Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
76 results about "4-methylthiazole" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
4-Methylthiazole is found in animal foods. 4-Methylthiazole is a flavouring ingredient. 4-Methylthiazole is present in asparagus, cooked beef, cooked pork, pork liver, coffee, roast barley, roast peanut and cooked shrimp.
Preparation method for febuxostat
InactiveCN103304512ALow costReduce operation processOrganic chemistryHydroxylamine HydrochlorideToxic material
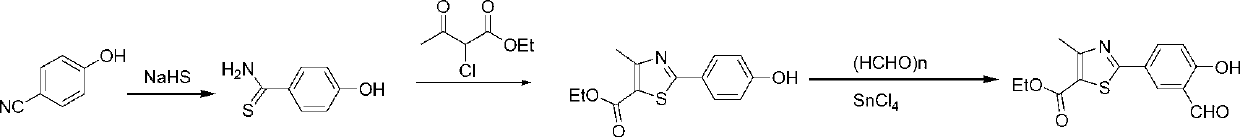
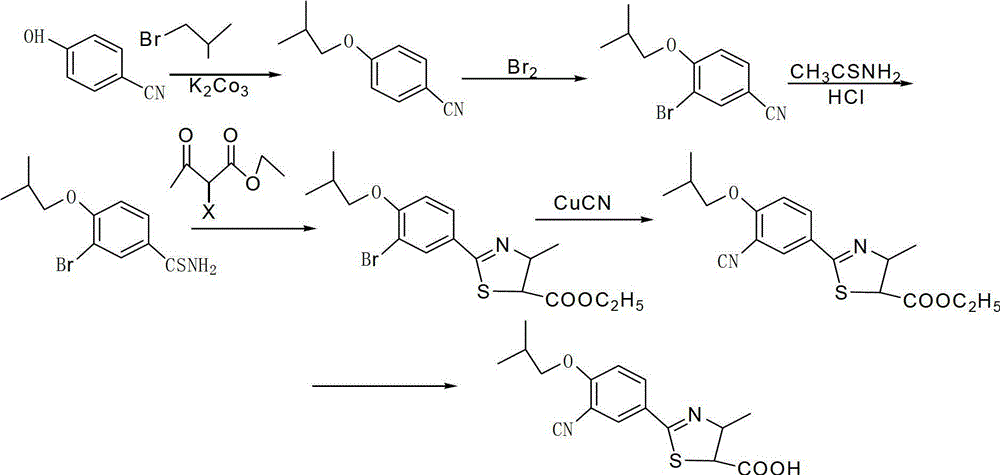
The invention discloses a preparation method for febuxostat. The preparation method for febuxostat comprises the following steps of: by using 4-hydroxybenzonitrile and thioacetamide as raw materials, and reacting in hydrochloric acid solution to prepare 4-hydroxythiobenzamide; carrying out a reaction on 4-hydroxythiobenzamide and 2-chloroacetoacetic acid ethyl ester to prepare 2-(4-hydroxylphenyl)-4-methylthiazol-5-carboxylic acid ethyl ester; carrying out a reaction on the obtained compound and hexamine in the mixed acid system of methanesulfonic acid and trifluoroacetic acid to prepare 2-(3-formyl-4-hydroxylphenyl)-4-methylthiazole-5-carboxylic acid ethyl ester; synthesising 2-(3-nitrile-4-isobutoxylphenyl) -4-methylthiazole-5-carboxylic acid ethyl ester from the compound, hydroxylamine hydrochloride, potassium carbonate, iso-butyl bromide and the like in a polar protonic solvent via a one-pot method; and finally hydrolyzing in an alkaline condition to obtain the target product, namely, febuxostat. The total yield of the preparation method for febuxostat disclosed by the invention is increased to 66%, the separation steps are reduced, any extremely toxic substance is not involved, and the environmental pollution is less.
