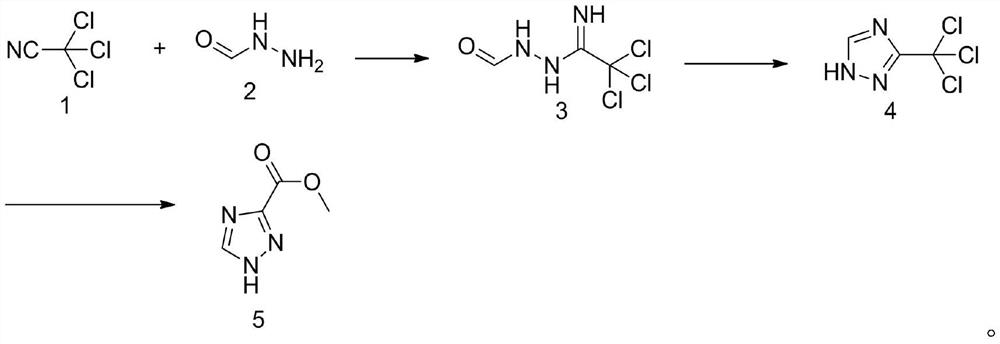
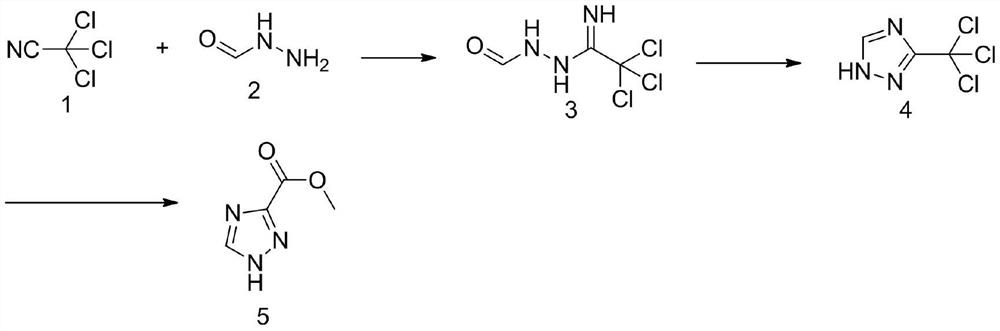
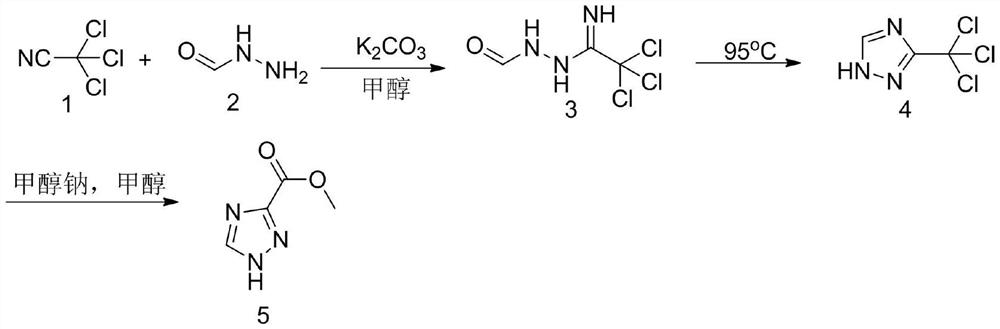
Method for synthesizing 1, 2, 4-triazole-3-carboxylic acid methyl ester
A technology of methyl carboxylate and triazole, which is applied in the synthesis of five-membered nitrogen-containing heterocycles and the field of synthesizing 1,2,4-triazole-3-methyl carboxylate, can solve the problem of difficult to achieve and impossible to carry out. Problems such as industrial production and environmental protection problems of diethyl oxalate method are difficult to deal with
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028]
[0029] In the first step, add 200mL of methanol into the reaction bottle, cool down to 0°C and add 1.4g (0.01mol) of potassium carbonate, then keep the temperature of the reaction solution at 0-5°C, and slowly drop in 28.9g (0.2mol) of trichloroacetonitrile After the dropwise addition, the reaction was incubated for 20 minutes, and then a methanol solution of 12.0 g (0.2 mol) of formic hydrazide was added dropwise. After the dropwise addition, the temperature was raised to 25°C for 4 hours. At this time, a large amount of white solids precipitated out. The temperature was lowered to 10°C for suction filtration, and the filter cake was rinsed with a small amount of methanol. The collected filter cake was dried under reduced pressure to obtain 36.5 g (0.180 mol) of the addition intermediate 3. The yield is 90%.
[0030] In the second step, add the above-mentioned addition intermediate 3 (0.18mol) into the three-necked reaction flask, raise the temperature to 95°C unt...
Embodiment 2
[0033]
[0034] In the first step, add 200mL of methanol into the reaction bottle, cool down to 0°C and add 3.3g (0.01mol) of cesium carbonate, then keep the temperature of the reaction solution at 0-5°C, and then slowly drop in 28.9g (0.2mol) of trichloroacetonitrile After the dropwise addition, the reaction was incubated for 20 minutes, and then a methanol solution of 12.0 g (0.2 mol) of formic hydrazide was added dropwise. After the dropwise addition, the temperature was raised to 25°C for 4 hours. At this time, a large amount of white solids precipitated out. The temperature was lowered to 10°C for suction filtration, and the filter cake was rinsed with a small amount of methanol. The collected filter cake was dried under reduced pressure to obtain 37.5 g (0.181 mol) of the addition intermediate 3. The yield is 91%.
[0035] In the second step, add the above-mentioned addition intermediate 3 (0.181mol) into the three-necked reaction flask, raise the temperature to 95°C ...
Embodiment 3
[0038]
[0039] In the first step, add 200mL of ethanol to the reaction bottle, cool down to 0°C and add 1.4g (0.01mol) of potassium carbonate, then keep the temperature of the reaction solution at 0-5°C, and then slowly drop in 28.9g (0.2mol) of trichloroacetonitrile After the dropwise addition, the reaction was incubated for 20 minutes, and then an ethanol solution of 12.0 g (0.2 mol) of formic hydrazide was added dropwise. After the dropwise addition, raise the temperature to 25°C and react for 4 hours. At this time, a large amount of white solid precipitates out. Cool down to 10°C and filter with suction. The filter cake is rinsed with a small amount of ethanol. The collected filter cake is dried under reduced pressure to obtain 35.5 g (0.175 mol) of the addition intermediate 3. The yield is 87.5%.
[0040] In the second step, add the above-mentioned addition intermediate 3 (0.175mol) into the three-necked reaction flask, raise the temperature to 95°C until the solid me...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com