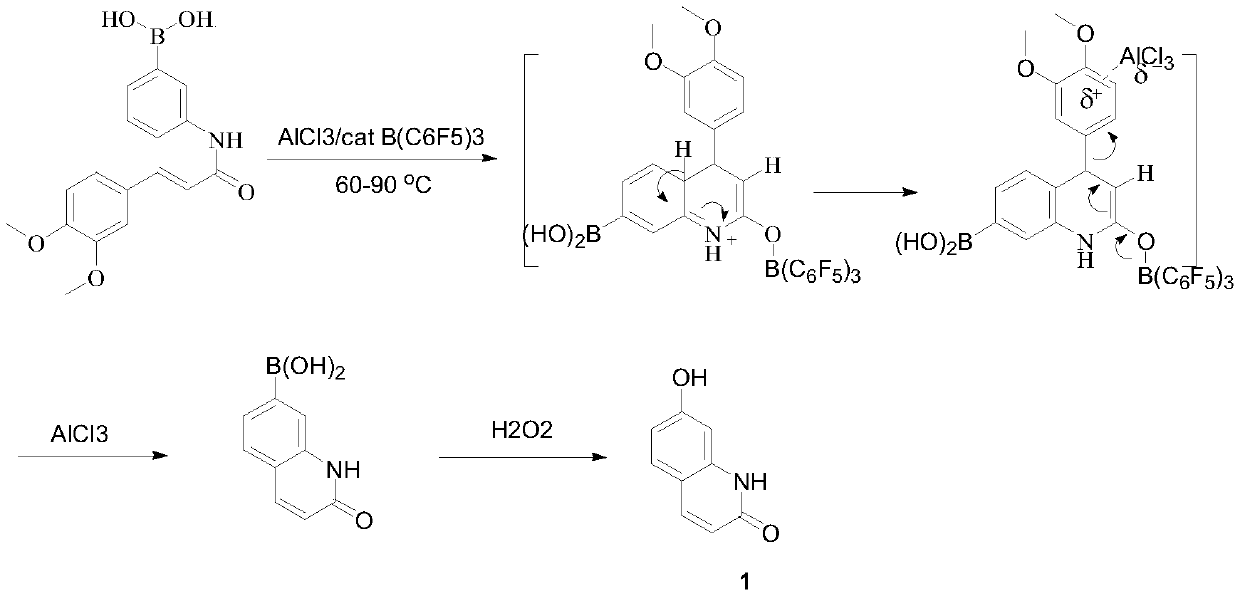
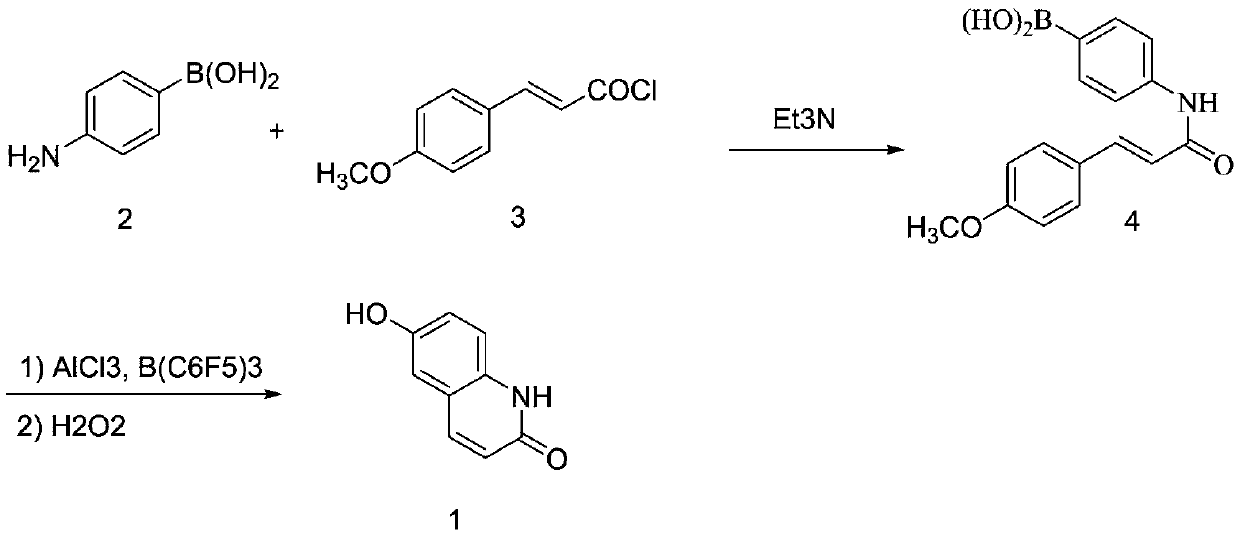
A kind of method for preparing hydroxy-2(1h)-quinolinone
A technology of quinolinone and hydroxyl, which is applied in the field of preparation of hydroxy-2-quinolinone, can solve the problems of difficult post-processing, high price, serious environmental pollution, etc., and achieve difficult post-processing, reduced production costs, and simple operation easy effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028]
[0029] Synthesis of compound (3):
[0030] In a 100mL three-neck flask equipped with a gas absorption device and a reflux condenser, add trans-4-methoxyphenylacrylic acid (17.8g, 0.10mol) and toluene (55mL), add thionyl chloride (11.9g , 0.10mol), slowly heated to 70°C, and reacted at constant temperature for 4 hours. After cooling, it was concentrated under reduced pressure to remove excess thionyl chloride, and the residue was frozen to precipitate a light yellow solid, which was recrystallized with dichloromethane to obtain 17.3 g of white crystals, with a yield of 88.0%.
[0031] Synthesis of compound (4):
[0032] Add 60 mL of tetrahydrofuran, 4-aminophenylboronic acid (12.1 g, 88.0 mmol), and triethylamine (13.3 g, 132.0 mmol) into a 250 mL three-necked flask, and add the compound dissolved in tetrahydrofuran (70 mL) dropwise at -5 to 0°C (3) (17.3g, 88.0mmol), after dropping, stir at room temperature for 2 hours, acidify the reaction solution with concentr...
Embodiment 2
[0036]
[0037] Synthesis of compound (3):