Owner:SOUTH CHINA UNIV OF TECH
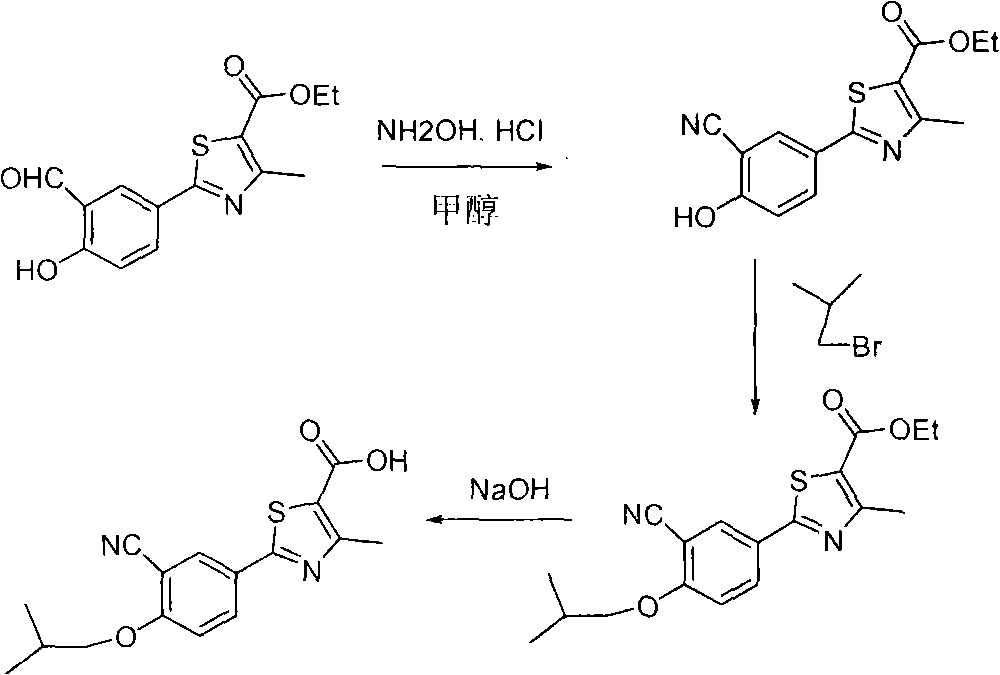
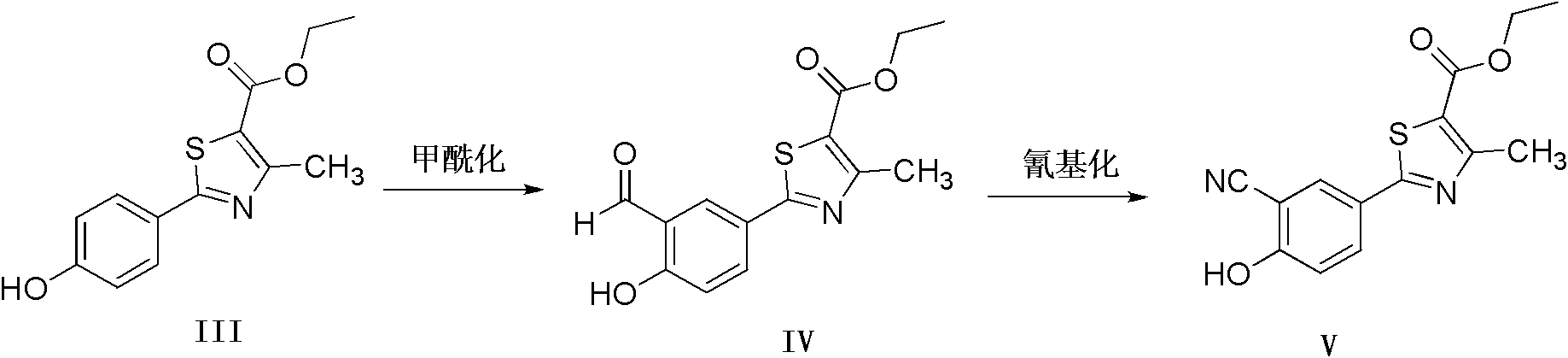
Method for preparing febuxostat intermediate
The invention relates to a method for preparing a febuxostat intermediate, which comprises the following steps of: dissolving 2-[4-hydroxyphenyl]-4-methylthiazol-5-ethyl formate in a mixed acid reaction solvent, adding a certain amount of urotropine, heating to react for 1-36 h at certain temperature, and treating the reaction liquid to obtain corresponding heterocyclic aldehyde.
