method comprises the following steps: uniformly mixing 2,4,6-trihydroxy-1,3,5-s-tribenzaldehyde and2,5-dialkoxy-1,4-phthaloyl hydrazine in an organic solvent, and reacting under the catalysis of acetic acid to obtain the covalent organic framework material, and alkoxyl is methoxy, ethoxyl, propoxyl, butoxyl or pentoxyl. The covalent organic framework material is applied to fluorescence detection of Cu<2+>, Co<2+>, Cr<3+> and Pb<2+>. The covalent organic framework material synthesized by the method has a long-range ordered two-dimensional hexagonal structure and regular pore passages; the material can be used as a fluorescent probe, can be recognized by heavy metal ions Cu<2+>, Co<2+>, Cr<3+> and Pb<2+> to quench fluorescence, and has sensitive fluorescence response behaviors.
Owner:LINYI UNIVERSITY
Ligustrazine acylhydrazone derivatives, and preparation method and application thereof
ActiveCN107118166AGood antitumor activityLow toxicityOrganic chemistryAntineoplastic agentsPyrazineNormal cell
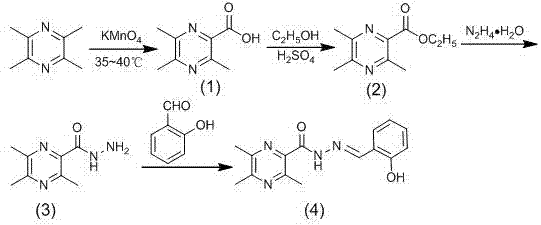
The invention discloses a preparation method and application of ligustrazine acylhydrazone derivatives. The structural formula of the ligustrazine acylhydrazone derivatives is as shown in the description; and the chemical name of the ligustrazine acylhydrazone derivatives is N-(2-hydroxybenzmethylene)-3,5,6-trimethyl pyrazine-2-formylhydrazine, the molecular formula is C15H16N4O2, and the relative molecular weight is 284.13. The preparation method of the ligustrazine acylhydrazone derivatives is simple, convenient and high in yield. The ligustrazine acylhydrazone derivatives have anti-tumor effect, have low toxicity on normal cells, and may become a novel, efficient and low-toxicity anti-tumor medicine.
Owner:WEIFANG MEDICAL UNIV
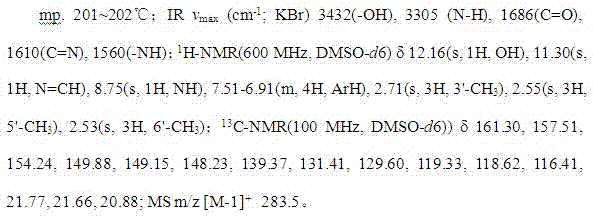
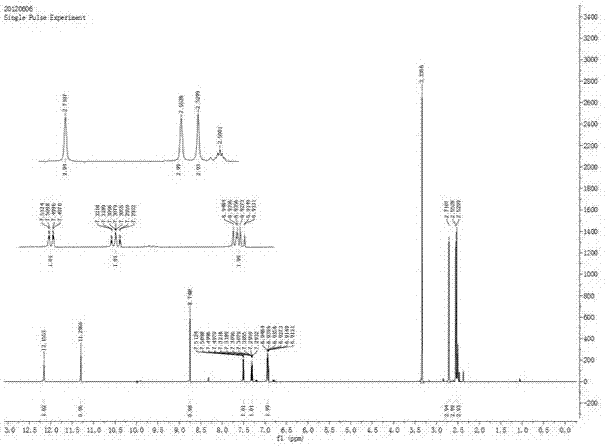
Synthetic method of (trifluoromethoxy) anisidine formylhydrazine
ActiveCN102584639AReduce dosageGuaranteed final yieldOrganic chemistryOrganic solventOrganic synthesis
The invention belongs to the technical field of organic synthesis and particularly provides a method for synthesizing (trifluoromethoxy) anisidine formylhydrazine with a little amount of hydrazine hydrate as reactants, inexpensive organic solvents not mutually dissolving with water as reaction system solvents and phase transfer catalysts as catalysts. The synthetic method quickens reaction, improves product quality and yield, avoids highly virulent hydrazine hydrate pollution, and has the advantages of being high in yield, small in environment pollution and low in cost.
Owner:JINGBO AGROCHEM TECH CO LTD
Novel anti-yellowing agent and preparation method thereof
ActiveCN108468211ANo risk of adsorptionMeet environmental protection requirementsBiochemical fibre treatmentLight resistant fibresEnvironmental resistanceDistilled water
The invention discloses a novel anti-yellowing agent and a preparation method thereof, wherein the novel anti-yellowing agent comprises, in parts by mass, 13-45 parts of an adamantane formylhydrazinederivative, 3-12 parts of alkylol amine, 2-8 parts of 1,2-aminoazophenylene, 3-15 parts of o-acetylsalicylic acid, 1-10 parts of lauryl alcohol polyoxyethylene ether, 10-40 parts of urea and 30-60 parts of distilled water; the adamantane formylhydrazine derivative is N-(chloroacetyl)-adamantane formylhydrazine or N-(2-phenylamino-acetyl)-adamantane formylhydrazine. The novel anti-yellowing agent has the advantages of low cost, no containing of harmful by-products (especially ADH), lasting anti-yellowing effect and no formaldehyde adsorption risk, and meets the environmental-protection requirements.
Owner:多恩生物科技有限公司
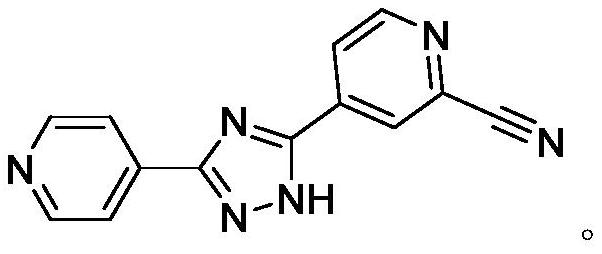
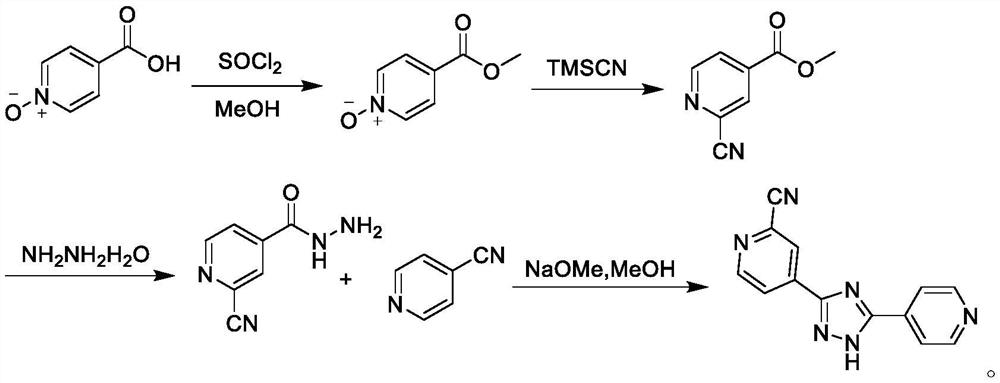
Preparation method of topiroxostat
PendingCN113666909ASimple and efficient operationHigh reaction yieldOrganic chemistryPtru catalystAcyl group
The invention belongs to the field of pharmaceutical chemicals, and particularly relates to a preparation method of topiroxostat. The preparation method disclosed by the invention comprises the following steps: 2-cyanopyridine and formamide are taken as raw materials to react to prepare 2-cyano-4-carbamoyl-pyridine; the 2-cyano-4-carbamoyl-pyridine continues to react with isoniazide to obtain a key intermediate 4-picolinic acid hydrazide-N'-(2-cyanopyridine-4-carbodiimide), and the key intermediate 4-picolinic acid hydrazide-N'-(2-cyanopyridine-4-carbodiimide) is subjected to ring closing to obtain the topiroxostat. The invention provides the novel method for synthesizing topiroxostat, which avoids the use of highly toxic chemical reagents, replaces a traditional catalyst with a green catalyst, is milder in reaction, is economical and environment-friendly, is higher in yield, and is suitable for industrial production.
