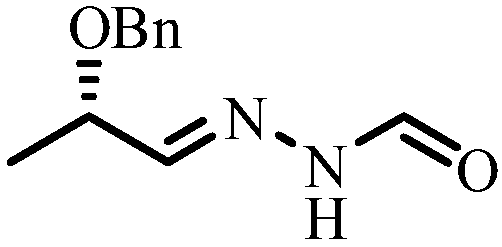
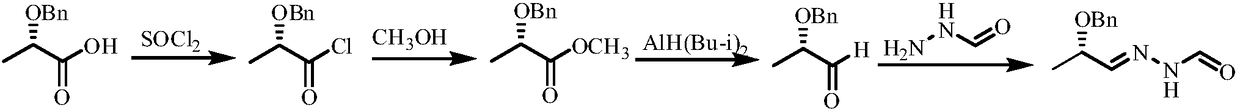Method of synthesizing (S)-N'-(2-benzyloxy propylidene) formylhydrazine
A technology of benzyloxypropylene and benzyloxyethyl, which is applied in the field of synthesis of posaconazole intermediates, can solve the problems of harsh anhydrous conditions, high production risk, and long preparation steps, and achieve less side reactions , less preparation steps, easy to operate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] 1. Add (S)-2-benzyloxypropionic acid (18.02 grams, 0.10mol), ethylenediamine (7.21 grams, 0.12mol) and 150ml toluene to a 250ml round bottom flask equipped with a water separator, and heat to reflux And divide the water. When the separated water reached 1.60ml (0.09mol), the heating was stopped, and the toluene was removed by rotary evaporation, and the resulting reaction mixture was heated to 150°C for two hours under a pressure of 10 mm Hg to obtain (S)-2-(1-benzyl Oxyethyl)-4,5-dihydro-1H-imidazole 17.77 g (0.087mol), yield 87%; (S)-2-(1-benzyloxyethyl)-4,5-dihydro The NMR data of -1H-imidazole are: 1H NMR (400MHz, CDCl3): δ=1.19(d, 3H), 2.77(t, 2H), 3.41(t, 2H), 3.92(q, 1H), 4.49(s , 2H), 6.97(s, 1H), 7.27~7.35(br, 5H); HRMS(ESI) calcd for C12H16N2O[M+H]+205.1336, found 205.1358; fully confirmed (S)- The structure of 2-(1-benzyloxyethyl)-4,5-dihydro-1H-imidazole is consistent.
[0030] 2. Dissolve 20.43 g (0.10 mol) of (S)-2-(1-benzyloxyethyl)-4,5-dihydro-1H-imid...
Embodiment 2
[0033] 1. Add (S)-2-benzyloxypropionic acid (0.10mol), ethylenediamine (0.13mol) and 160ml toluene into a 250ml round bottom flask equipped with a water separator, heat to reflux and separate water. When the separated water reached 1.70ml, the heating was stopped, and the toluene was removed by rotary evaporation, and the resulting reaction mixture was heated to 150° C. for two hours under a pressure of 10 mm Hg to obtain (S)-2-(1-benzyloxyethyl )-4,5-dihydro-1H-imidazole 17.36 g, yield 85%; (S)-2-(1-benzyloxyethyl)-4,5-dihydro-1H-imidazole NMR data is : 1H NMR (400MHz, CDCl3): δ=1.19(d, 3H), 2.77(t, 2H), 3.41(t, 2H), 3.92(q, 1H), 4.49(s, 2H), 6.97(s, 1H), 7.27~7.35 (br, 5H); HRMS (ESI) calcd forC12H16N2O[M+H]+205.1336, found 205.1358; fully confirmed (S)-2-(1-benzyloxyethyl base)-4,5-dihydro-1H-imidazole structure is consistent.
[0034] 2. Dissolve 0.10mol of (S)-2-(1-benzyloxyethyl)-4,5-dihydro-1H-imidazole in 100ml of absolute ethanol, add metal sodium wire, 0.30mol unde...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com