Owner:CHINA RESOURCES SAIKE PHARMA
Peach essence
ActiveCN104957584AReduce manufacturing costBroad sales marketFood ingredientsFood preparationAdditive ingredientPhenylacetic acid
The invention discloses a peach essence. The peach essence consists of the following ingredients: propanediol, hexanol, hexenyl phenylacetate, benzaldehyde, ethyl acetate, phenylacetic acid cis-3-ester hexane, methyl acetate, hexyl acetate, hexyl trans-2-ester hexane, ethyl maltol, leaf alcohol, hexanal, acetic acid, linalool, gamma-caprylolactone, theta-decalactone, gama-decalactone, gamma-undecalactone, 8-mercaptomenthone, 2-isopropyl-4-methylthiazole, beta- damascenone, ethyl caprate and ethyl benzoate. The peach essence is moderate in fragrance, saturated, thick, stable in quality, natural in flavor and approximat to the natural fragrance of peach.
Owner:ZHEJIANG GREEN CRYSTAL FLAVOR
Preparation method of high-purity febuxostat
The invention relates to a preparation method of high-purity febuxostat. The method comprises the steps: carrying out etherification reaction on ethyl 2-(3-formyl-4-hydroxyphenyl)-4-methylthiazole-5-carboxylate and refined bromo-isobutane, then conducting cyaniding and hydrolysis to obtain other crude febuxostat, and recrystalizing to obtain high-purity febuxostat. In febuxostat prepared by using the method, the content of impurity 2-(3-cyano-4-n-propoxy phenyl)-4-methylthiazole-5-formic acid is smaller than 0.10%.
Owner:CHINA RESOURCES SAIKE PHARMA
Preparation methods of compound 2-(3-formyl-4-isobutoxy phenyl)-4-methyl thiazole-5-ethyl formate and febuxostat
The invention provides a preparation method of 2-(3-formyl-4-isobutoxy phenyl)-4-methyl thiazole-5-ethyl formate which is obtained by using 4-isobutoxy cyanophenyl as an initial raw material and through a series of reactions. The invention also provides a preparation method of febuxostat, which comprises the following steps: reacting 2-(3-formyl-4-isobutoxy phenyl)-4-methyl thiazole-5-ethyl formate with hydroxylamine hydrochloride under the action of a catalyst to obtain a compound with a structure as shown in formula (VIII); hydrolyzing the compound with the structure as shown in formula (VIII) under an alkaline condition, and performing acidification to obtain febuxostat. The preparation method of the invention prepares febuxostat without using cyanides, and is high in safety. The preparation methods of the invention are simple in operation and high in yield. Experiment results show that the yield of step (A) is up to 90%, the yield of step (B) is up to 85%, the yield of step (C) is up to 90%, the yield of step (D) is up to 90%, and the yield of step (E) is up to 97%.
Owner:ZHEJIANG AUSUN PHARMA
Synthetic method of febuxostat
InactiveCN102964313ARaw materials are cheap and easy to getHigh yieldOrganic chemistrySalicylaldehydePhenyl Ethers
The invention discloses a synthetic method of febuxostat. The synthetic method of febuxostat comprises the following steps: (1) preparing 4-hydroxy-1, 3-phthalic aldehyde; (2) preparing 4-hydroxy-1, 3-diphenyl nitrile; (3) preparing 4-isobutoxy-1, 3-diphenyl nitrile; (4) preparing 2-isobutoxy-5-sulfobenzyl acylamino cyanophenyl; (5) preparing 2-(3-cyan-4-isobutyl phenyl ether)-4-methylthiazole-5-ethyl formate; and (6) preparing febuxostat. According to the synthetic method, the low-price salicylaldehyde is adopted as the starting material and is subjected to aldehydizing, alkylating, thioacid amidating, cyclizing, and hydrolyzing so as to obtain the end product; the toxic agents such as sodium cyanide and the highly-corrosive agent like trifluoroacetic acid are not used; the raw materials in use are low in price and easy to obtain; the reaction condition is mild; the operation is simple and convenient; the total recovery is high; the obtained product is easy to purify; and the industrial production is easy to realize.