Owner:LUNAN PHARMA GROUP CORPORATION
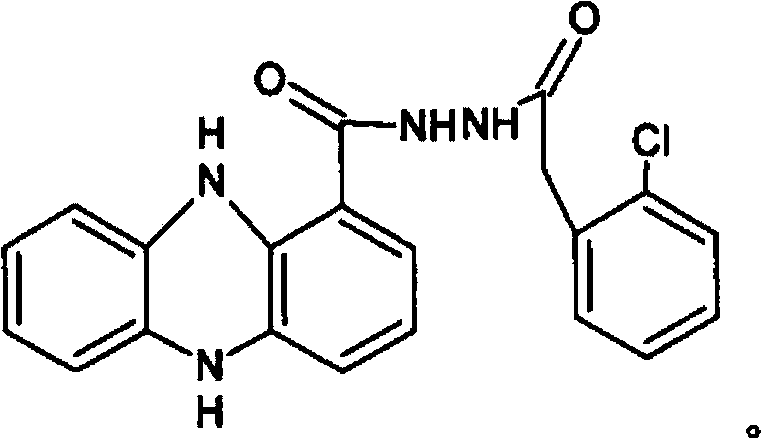
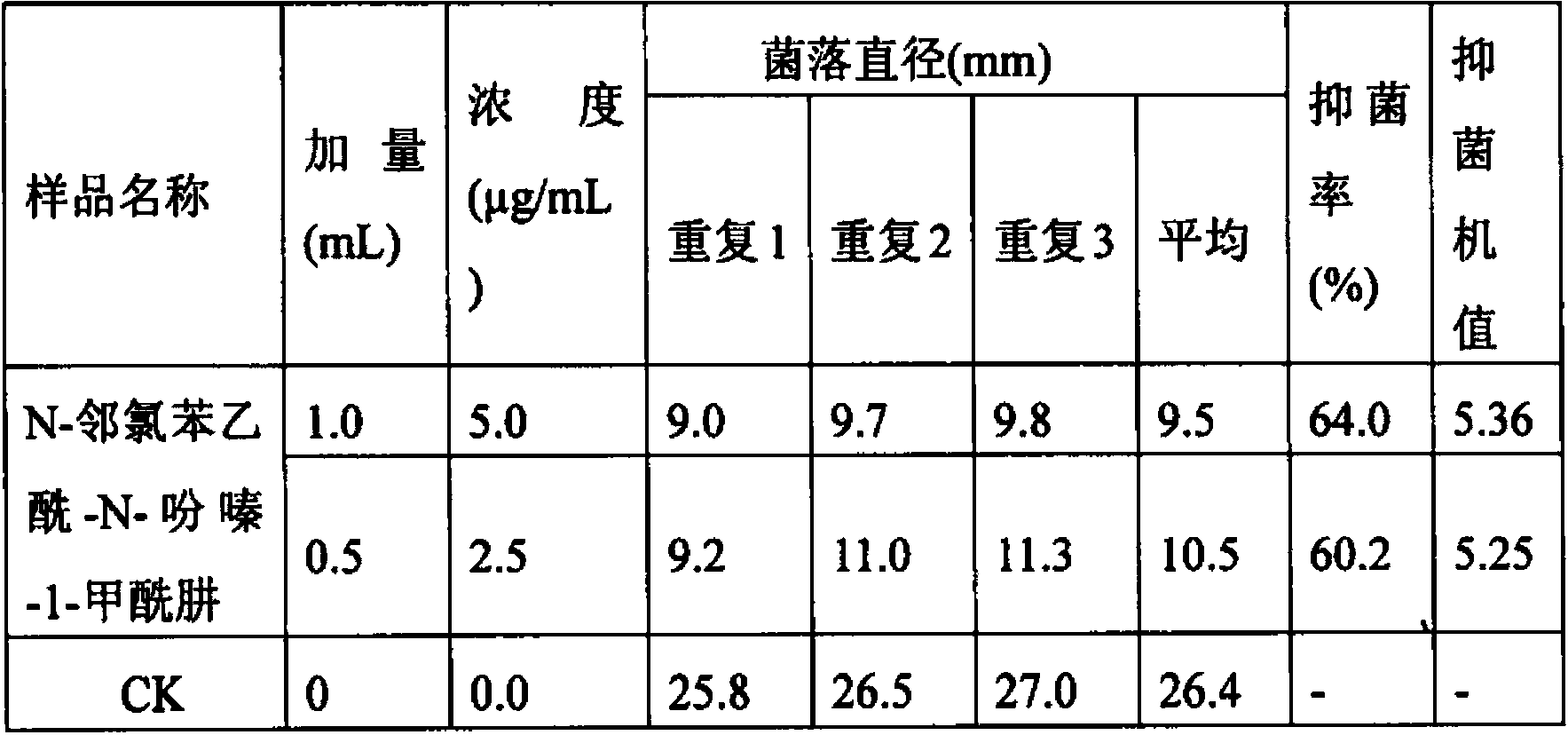
Biological bactericide and preparation method thereof
PendingCN103834704AIncrease concentrationIncrease productionOrganic chemistryMicroorganism based processesTwo stepPseudomonas
The invention relates to a biological bactericide and a preparation method thereof. The biological bactericide is N-o-chlorophenylacetyl-N-phenazine-1-formylhydrazine which is obtained by virtue of two-step fermentation culture of a genetically improved strain SN36 of pseudomonas choloeaphtis in a liquid culture medium; the collection number of the obtained SN36 in the China General Microbiological Culture Collection Center is CGMCC No.7846. Compared with the prior art, the biological fermentation product has remarkable effects on prevention and treatment of fungal diseases of a plurality of crops, and mass production of the biological bactericide can be realized by virtue of introduction.
Owner:河北三农农用化工有限公司
Synthesis method and application of disalicylaldehyde condensation 2-pyridine formylhydrazine schiff base
ActiveCN110204485ANovel structureThe synthesis method is simpleOrganic chemistryFluorescence/phosphorescenceAluminum IonSynthesis methods
The invention relates to a synthesis method and application of disalicylaldehyde condensation 2-pyridine formylhydrazine schiff base, and relates to a synthesis method and application of 5,5'-methylethylidene disalicylaldehyde condensation 2-pyridylformylhydrazine schiff base. The method constructs a novel disalicylaldehyde condensation 2-pyridine formylhydrazine schiff base fluorescent probe which is applied to aluminum ion detection. The preparation method comprises dripping a disalicylaldehyde solution into a 2-pyridine formylhydrazine solution, heating and refluxing to separate out a solid, cooling, filtering by suction, and washing to obtain the fluorescent probe. The detection limit of the probe for aluminum ions in a DMF solution is 6.21* 10<-8> mol / L, the fluorescence intensity isenhanced by 302 times, and the complexing ratio of probe molecules to aluminum ions is 1: 2. The probe has good selectivity and anti-interference performance, and can be widely applied to the analysisand detection of aluminum ions in industries such as environment, food, medicine and the like.
Owner:HARBIN UNIV OF SCI & TECH
Phenoxyl tritriazole compound, preparation method and applications thereof
InactiveCN104402835AHigh reaction yieldHigh purityOrganic chemistryAzo dyesHydrazine compoundAniline
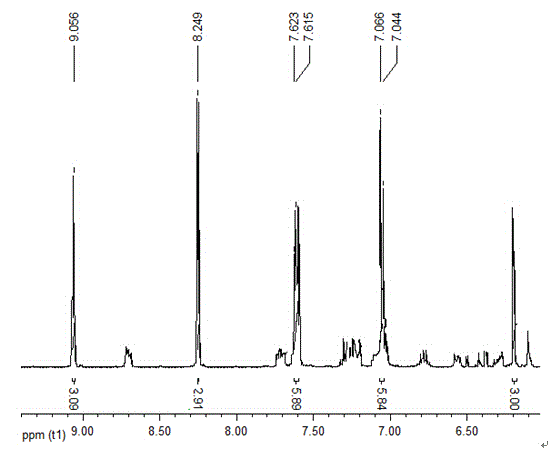
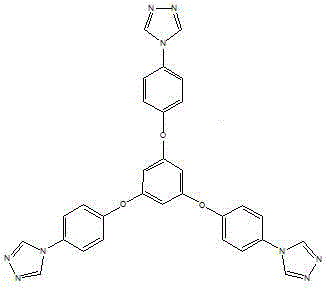
The invention provides a phenoxyl tritriazole compound, a preparation method and applications thereof. The invention discloses a preparation method of 4-(4-(3,5-di(4-(4H-1,2,4-triazole-4-yl)phenoxyl)phenoxyl)phenyl)-4H-1,2,4-triazole. The compound is prepared by a one-pot method, namely heating 4-(3,5-di(4-aminophenoxyl)phenoxyl)aniline and diformyl hydrazine to prepare the compound. The preparation method has the characteristics of simple operation, low production cost, and little environment pollution, and is suitable for massive industrial production. The prepared 4-(4-(3,5-di(4-(4H-1,2,4-triazole-4-yl)phenoxyl)phenoxyl)phenyl)-4H-1,2,4-triazole can be applied to fields of photoelectric materials and luminescent agents.
Owner:TIANJIN NORMAL UNIVERSITY
Synthesis method of phthalazine derivative
InactiveCN102558184AThe synthesis method is simpleEfficient synthesis methodOrganic chemistryDimedonePtru catalyst
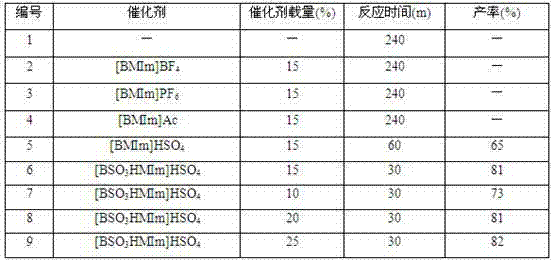
The invention discloses a synthesis method of a phthalazine derivative. In the method, phthalylhydrazine and dimedone or 1,3-cyclohexanedione react with aromatic aldehyde or aliphatic aldehyde in the conditions that the ionic liquid [BSO3HMIm]HSO4 is used as a catalyst, PEG (polyethylene glycol) 600 is used as a solvent and the temperature is 100-160 DEG C; and the phthalazine derivative is synthesized from three components in one pot. The synthesis method has the advantages of low cost, good application prospect and the like, and is efficient, non-toxic and environment-friendly; and the catalyst and solvent are easily recycled and can be recycled.
