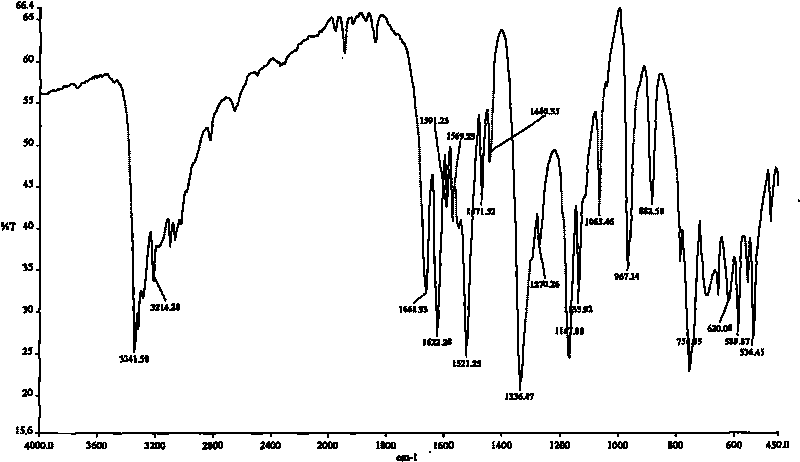
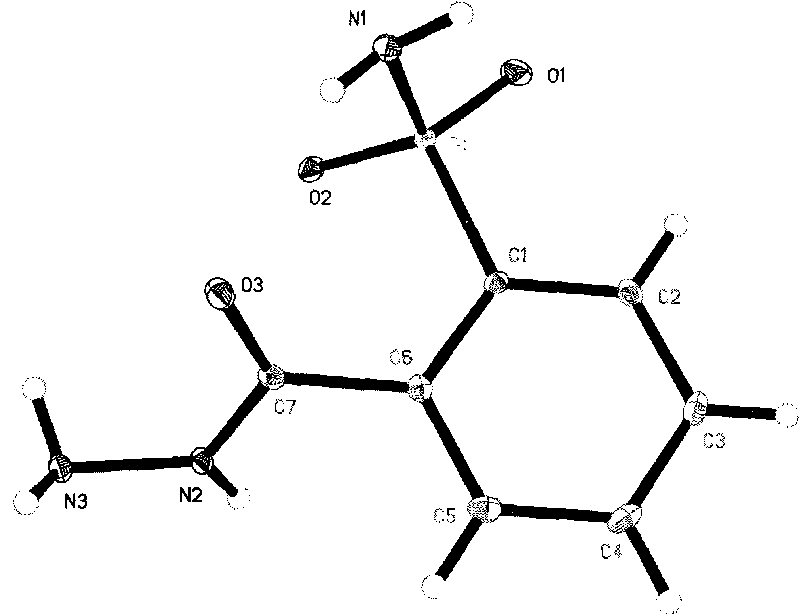
Preparation method of o-aminosulfonyl-benzoyl hydrazine
A technology of o-aminosulfonylbenzoyl hydrazide and aminosulfonylbenzoyl hydrazide, applied in the field of intermediate preparation of organic synthesis, can solve the problem that o-methoxycarbonylbenzenesulfonamide is not easy to obtain, the solvent is expensive, and the reaction steps are cumbersome and other problems, to achieve the effect of easy control, simple operation and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] This example is to illustrate the preparation method of o-aminosulfonylbenzohydrazide of the present invention.
[0024] (1) Suspend 1 mole of saccharin in 200 milliliters of absolute ethanol, and add 3 moles of 30% hydrazine hydrate dropwise under stirring at -5 to 5°C in an ice bath, and the addition is completed within 10 minutes;
[0025] (2) be warming up to reflux within 30 minutes; Continue reaction 5 hours under the reflux temperature;
[0026] (3) Transfer the reaction solution to a beaker, cool naturally to room temperature, let stand, and separate out granular white crystals, wash with water, wash with alcohol, and dry to obtain 0.65 moles of o-aminosulfonylbenzohydrazide of the present invention.
[0027] Test melting point: 195°C.
Embodiment 2
[0029] This example is to illustrate the preparation method of o-aminosulfonylbenzohydrazide of the present invention.
[0030] (1) Suspend 1 mole of saccharin in 200 milliliters of anhydrous methanol, and add 1 mole of 80% hydrazine hydrate dropwise under stirring under an ice-bath condition of -5 to 0°C, and the addition is completed within 10 minutes;
[0031] (2) Warming up to reflux in 30 minutes; Continue to react for 6 hours at the reflux temperature;
[0032] (3) The reaction solution was transferred to a beaker, cooled naturally to room temperature, left to stand, and granular white crystals were precipitated, washed with water, washed with alcohol, and dried to obtain 0.6 moles of o-aminosulfonylbenzohydrazide of the present invention.
Embodiment 3
[0034] This example is to illustrate the preparation method of o-aminosulfonylbenzohydrazide of the present invention.
[0035] (1) Take 1 mole of saccharin and suspend it in 200 milliliters of anhydrous isopropanol, and add 5 moles of 50% hydrazine hydrate dropwise under stirring under an ice bath condition of 0 to 5°C, under nitrogen protection, and complete the dropwise addition within 10 minutes;
[0036] (2) be warming up to reflux in 10 minutes; Continue reaction 15 hours under the reflux temperature;
[0037] (3) The reaction solution was transferred to a beaker, cooled naturally to room temperature, left to stand, and granular white crystals were precipitated, washed with water, washed with alcohol, and dried to obtain 0.7 moles of o-aminosulfonylbenzohydrazide of the present invention.
[0038] Test melting point: 195°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com