Owner:周广连
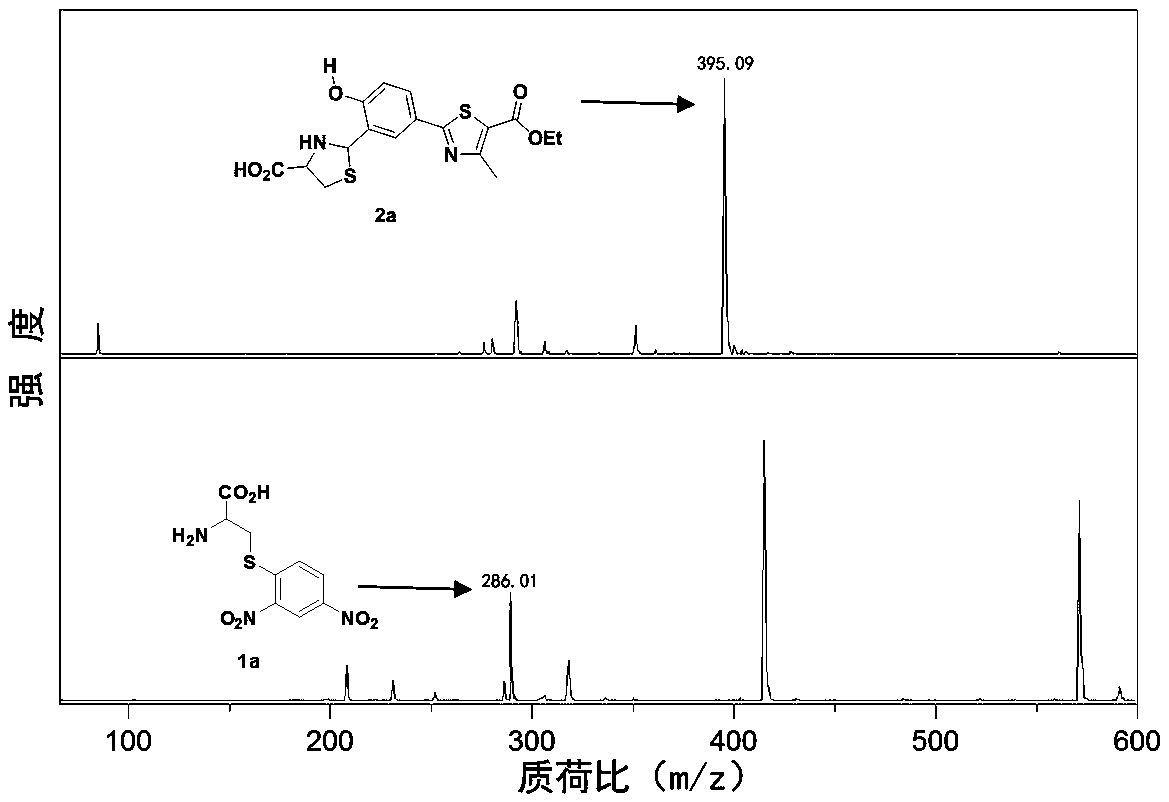
ESIPT (excited state intramolecular proton transfer) type fluorescent probe for biological mercaptan detection and application
ActiveCN107602502AThe synthesis steps are simpleEasy to operateOrganic chemistryFluorescence/phosphorescenceThiolDinitrophenyl
The invention discloses an ESIPT (excited state intramolecular proton transfer) type fluorescent probe for biological mercaptan detection. The ESIPT type fluorescent probe is 2-(4-(2,4-dinitrophenyl sulfonyloxy)-3-formylphenyl)-4-methylthiazole-5-ethyl carboxylate, wherein 2,4-dinitrophenyl sulfonyl serves as a recognition group, and 2-(3-formyl-4-hydroxyphenyl)-4-methylthiazole-2-ethyl formate serves as an information reporter group. A preparation method of the ESIPT type fluorescent probe is simple, the fluorescent probe can enter cells simply and rapidly and specifically bound with biological mercaptan in the cells, so that the fluorescent probe has an obvious fluorescence enhancement effect, can realize distinguishing by naked eyes, has high anti-interference capacity on common biological molecules, has quite efficient selectivity and can perform analysis through ultraviolet absorption and fluorescence spectrophotometry. The ESIPT type fluorescent probe is good in stability, can bestored and used for a long time, is applicable to growing environments of various living cells, can realize high-sensitivity detection of trace biological mercaptan in the cells, can be applied to cell and living imaging and has quite important application value.