Owner:SOUTHWEST UNIVERSITY
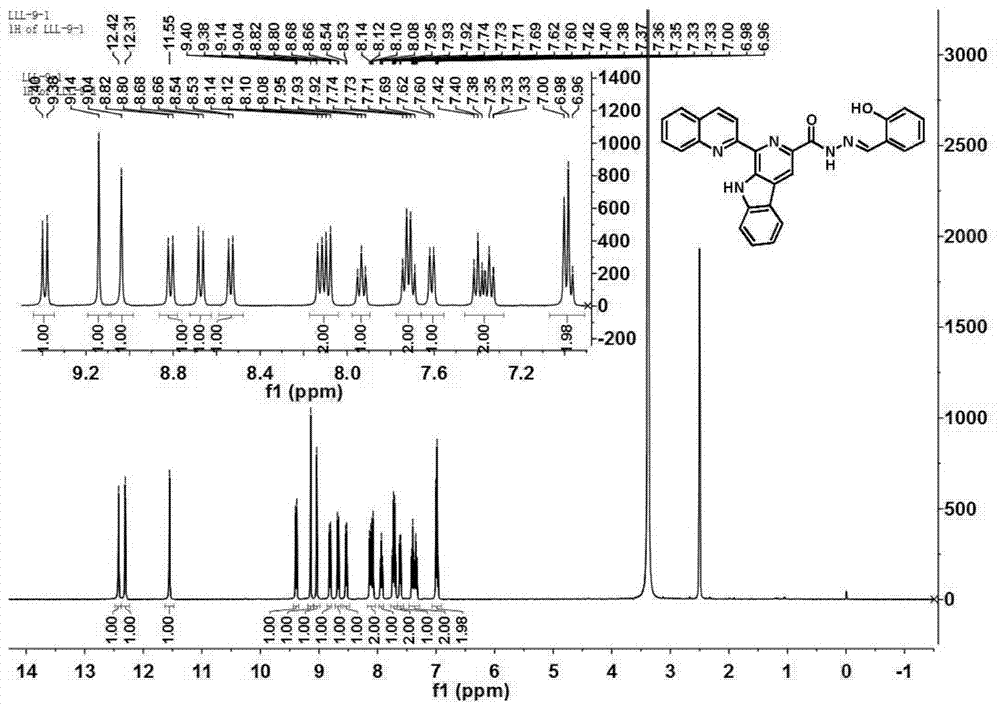
Mercury ion fluorescence probe of benzimidazole [1, 2-a] pyridines derivative and application thereof
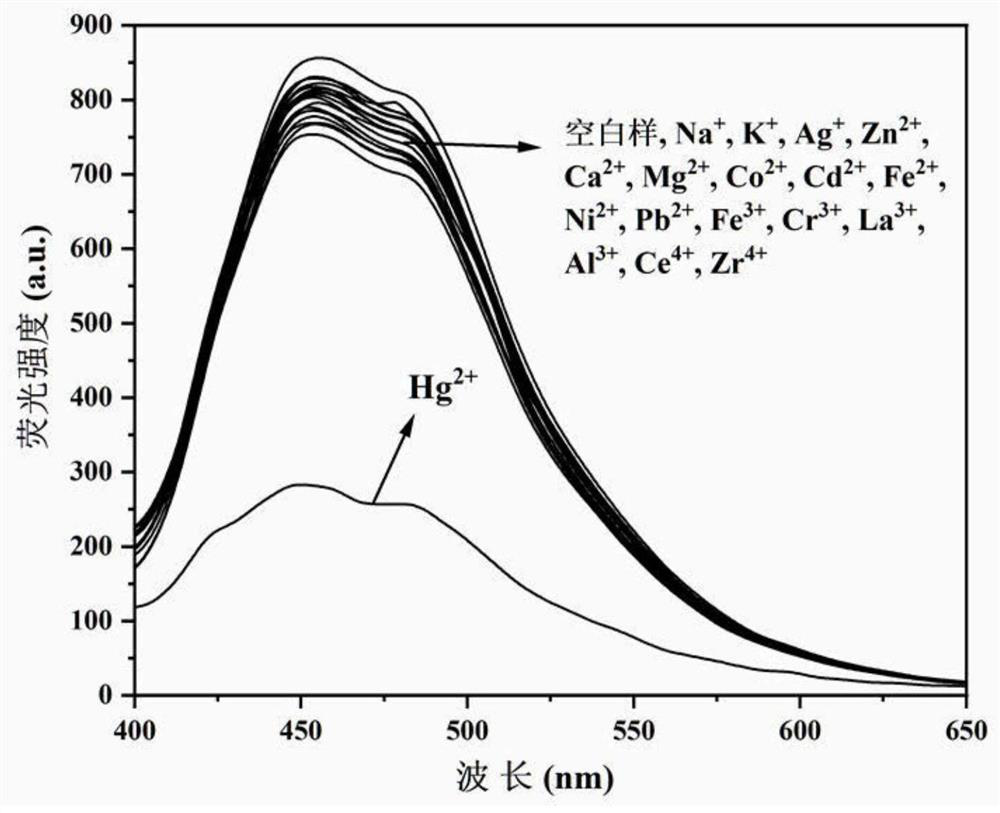
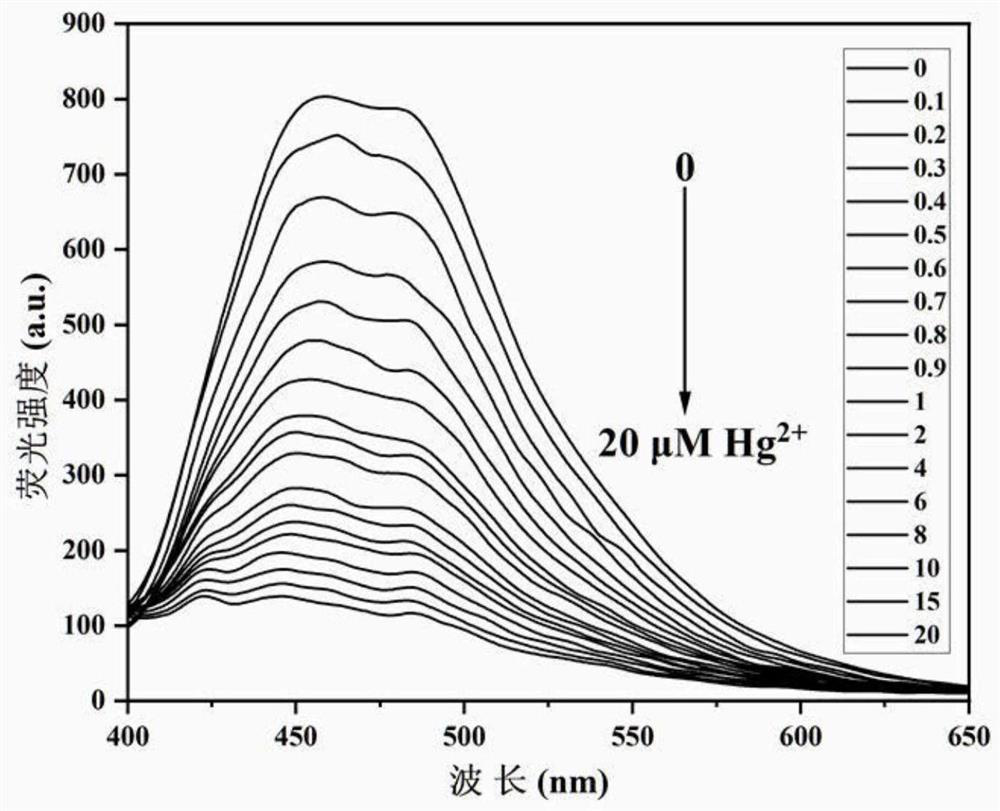
The invention discloses a benzimidazole [1, 2-a] pyridines hydrazine derivative Hg<2+> fluorescence probe, the probe is benzoyl isothiocyanate-substituted 2-phenylbenzimidazole [1, 2-a] pyridine-3-formylhydrazine, and the chemical structural formula is shown in the formula (1). The fluorescence probe has better fluorescence selectivity, higher sensitivity and stronger ability to resist other ion interference for Hg<2+> in a PBS / EtOH(8 / 2 v / v) buffered solution (a pH value equals to 7.40), and has a wide application prospect.
Owner:TAISHAN MEDICAL UNIV
High-sensitivity fluorescent probe for detecting Hg<2+>, preparation method and application thereof
ActiveCN111825655ARapid quenchingOrganic chemistryFluorescence/phosphorescenceFluoProbesFluorescence
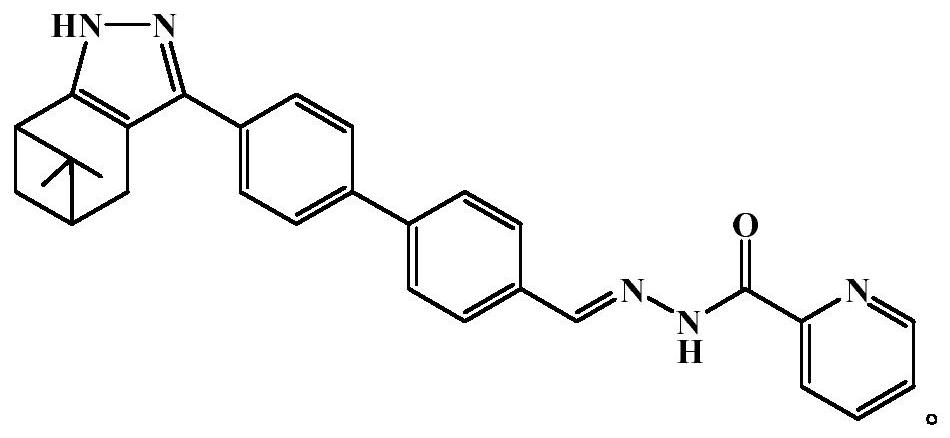
The invention discloses a high-sensitivity fluorescent probe for detecting Hg<2+>, a preparation method and application thereof. The N'-(4'-(6, 6-dimethyl-4, 5, 6, 7-tetrahydro-1H-5, 7-bridge methylene indazole-3-yl)-[1, 1'-biphenyl]-4-methylene)pyridine-2-formylhydrazine is prepared by taking a natural renewable resource beta-pinene derivative nopinone as a raw material. The compound can be selectively complexed with Hg<2+>, cyan fluorescence of the compound is rapidly quenched, the detection limit reaches 17nM, and the compound can be used as a specific fluorescent probe for detecting Hg<2+>and has good application value.