[0038] In a 100mL three-neck flask equipped with a gas absorption device and a reflux condenser, add trans-3,4-dimethylphenylacrylic acid (17.6g, 0.10mol) and toluene (55mL), dropwise add thionyl chloride ( 11.9g, 0.10mol), slowly heated to 70°C, and reacted at constant temperature for 4 hours. After cooling, it was concentrated under reduced pressure to remove excess thionyl chloride, and the residue was frozen to precipitate a light yellow solid, which was recrystallized with dichloromethane to obtain 17.2 g of white crystals, with a yield of 88.6%.
[0039] Synthesis of compound (4):
[0040] Add 60 mL of tetrahydrofuran, 4-aminophenylboronic acid (12.1 g, 88.6 mmol), and triethylamine (22.4 g, 0.22 mol) into a 250 mL three-necked flask, and drop the compound dissolved in tetrahydrofuran (70 mL) at -5 to 0°C (3) (17.2g, 88.6mmol), after dropping, stir at room temperature for 2 hours, acidify the reaction solution with concen...
Embodiment 3
[0044]
[0045] Synthesis of compound (3):
[0046] In a 100mL three-necked flask equipped with a gas absorption device and a reflux condenser, add trans-3,4,5-trimethylphenylacrylic acid (19.0g, 0.10mol) and toluene (55mL), dropwise add chlorinated Sulfone (11.9 g, 0.10 mol) was slowly heated to 70°C and reacted at constant temperature for 4 hours. After cooling, it was concentrated under reduced pressure to remove excess thionyl chloride, and the residue was frozen to precipitate a light yellow solid, which was recrystallized with dichloromethane to obtain 18.4 g of white crystals, with a yield of 88.3%.
[0047] Synthesis of compound (4):
[0048] Add 60mL of tetrahydrofuran, 4-aminophenylboronic acid pinacol ester (19.3g, 88.3mmol), pyridine (17.5g, 0.22mol) into a 250mL three-necked flask, and add the solution in tetrahydrofuran (75mL) dropwise at -5 to 0°C Compound (3) (18.4g, 88.3mmol), after dropping, stirred at room temperature for 2 hours, acidified the reaction...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com