Owner:ANHUI AGRICULTURAL UNIVERSITY
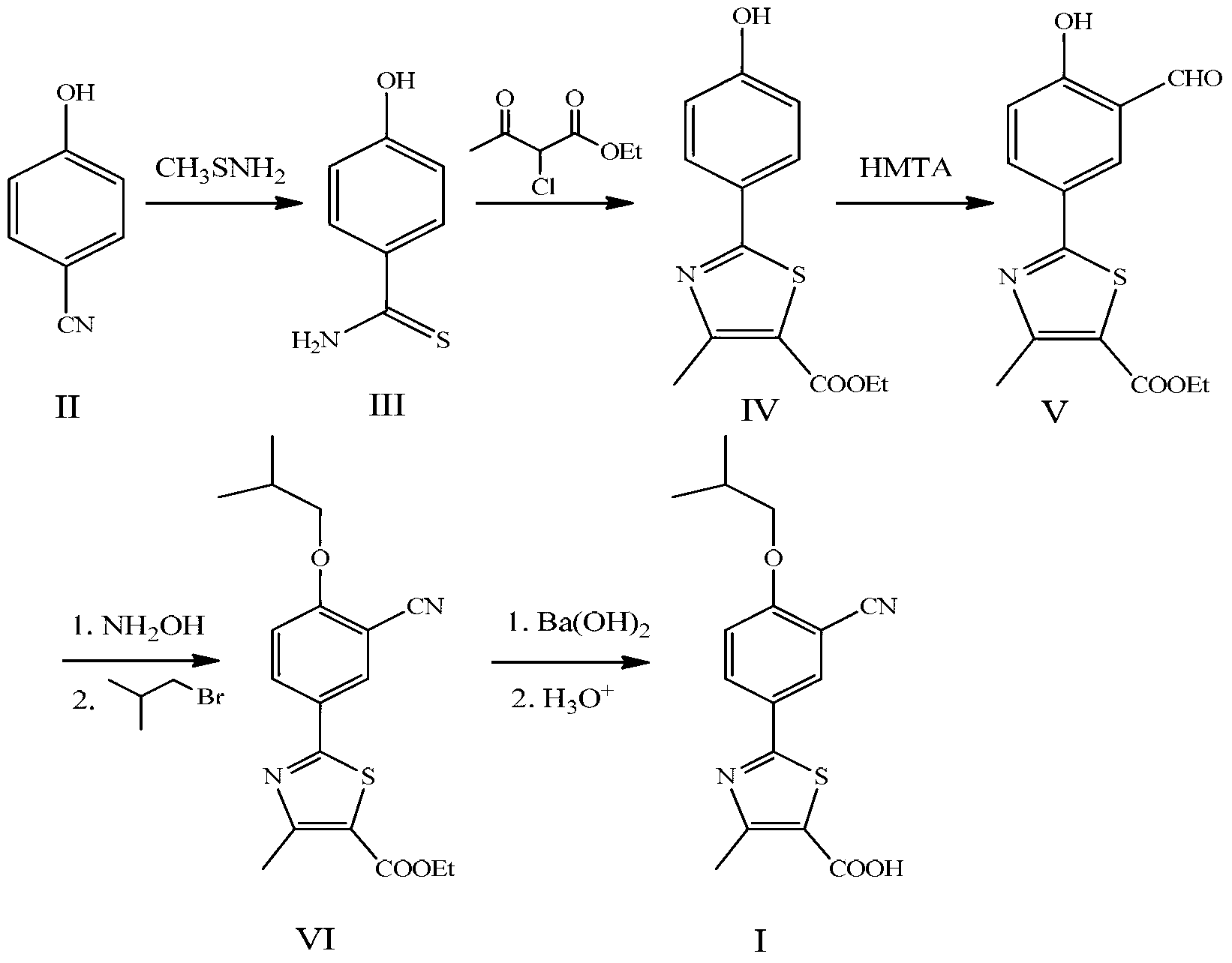