Owner:NANJING FORESTRY UNIV
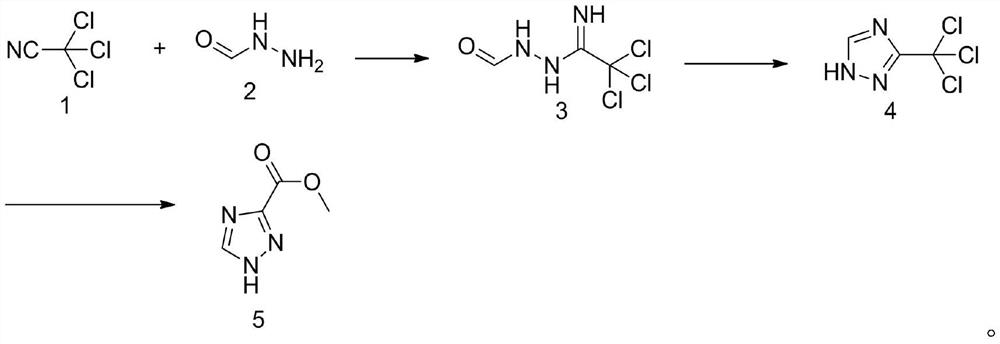
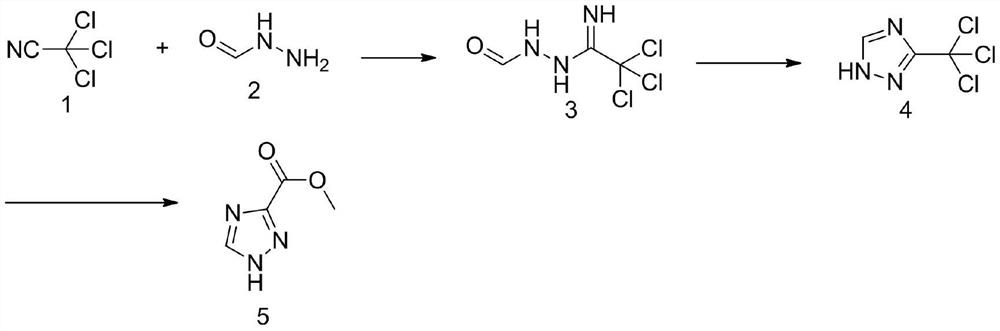
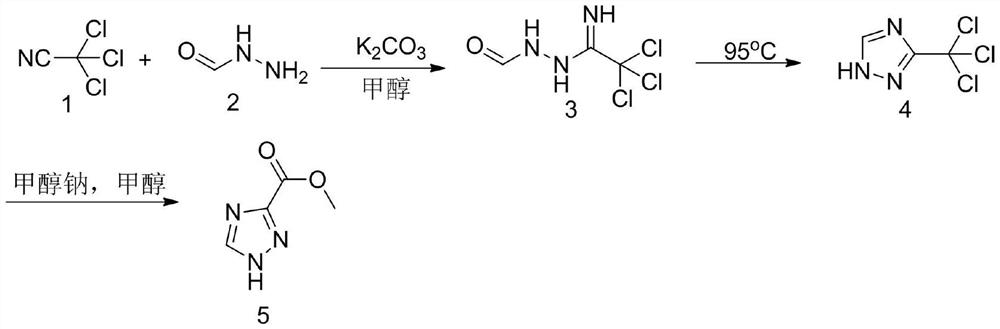
Method for synthesizing 1, 2, 4-triazole-3-carboxylic acid methyl ester
ActiveCN111808034ANovel design routeReduced risk of reactionOrganic chemistryTrichloroacetonitrileMetaclazepam
The invention discloses a method for synthesizing 1, 2, 4-triazole-3-carboxylic acid methyl ester, and belongs to the field of organic chemistry. The method comprises the following reaction steps: reacting trichloroacetonitrile 1 with formylhydrazine 2 to generate an intermediate 3, then carrying out cyclization reaction to obtain an intermediate 4, and finally carrying out alcoholysis reaction togenerate 1, 2, 4-triazole-3-carboxylic acid methyl ester 5. According to the method, only three steps of reaction are needed, the overall yield is high, dangerous diazotization deamination reaction in a traditional synthesis process is avoided, the safety risk in the reaction process is reduced, and meanwhile the production cost can be reduced.
Owner:TUOXIN GROUP +1
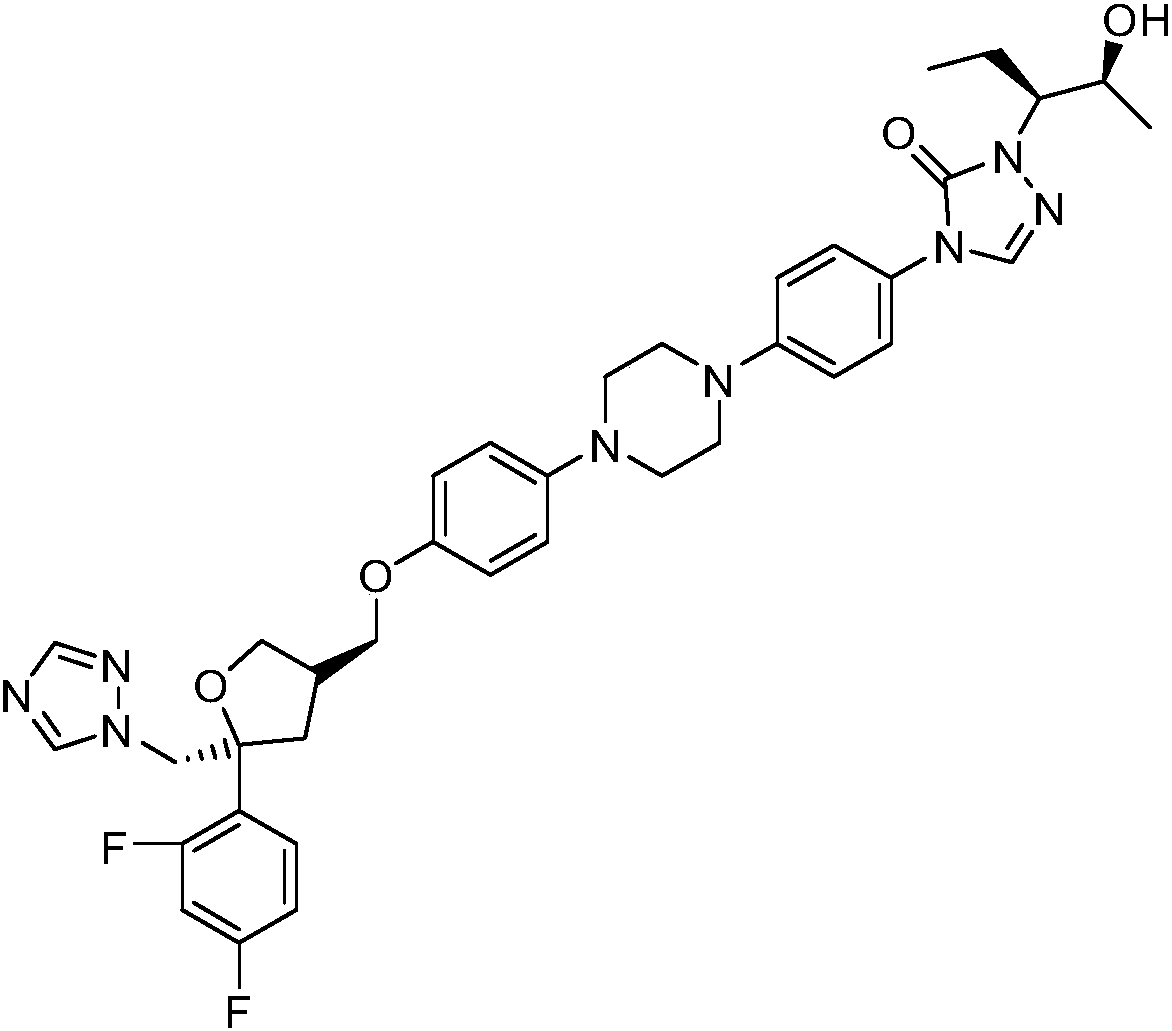
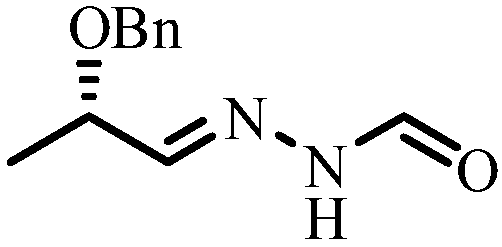
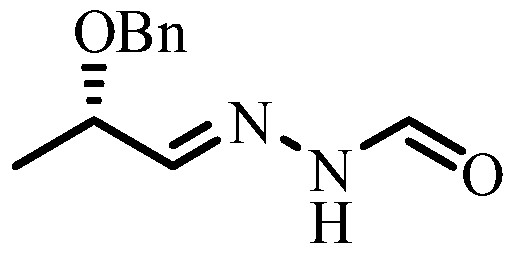
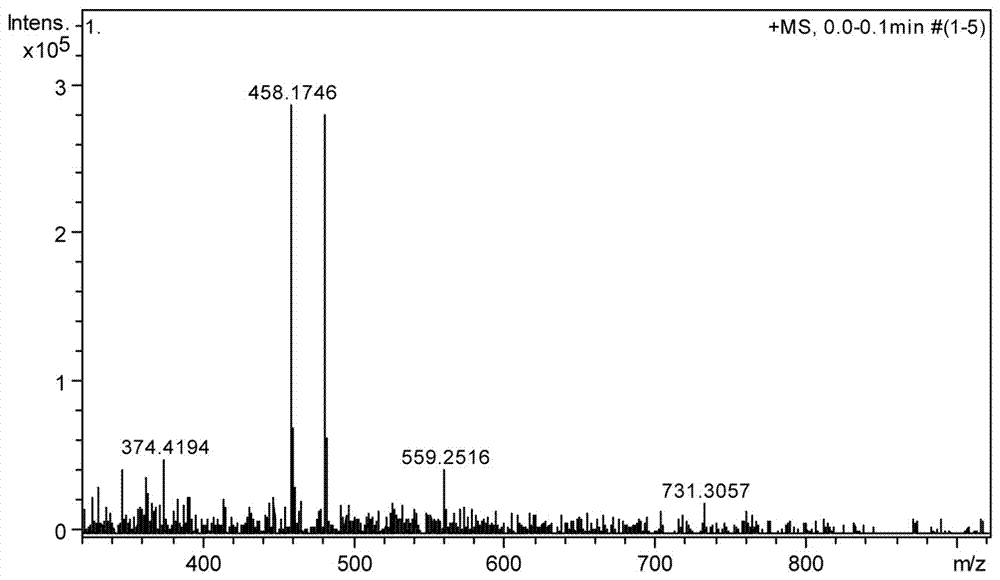
Method for synthesizing N'-[(2S, 3S)-2-(benzyloxy) pentyl-3-base] formylhydrazine
ActiveCN109796368ALower synthesis costReduce manufacturing costHydrazide preparationChemical recyclingPropionyl chlorideHydrogen atmosphere
A method for synthesizing N'-[(2S, 3S)-2-(benzyloxy) pentyl-3-base] formylhydrazine comprises the following steps: reacting (S)-2-benzyloxypropionic acid with an acylation reagent to obtain (S)-2-benzyloxy propionyl chloride; adding a palladium barium sulfate catalyst into o-xylene and reacting in hydrogen atmosphere for 15-30 min; adding (S)-2-benzyloxy propionyl chloride hydrogen atmosphere forreflux reaction until hydrogen is not absorbed; after the reaction is finished, the catalyst is filtered and the o-xylene is removed to obtain (S)-2-benzyloxypropionaldehyde; reacting the (S)-2-benzyloxypropionaldehyde with formylhydrazine, removing the solvent after finishing the reaction, and post-treating to obtain (S)-N'-(2 benzyloxypropyl) formylhydrazine; reacting the (S)-N'-(2 benzyloxypropyl) formylhydrazine with a Grignard reagent, and post-treating to obtain N'-((2s,3s)- 2-(benzyloxy) pentyl-3-base] formylhydrazine. According to the invention, an acylating reagent which is low in price and safer and more environment-friendly in reaction and a palladium barium sulfate catalyst which can be recycled for a plurality of times are used as reaction raw materials, so that the reaction process more conforms to the principle of atom economy, and the reaction is milder.
