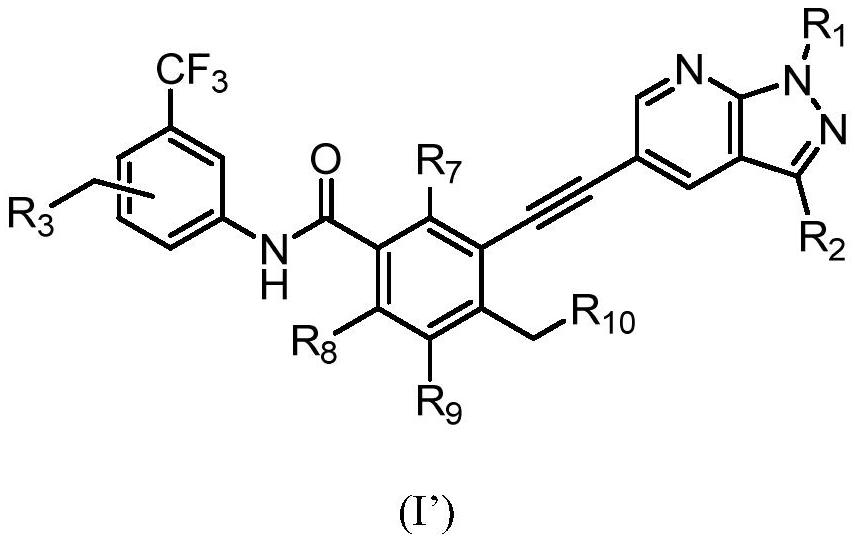
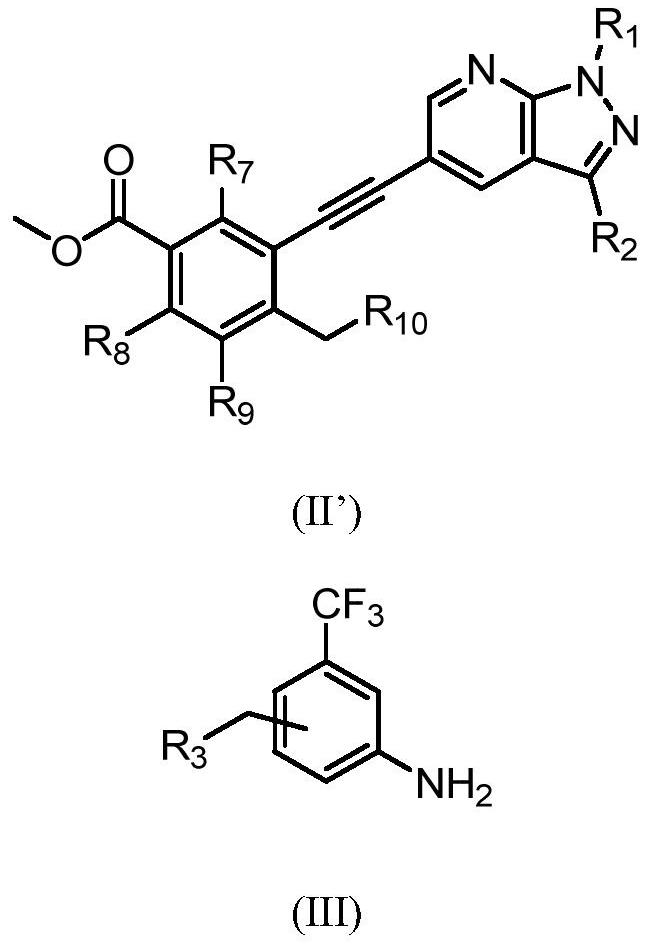
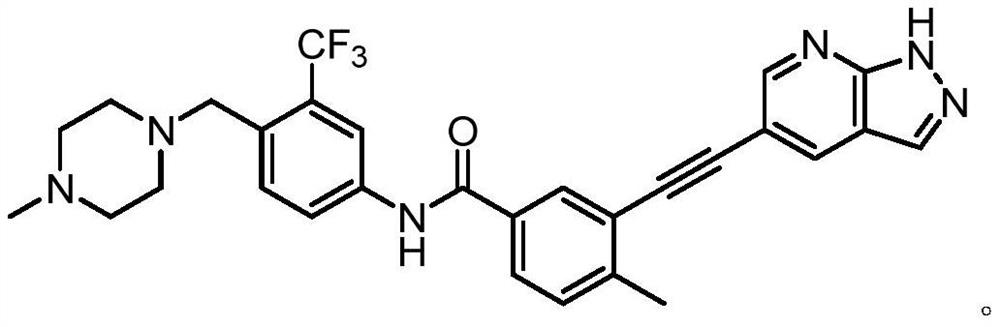
Preparation method of alkynyl-containing compound and intermediate thereof
A compound and alkyl technology, which is applied in the field of preparation of alkyne-containing compounds and their intermediates, can solve the problems of restricting large-scale production, and achieve the effects of novel design routes, convenient utilization and operation, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0264] step 1:
[0265]
[0266] Under the protection of nitrogen, N-methylpyrrolidone (137.6g) was heated to 30-35°C to obtain compound of formula 1 (14.4g, 1.3eq), compound of formula 2 (19.14g, 1eq), and dichloride bis Palladium (triphenylphosphate) (0.46g, 0.01eq) and cuprous iodide (0.113g, 0.01eq) were then added triethylamine (9.45g, 1.5eq) under nitrogen. The reaction mixture was heated to 65 to 75°C and maintained at this temperature for 2 hours. The process control of the reaction is carried out by liquid phase detection. When the content of the compound of formula 2 is ≤0.1%, the reaction is terminated. After the reaction, the reaction solution was cooled to 35-45°C, and N-acetyl-L-cysteine (1g, 0.1eq) was added directly. The reaction was carried out with stirring for 4 to 5 hours. The resulting product was cooled to room temperature, precipitated by adding water, centrifuged, and washed with pure water to obtain a coarse filter cake. After the coarse filt...
Embodiment 2
[0278] Carry out Sonogashira reaction (other parameters are with the first step of embodiment 1) with reference to each row reaction parameter of table 4, calculate yield by formula 2 compound and be shown in the last column of table 4.
[0279] Table 4
[0280]
[0281]
Embodiment 3
[0283] The amidation reaction is carried out with reference to the reaction parameters of each row in Table 5 (other parameters are the same as the third step in Example 1), and the calculated yield of the compound of formula 4 or 5 in moles is shown in the last column of Table 5.
[0284] table 5
[0285]
[0286]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com