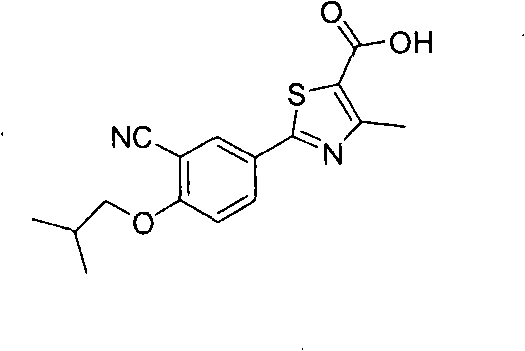
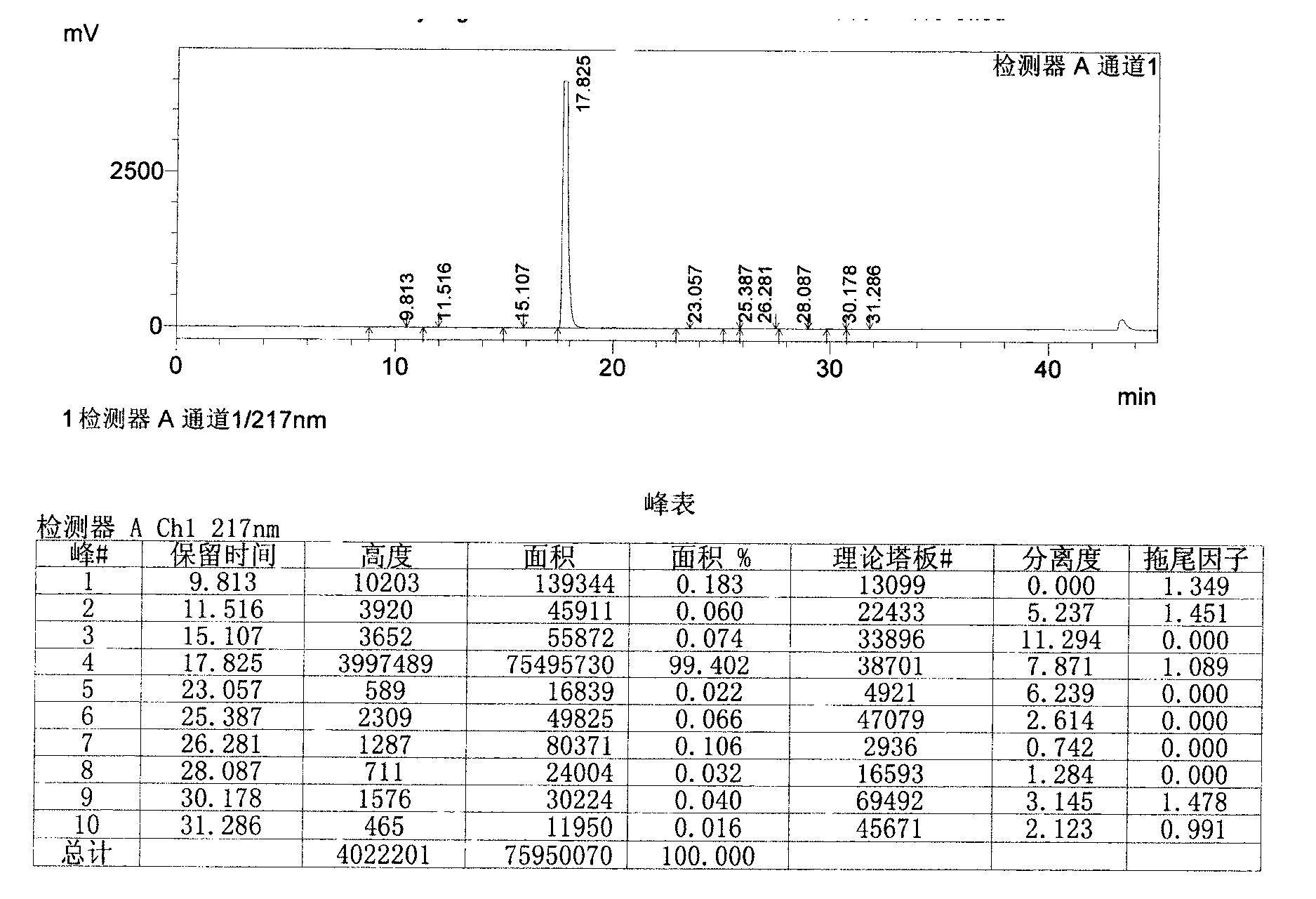