Owner:NINGBO INST OF TECH ZHEJIANG UNIV ZHEJIANG
Method of synthesizing (S)-N'-(2-benzyloxy propylidene) formylhydrazine
ActiveCN108191703AFew preparation stepsLess side effectsOrganic compound preparationHydrazide preparationEthylenediaminePropanoic acid
The invention discloses a method of synthesizing (S)-N'-(2-benzyloxy propylidene) formylhydrazine. The method comprises the steps of: (1) allowing (S)-2-benzyloxy propionic acid and ethidene diamine to react to form (S)-2-(1-benzyloxy ethyl)-4,5-dihydro-1H-imidazole, (2) dissolving (S)-2-(1-benzyloxy ethyl)-4,5-dihydro-1H-imidazole in absolute ethyl alcohol, adding metallic sodium for stirring andreaction under nitrogen protection, removing alcohol after the reaction, slowly adding residue into a saturated oxalic acid solution under the nitrogen protection, giving a backflow reaction after uniform stirring, performing extraction, drying and filtration after the reaction, and removing a solvent to form (S)-2-benzyloxy propionaldehyde, and (3) allowing (S)-2-benzyloxy propionaldehyde and formylhydrazine to react, removing a solvent after the reaction and performing post treatment to form the product (S)-N'-(2-benzyloxy propylidene) formylhydrazine. The method has the advantages that anorgano-aluminum compound that is high in price and unsafe is not used, the preparation cost is low, the post treatment is simple, and the method is easy and simple to operate.
Owner:东营睿港投资服务有限责任公司
Preparation method and application of m-phenyl-4-substituted-bitriazole compound
InactiveCN104370840AHigh reaction yieldHigh purityOrganic chemistryAzo dyesSimple Organic CompoundsBis triazole
The invention discloses a preparation method and application of an m-phenyl-4-substituted-bitriazole compound, and particularly discloses a preparation method of 4-(3-(4H-1, 2, 4-triazole-4-yl)phenyl)-4H-1, 2, 4-triazole. The organic compound is prepared by using a one-pot method, namely heating m-phenylenediamine and diformylhydrazine. The preparation method disclosed by the invention has the characteristics of simple technological operation, low production cost and little environmental pollution and is suitable for large-scale industrial production. The 4-(3-(4H-1, 2, 4-triazole-4-yl)phenyl)-4H-1, 2, 4-triazole prepared by using the preparation method can be applied to the aspects of photoelectric materials and luminescent agents.
Owner:TIANJIN NORMAL UNIVERSITY
Preparation method of novel acylhydrazone bond gel through one-step cross-linking polymerization
PendingCN114316171ACutting-edge and novel design thinkingThe synthesis method is simplePolymer sciencePolymer chemistry
The invention provides a preparation method of novel acylhydrazone bond gel through one-step cross-linking polymerization. The method comprises the following steps: by taking 1, 3, 5-benzoyl chloride as an initial raw material, firstly reacting with absolute ethyl alcohol to generate triethyl trimellitate, and then reacting triethyl trimellitate with hydrazine hydrate to generate trimesoyl hydrazine. Finally, trimesoyl hydrazine and aldehyde are adopted as organic monomers to construct a module, and according to the Schiff base reaction principle, an acylhydrazone covalent bond is adopted as a cross-linking point, dimethyl sulfoxide is adopted as a solvent medium, and a series of novel acylhydrazone bond polymer gels are rapidly and directly synthesized at room temperature in one step. Compared with an existing preparation method of an acylhydrazone bond gel material, the novel acylhydrazone bond gel is completed through one-step cross-linking polymerization of organic monomers of hydrazide and aldehyde, the process that a gel factor is designed and synthesized firstly and then a cross-linking group is searched for in the existing acylhydrazone bond gel material is omitted, and the design idea of the novel acylhydrazone bond gel is more advanced and novel. The synthesis method disclosed by the invention is simple, low in cost, mild in condition, high in reaction rate and high in yield, and has good development and application potential in the aspect of intelligent materials.
Owner:ZUNYI MEDICAL UNIVERSITY
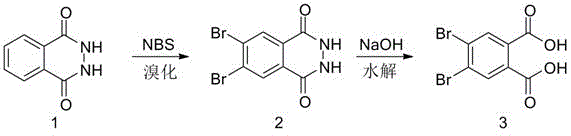
Preparation method of 4,5-dibromophthalic acid
InactiveCN105646176AEnvironmental Concerns for Reducing EmissionsEasy to prepareOrganic compound preparationCarboxylic compound preparationImideAcetic acid
The invention discloses a preparation method of 4,5-dibromophthalic acid. The preparation method comprises the following steps: (1) after dissolving phthalylhydrazine into glacial acetic acid, adding N-bromo-succinimide (NBS), heating the mixture to 80 to 100 DEG C and reacting for 0.5 to 1 hour; after the reaction is finished, cooling the system to a room temperature and pouring the mixture into ice water; separating out solids and filtering to obtain dibromophthalhydrazide and filtrate; (2) dissolving the dibromophthalhydrazide into a sodium hydroxide water solution, and heating the mixture to 60 to 70 DEG C and carrying out a hydrolysis reaction; after the hydrolysis reaction is finished, adjusting the pH of the hydrolysis reaction system to be 6 to 7; separating out white solids, and filtering and washing with water to prepare the 4,5-dibromophthalic acid. The preparation method of the 4,5-dibromophthalic acid has the advantages that the cost is low; products of two steps are both separated out in a solid manner; reaction is easy to treat and the purity of the products is high; industrial requirements on the products can be met completely, and sufficient raw materials are provided for research and development of downstream chemical products and materials.
Owner:ZHENGZHOU UNIVERSITY OF LIGHT INDUSTRY
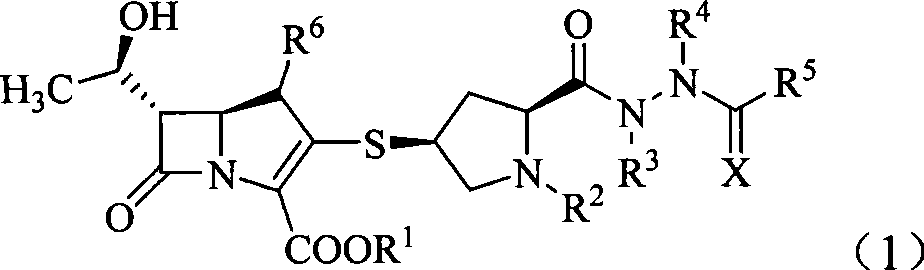
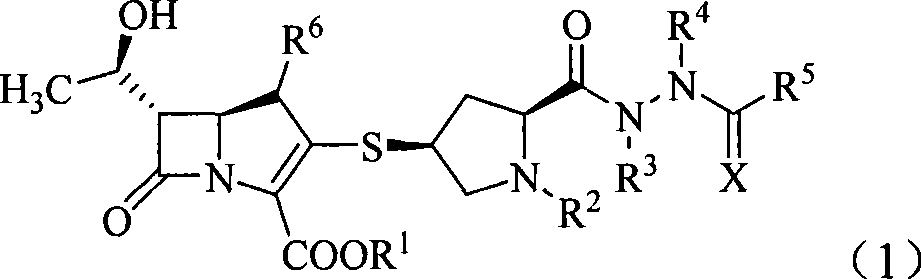
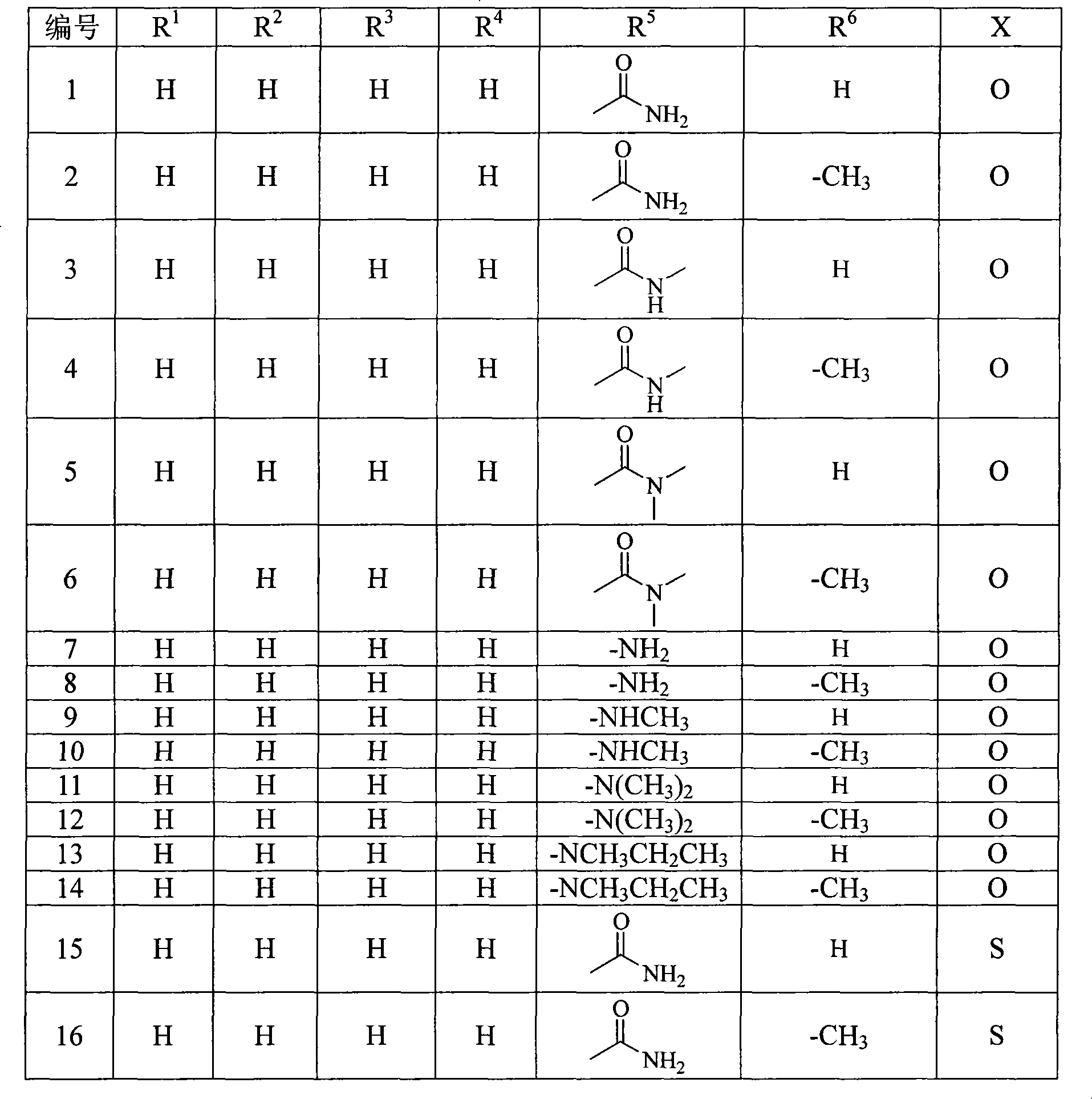
Penem derivant containing sulfhydryl pyrrolidine formhydrazide
ActiveCN101367809AHigh antibacterial activityLow toxicityAntibacterial agentsOrganic active ingredientsDiseaseFormylhydrazine
The present invention belongs to the technical field of medicine, in particular to a penem derivative containing mercaptopyrrolidine formylhydrazine shown in the formula (1) and a pharmaceutically acceptable salt, an easily hydrolyzed ester, an isomer, a hydrate and a hydrate of the ester or the salt thereof: wherein, R<1>, R<2>, R<3>, R<4>, R<5>, R<6> and X are defined in the description. The present invention also relates to preparation methods of the compounds, drug combinations and applications of the compounds in the preparation of drugs for treating and / or preventing infective diseases.
