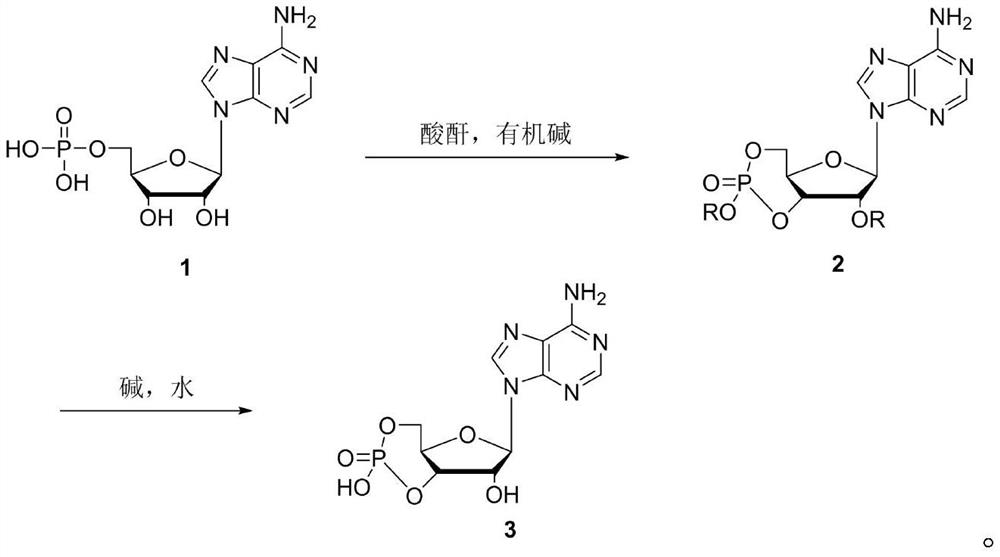
A method for synthesizing cyclic adenosine monophosphate
A technology for cyclic adenosine monophosphate and adenosine acid, which is applied in the field of synthesizing cyclic adenosine monophosphate, can solve the problems of solvent waste and unsuitability for industrial production, and achieve the effects of shortened steps, novel design routes, and simple operation processes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
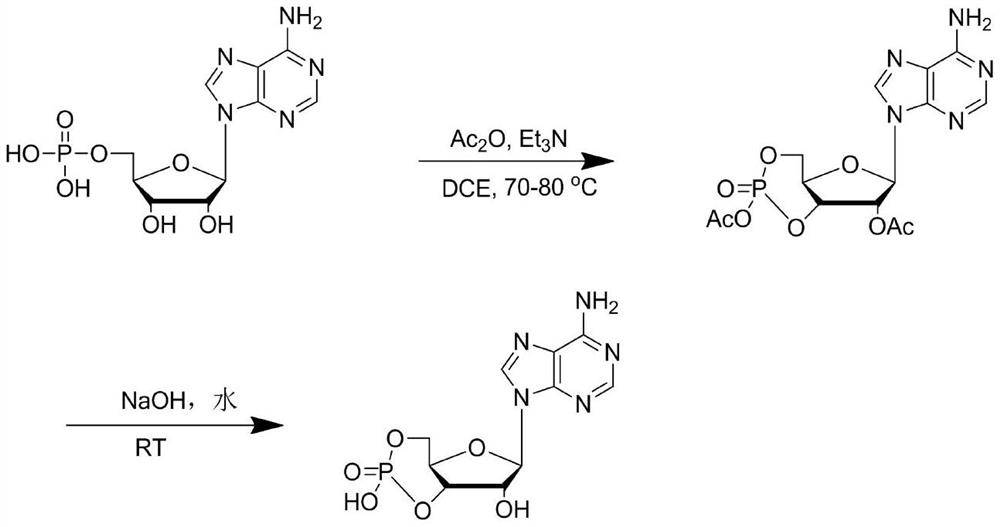
Embodiment 1
[0025]
[0026] In the first step, add 173.5g (0.5mol) of adenosine acid, 108g (1.05mol) of acetic anhydride, 111g (1.1mol) of triethylamine, and 500mL of dichloroethane into the reactor, and heat up to 70°C to 80°C for reaction After 4-6 hours, the liquid phase was tracked until the raw materials disappeared, and the unreacted adenylic acid was removed by cooling and filtering, and the filtrate was concentrated to obtain the crude acetyl-protected cyclic adenosine monophosphate, whose molecular weight was confirmed by LC-MS to be consistent with the described intermediate.