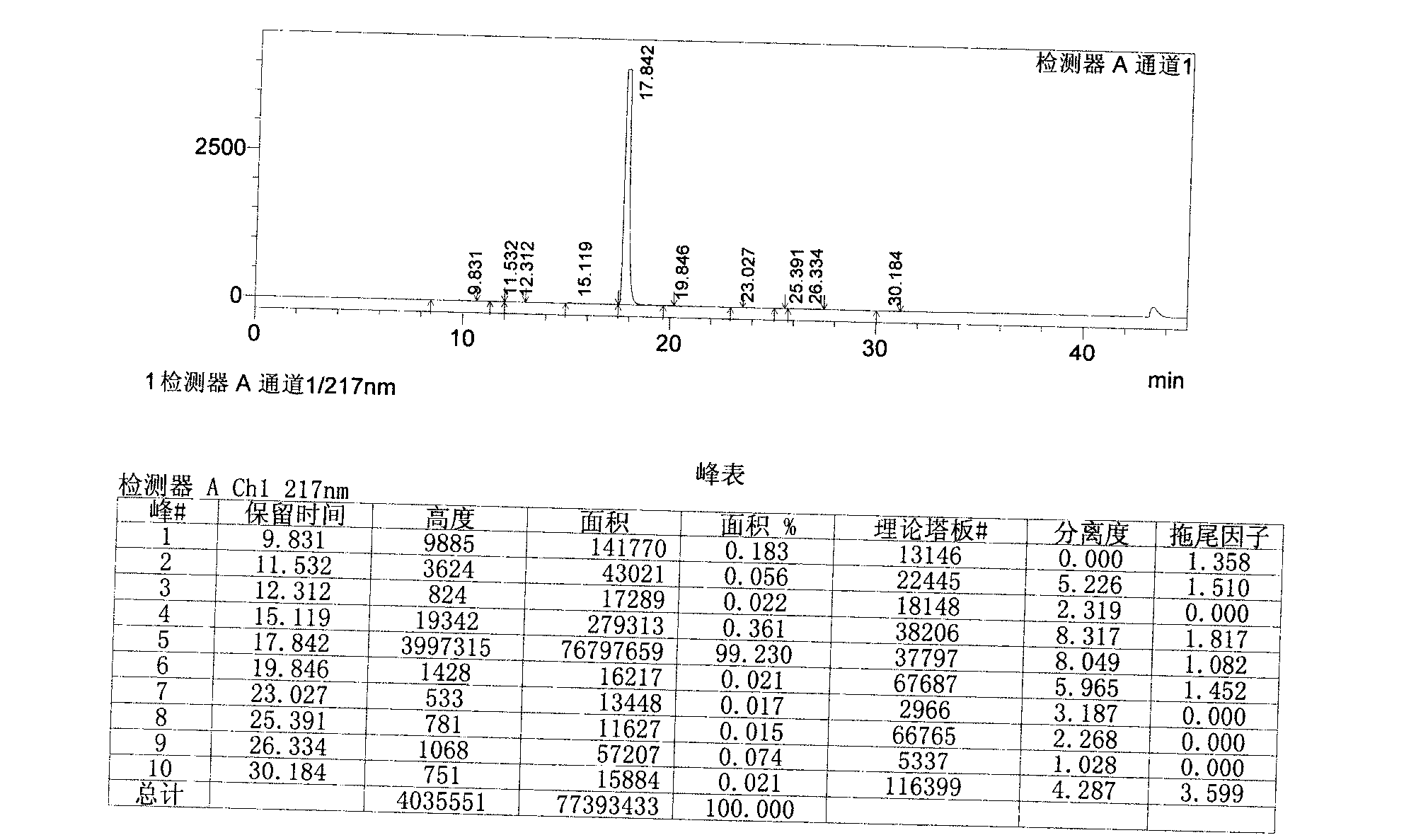