Owner:XUANZHU BIOPHARMACEUTICAL CO LTD
Curable fluoroelastomer composition
Fluoroelastomer compositions comprising fluoroelastomers having copolymerized units of a nitrile-containing cure site monomer are cured with a phthalhydrazide hydrazine or hydroxylamine salt curative.
Owner:EI DU PONT DE NEMOURS & CO
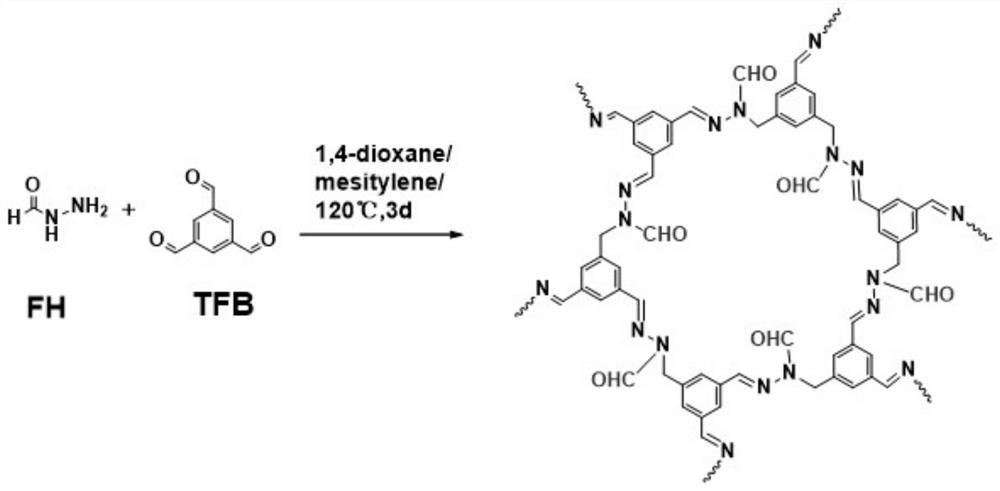
Aldehyde group modified covalent organic framework material, preparation and application thereof
ActiveCN112390924AImprove stabilityLarge specific surface areaProductsGas treatmentAcetic acidPolymer science
The invention particularly relates to preparation of an aldehyde group modified covalent organic frameworks (COFs) material, and the aldehyde group modified covalent organic framework material is applied to gas adsorption. The preparation method comprises the following steps of: firstly, selecting a precursor 1, 3, 5-benzenetricarboxaldehyde (TFB) which not only has good rigidity and is highly symmetrical, but also can react with a bifunctional monomer formylhydrazine (FH) through Schiff base, and taking acetic acid as a catalyst to prepare the novel COF with good performance. The prepared covalent organic framework material not only has relatively good stability and relatively large specific surface area, but also forms a hollow structure with relatively uniform size, and the prepared material can be applied to adsorption of carbon dioxide gas.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Preparation method of o-aminosulfonyl-benzoyl hydrazine
The invention relates to a preparation method of pharmaceutical intermediates, in particular to a preparation method of o-aminosulfonyl-benzoyl hydrazine, belonging to the intermediates preparation field of organic synthesis. The preparation method comprises the following steps of: (1), firstly, determining that the feed ratio of o-sulfonylbenzoylimine to hydrazine hydrate is 1mol to 1-10mol; at the room temperature, adding the o-sulfonylbenzoylimine to a container, adding anhydrous alcohol as a solvent, and stirring to fully mix; and under the ice-bath condition of 5 DEG C below zero-5 DEG C, dropwise adding the hydrazine hydrate while stirring; (2), heating the solution to back flow within 30 minutes when the solution changes clear; and continuously reacting