[0027] In the second step, in the reaction kettle, add crude acetyl-protected cyclic adenosine monophosphate, 100mL of water, dropwise add 250mL of 20% sodium hydroxide aqueous solution, control the temperature at 20-25 degrees, keep the reaction for 5 hours after the addition is completed, and follow the reaction in the liquid phase to The intermediate disappeared, the reaction liquid was adjusted to p...
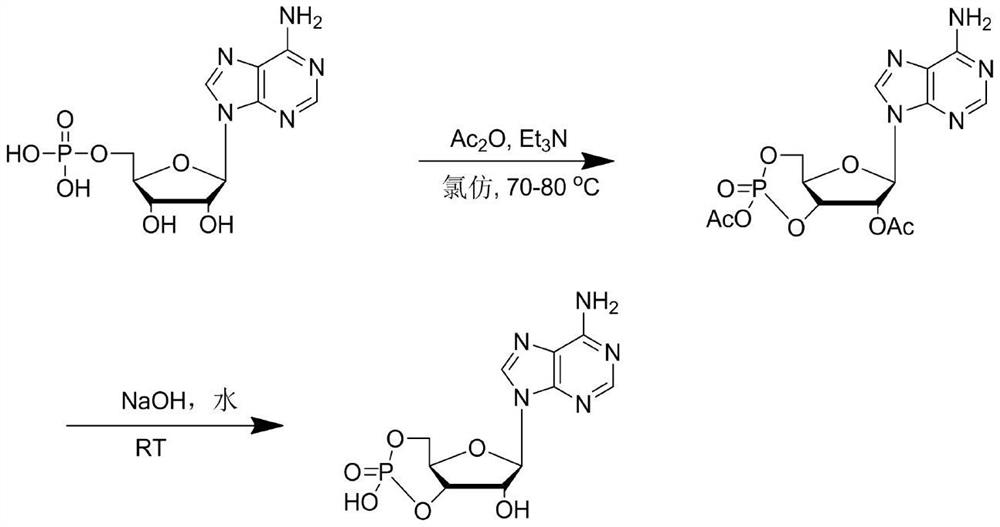
Embodiment 2
[0029]
[0030] In the first step, add 173.5g (0.5mol) of adenosine acid, 108g (1.05mol) of acetic anhydride, 111g (1.1mol) of triethylamine, and 500mL of chloroform into the reactor, and heat up to 70°C to 80°C for 4-6h , the liquid phase was tracked until the raw materials disappeared, the temperature was lowered and filtered to remove unreacted adenosine, and the filtrate was concentrated to obtain the crude acetyl-protected cyclic adenosine monophosphate.
[0031] In the second step, in the reaction kettle, add crude acetyl-protected cyclic adenosine monophosphate, 100mL of water, dropwise add 250mL of 20% sodium hydroxide aqueous solution, control the temperature at 20-25 degrees, keep the reaction for 5 hours after the addition is completed, and follow the reaction in the liquid phase to The intermediate disappeared, the reaction solution was adjusted to pH=7, concentrated under reduced pressure until the remaining 80mL of the reaction solution was adjusted to pH=4 to ...
Embodiment 3
[0033]
[0034] In the first step, add 173.5g (0.5mol) of adenosine acid, 108g (1.05mol) of acetic anhydride, 111g (1.1mol) of triethylamine, and 500mL of acetonitrile into the reactor, and heat up to 70°C to 80°C for 4-6h , the liquid phase was tracked until the raw materials disappeared, the temperature was lowered and filtered to remove unreacted adenosine, and the filtrate was concentrated to obtain the crude acetyl-protected cyclic adenosine monophosphate.
[0035] In the second step, in the reaction kettle, add crude acetyl-protected cyclic adenosine monophosphate, 100mL of water, dropwise add 250mL of 20% sodium hydroxide aqueous solution, control the temperature at 20-25 degrees, keep the reaction for 5 hours after the addition is completed, and follow the reaction in the liquid phase to The intermediate disappears, the reaction solution is adjusted to pH=7, concentrated under reduced pressure until the remaining 80mL of the reaction solution is adjusted to pH=4 to p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com