Preparation method and application of m-phenyl-4-substituted-bitriazole compound
A technology of triazole compound and triazole nucleus, applied in chemical instruments and methods, organic chemistry, azo dyes, etc., can solve the problem of high cost, achieve low production cost, suitable for large-scale industrial production, and large profit margins. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
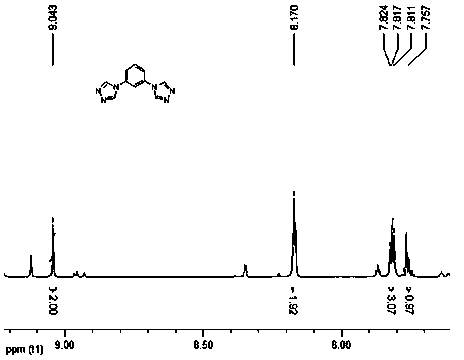
[0024] The molar ratio of m-phenylenediamine to bisformylhydrazide is 1:2.
[0025] Add m-phenylenediamine (1 mmol) and bisformylhydrazide (2 mmol) respectively into a 50 mL three-necked round-bottomed flask equipped with a magnet, reflux condenser, and thermometer, start stirring at 160 °C, and react for 12 hours. After the reaction was completed, the reaction solution was cooled to room temperature, and a large amount of precipitate was precipitated, which was recrystallized with water and ethanol, and the yield was 86%. Elemental Analysis C 10 h 8 N 6 Theoretical: C, 56.60; H, 3.80; N, 39.60. Experimental values: C, 56.56; H, 3.75; N, 39.56.
[0026] Preparation:
[0027] The molar ratio of m-phenylenediamine to bisformylhydrazide is 1:2.
[0028] Add m-phenylenediamine (1 mmol) and bisformylhydrazide (2 mmol) respectively into a 50 mL three-necked round-bottom flask equipped with a magnet, reflux condenser and thermometer, and start stirring at 160 ° C for 12 hours. ...
Embodiment 3
[0030] The luminescent agent has fluorescent properties; the specific method steps are as follows:
[0031] Scan the excitation wavelength and emission wavelength of the compound (Example 1) with a spectrofluorometer to select and determine the optimum wavelength.
[0032] Conclusion: The excitation and emission wavelengths of this compound are 375 nm and 550 nm, respectively.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com