for 1-15 hours at the backflow temperature; and (3), transferring the reaction liquid to a beaker for naturally cooling to the room temperature, standing to separate out granulous white crystals, washing with water and alcohol, and drying to obtain the final product, i.e. o-aminosulfonyl-benzoyl hydrazine. In the invention, the preparation method has the advantages of simple operation, easy control, one-step finished reaction, high purity, innocuity and the like.
Owner:BEIJING INSTITUTE OF TECHNOLOGYGY
Second-order nonlinear laser crystal N, N'-acetyl-(2-thenoyl) hydrazine crystal and preparation method and application thereof
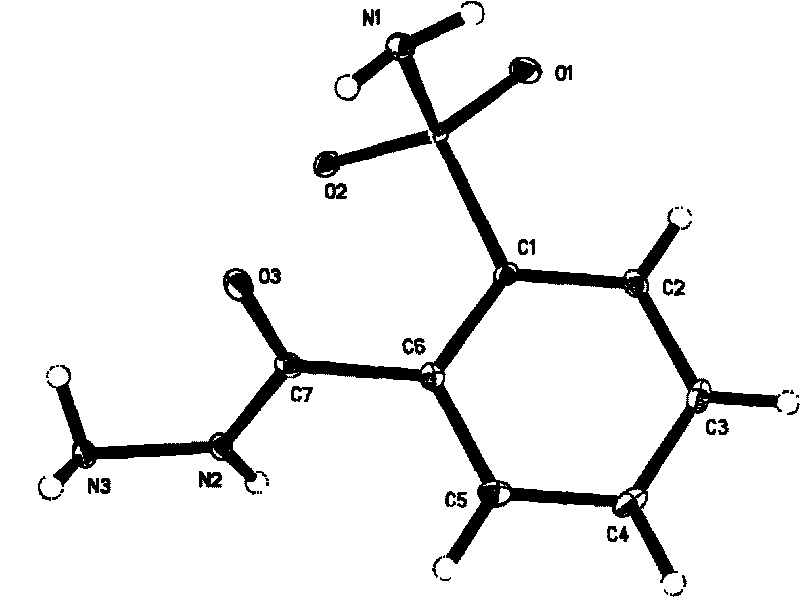
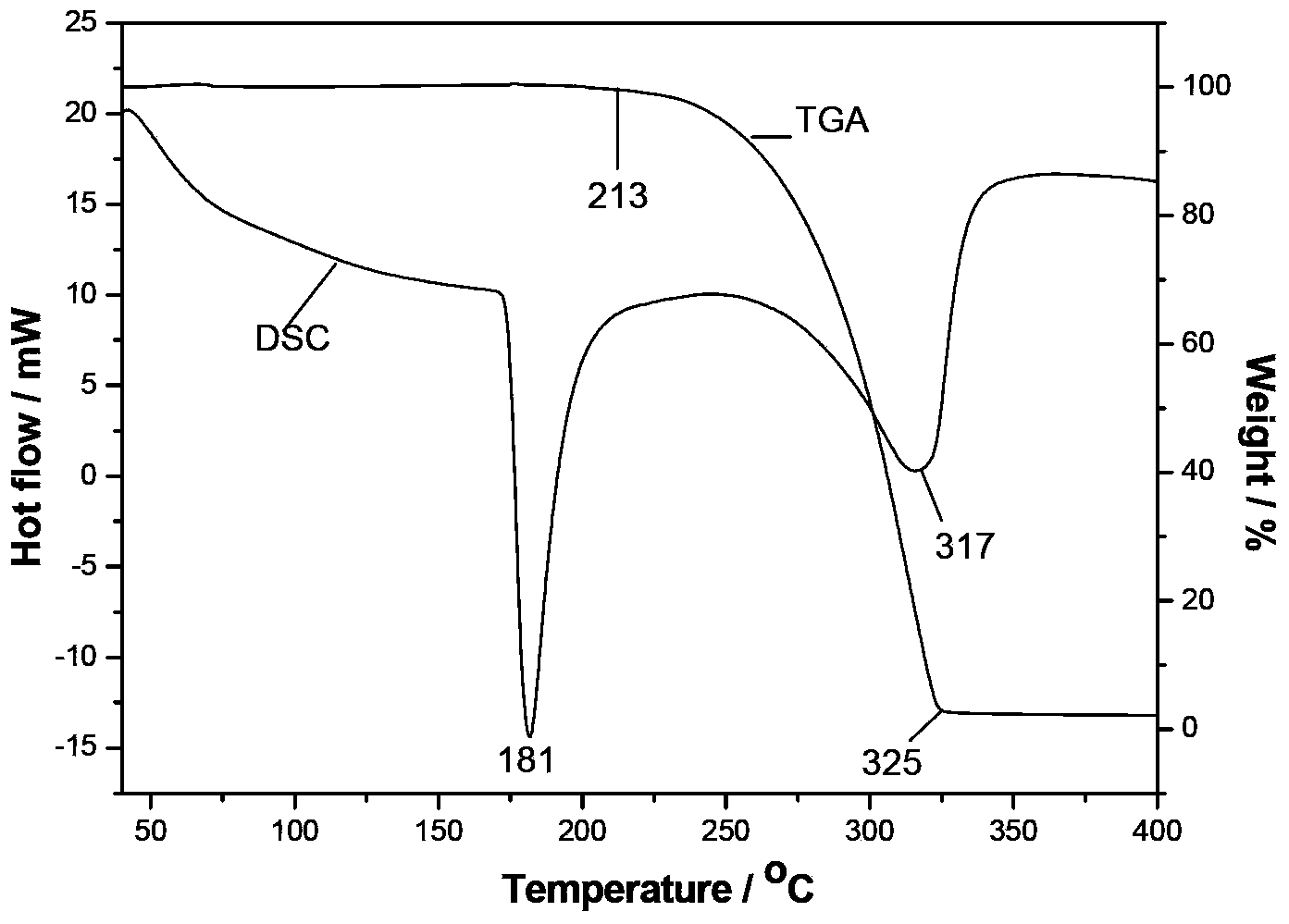
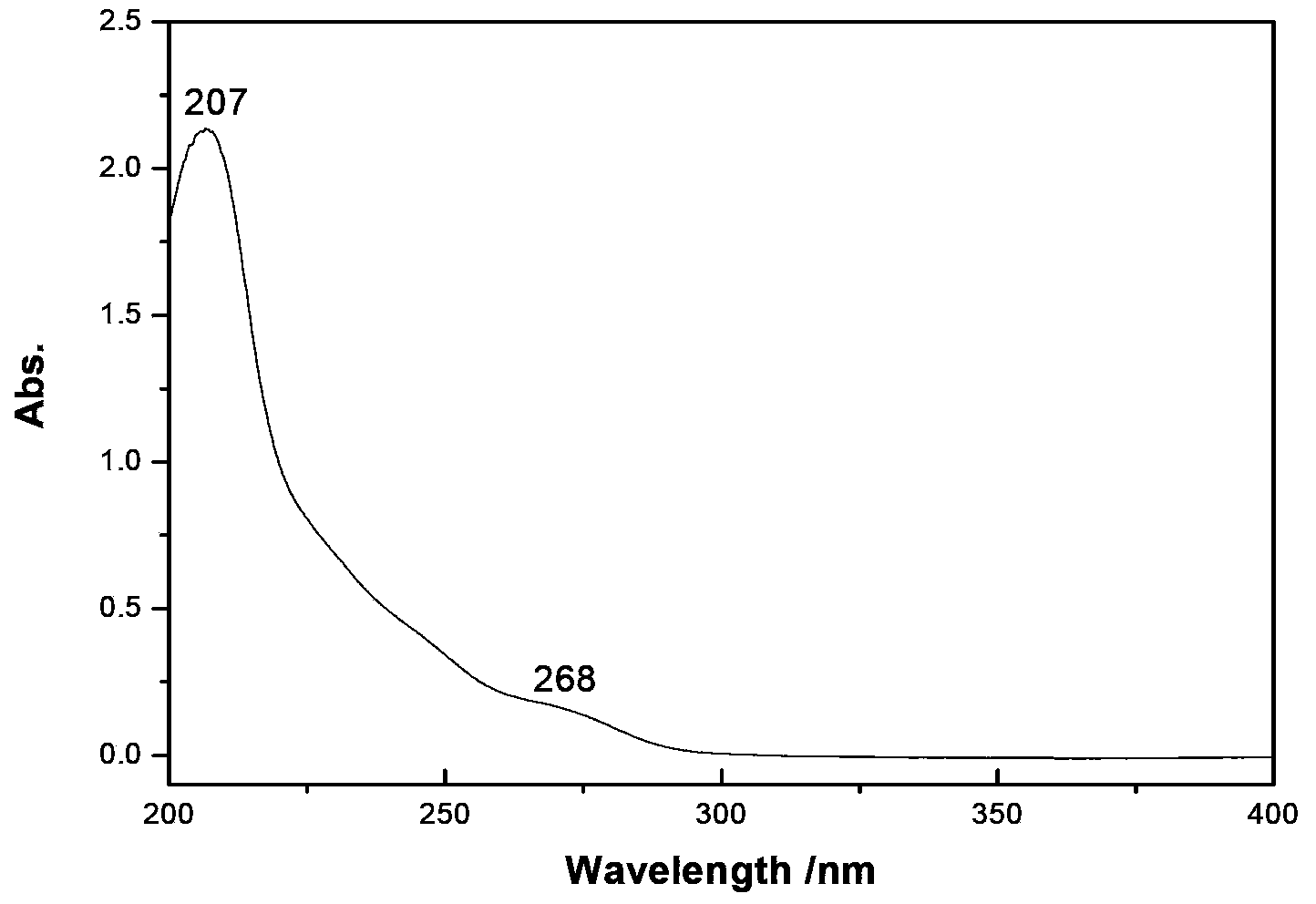
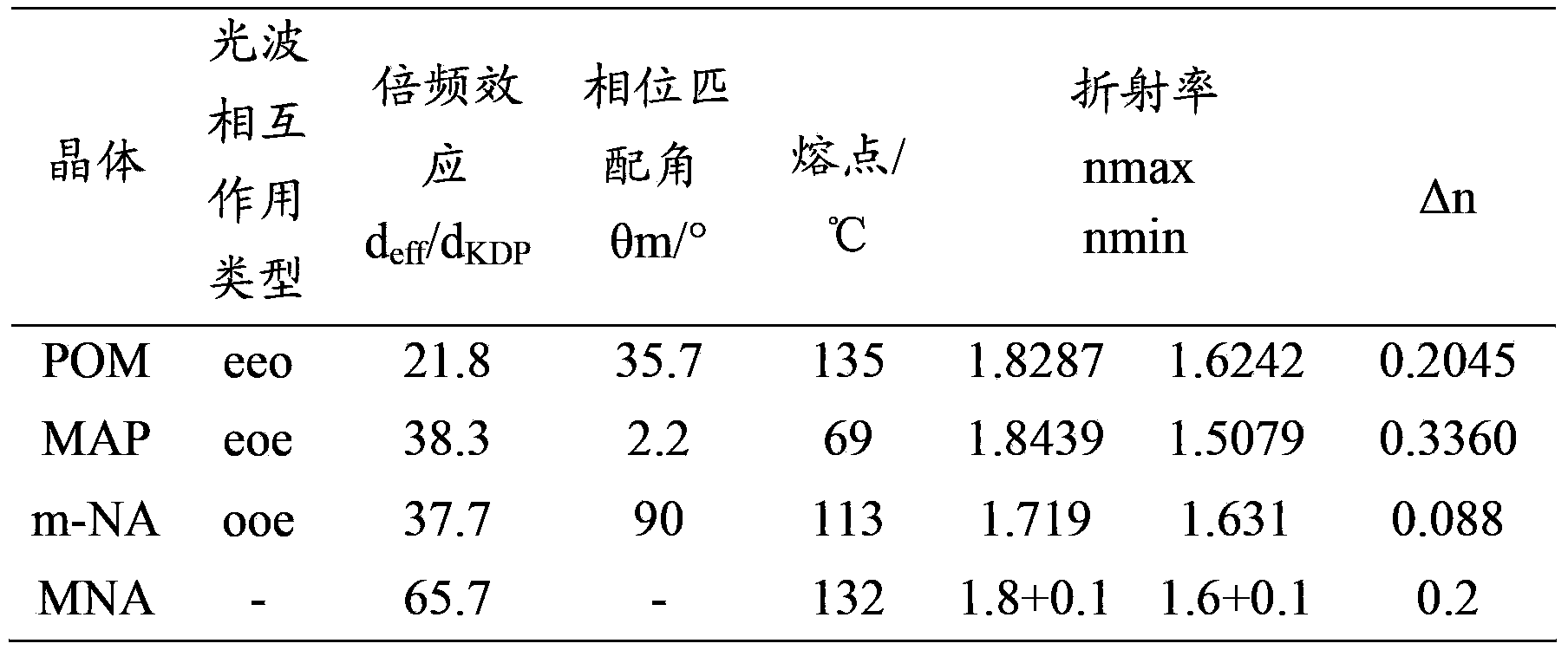
InactiveCN103351378AHigh mechanical strengthImprove thermal stabilityOrganic chemistryNon-linear opticsAlcoholSpace group
The invention provides a second-order nonlinear laser crystal N, N'-acetyl-(2-thenoyl) hydrazine crystal and a preparation method and application thereof. The crystal belongs to the orthorhombic crystal system, the space group is C<cc2>, the molecular formula is C7H8N2O2S, Mr equals to 184.21, the crystallography parameter is as follows: a equals to 14.027 angstroms, b equals to 15.572 angstroms, c equals to 8.1078 angstroms, V equals to 17771.0 cubic angstrom, and Z equals to 8, a molecule of the N, N'-acetyl-(2-thenoyl) hydrazine is formed by the N-(2-thenoyl) hydrazino plane and an acetyl plane which are mutually perpendicular; the preparation method of the hydrazine crystal comprises the following steps: acetyl is introduced onto the N-(2-thenoyl) hydrazine, then the produced N, N'-acetyl-(2-thenoyl) hydrazine is dissolved into the chloroform and methyl alcohol mixed solvent, the ration of chloroform and methyl alcohol both of the chloroform and methyl alcohol mixed solvent is 1 to 1, and the mixture is evaporated till approximate saturation under the room temperature, and is evaporated slowly under the temperature of 10 DEG C so as to obtain the tabular crystal N, N'-acetyl-(2-thenoyl) hydrazine crystal. According to the hydrazine crystal, the melting point of the crystal is high and reaches 180.5-181.5 DEG C, and the application range is wider.
Owner:HUAQIAO UNIVERSITY
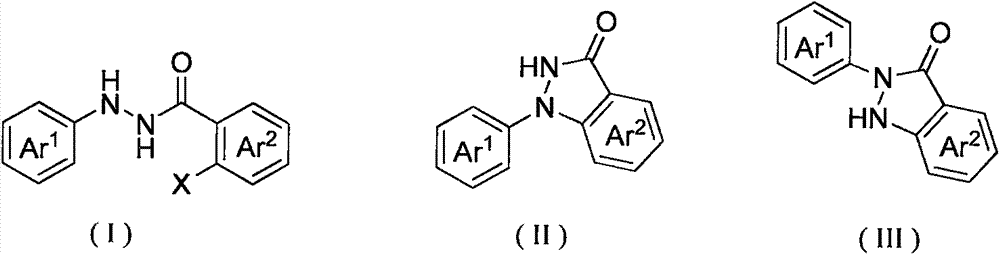
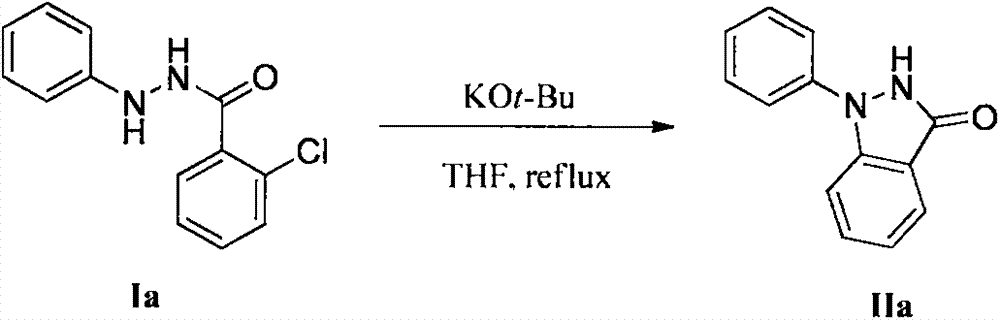
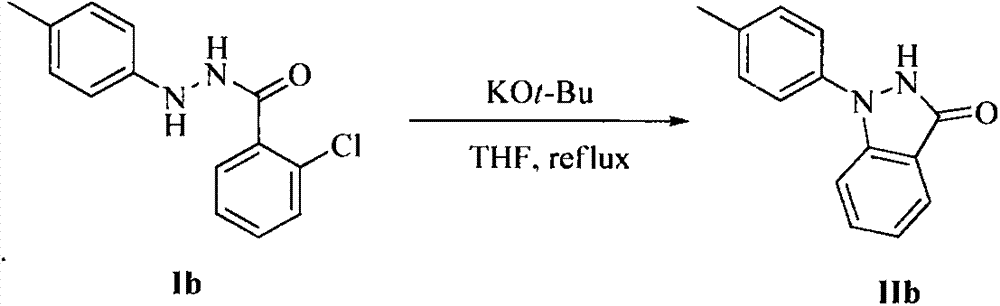
Method for synthesis of indazolone compound
The invention provides a novel method for synthesis of an indazolone compound. The method comprises that an alkali is added into o-halogenated aromatic formylhydrazine as a raw material, and the mixture is heated in a solvent so that a 1-aryl and 2-aryl indazolone compound having a high yield is obtained. The method is free of a transition metal catalyst, is simple in operation, has a high reaction yield and has a very high practical value in industrial preparation of the indazolone compound.
Owner:SUN YAT SEN UNIV
Covalent organic framework material containing oxadiazole connecting element
ActiveCN114085388ASolve problems that are difficult to synthesizeSimple manufacturing methodOrganic-compounds/hydrides/coordination-complexes catalystsHydrogen productionOrganosolvAcyl group
The invention relates to a covalent organic framework material containing an oxadiazole connecting element. The preparation method of the material comprises the following steps: (1) adding aromatic aldehyde, aromatic formylhydrazine, a first organic solvent and an inorganic acid aqueous solution into a Schlenk tube, and reacting the mixture at 100-130 DEG C for 3-7 days to obtain COFs containing an N-acylhydrazone linking element; (2) adding the COFs, copper salt and carbonate into a second organic solvent, and reacting the components at 90-120 DEG C for 3-7 days to obtain the COFs containing the oxadiazole connecting element. The post-synthesis modification strategy of COFs provided by the invention not only widens the absorption range of COFs in a visible light region, but also can better promote the separation and transportation of photon-generated carriers, thereby improving the performance of photocatalytic water production for hydrogen production.
Owner:HEBEI UNIV OF TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com

![Synthetic method of 5-acylbenzo[a]carbazole compound Synthetic method of 5-acylbenzo[a]carbazole compound](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/db47ca27-2c52-4d15-9a24-a2dc97b2b541/BDA0001721156590000011.png)
![Synthetic method of 5-acylbenzo[a]carbazole compound Synthetic method of 5-acylbenzo[a]carbazole compound](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/db47ca27-2c52-4d15-9a24-a2dc97b2b541/BDA0001721156590000021.png)
![Synthetic method of 5-acylbenzo[a]carbazole compound Synthetic method of 5-acylbenzo[a]carbazole compound](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/db47ca27-2c52-4d15-9a24-a2dc97b2b541/BDA0001721156590000051.png)
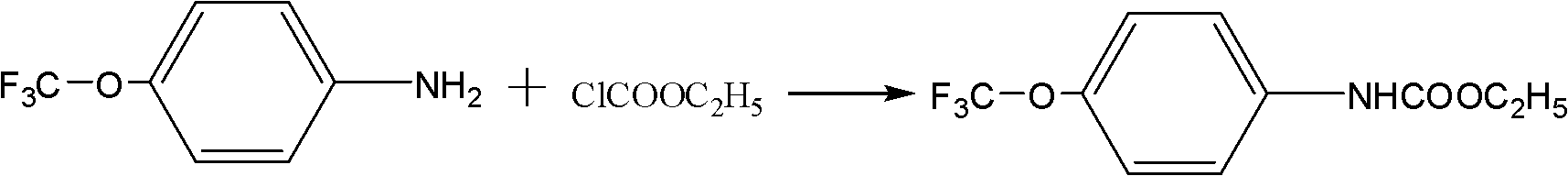
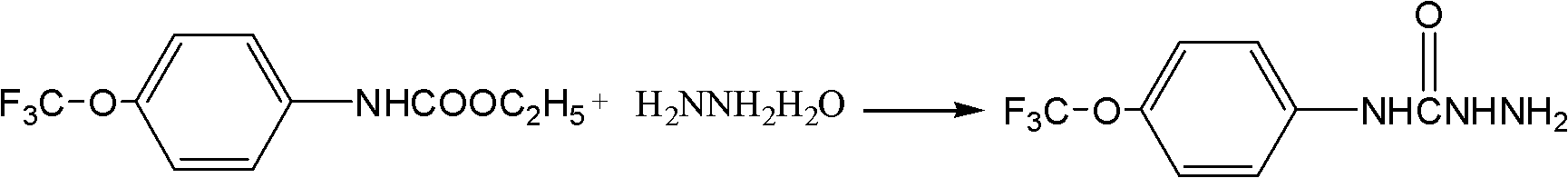




![Mercury ion fluorescence probe of benzimidazole [1, 2-a] pyridines derivative and application thereof Mercury ion fluorescence probe of benzimidazole [1, 2-a] pyridines derivative and application thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/de73db21-bfa6-4a82-b465-9feafd79485d/180525091547.png)
![Mercury ion fluorescence probe of benzimidazole [1, 2-a] pyridines derivative and application thereof Mercury ion fluorescence probe of benzimidazole [1, 2-a] pyridines derivative and application thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/de73db21-bfa6-4a82-b465-9feafd79485d/180525091553.png)
![Mercury ion fluorescence probe of benzimidazole [1, 2-a] pyridines derivative and application thereof Mercury ion fluorescence probe of benzimidazole [1, 2-a] pyridines derivative and application thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/de73db21-bfa6-4a82-b465-9feafd79485d/180525091559.png)
![Method for synthesizing N'-[(2S, 3S)-2-(benzyloxy) pentyl-3-base] formylhydrazine Method for synthesizing N'-[(2S, 3S)-2-(benzyloxy) pentyl-3-base] formylhydrazine](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/4f1e8b7b-cfe3-4c02-8f9d-6273c54e6367/FDA0001926477880000011.png)
![Method for synthesizing N'-[(2S, 3S)-2-(benzyloxy) pentyl-3-base] formylhydrazine Method for synthesizing N'-[(2S, 3S)-2-(benzyloxy) pentyl-3-base] formylhydrazine](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/4f1e8b7b-cfe3-4c02-8f9d-6273c54e6367/BDA0001926477890000011.png)
![Method for synthesizing N'-[(2S, 3S)-2-(benzyloxy) pentyl-3-base] formylhydrazine Method for synthesizing N'-[(2S, 3S)-2-(benzyloxy) pentyl-3-base] formylhydrazine](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/4f1e8b7b-cfe3-4c02-8f9d-6273c54e6367/BDA0001926477890000012.png)