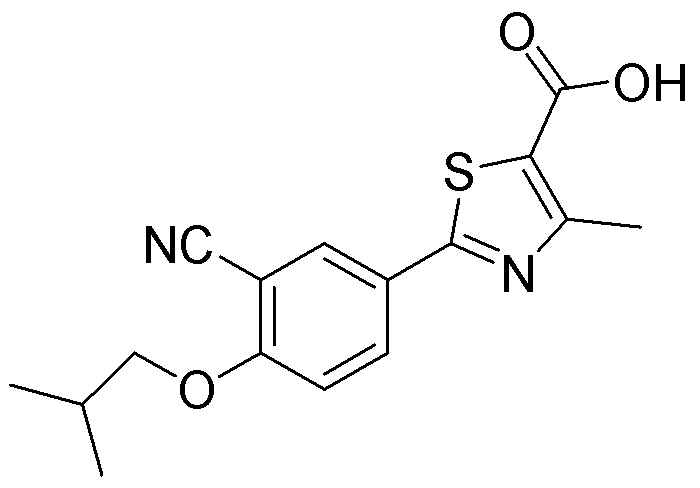
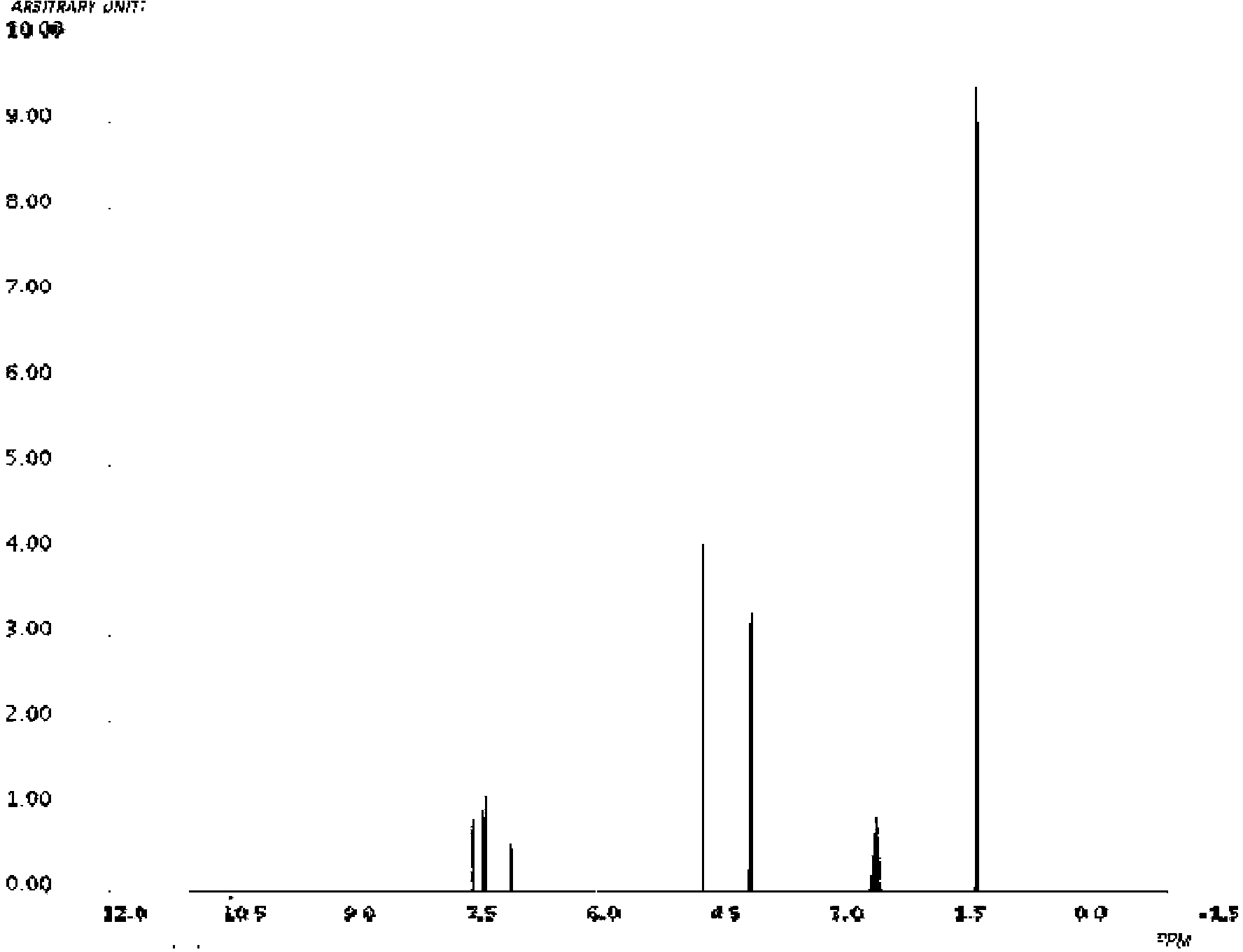
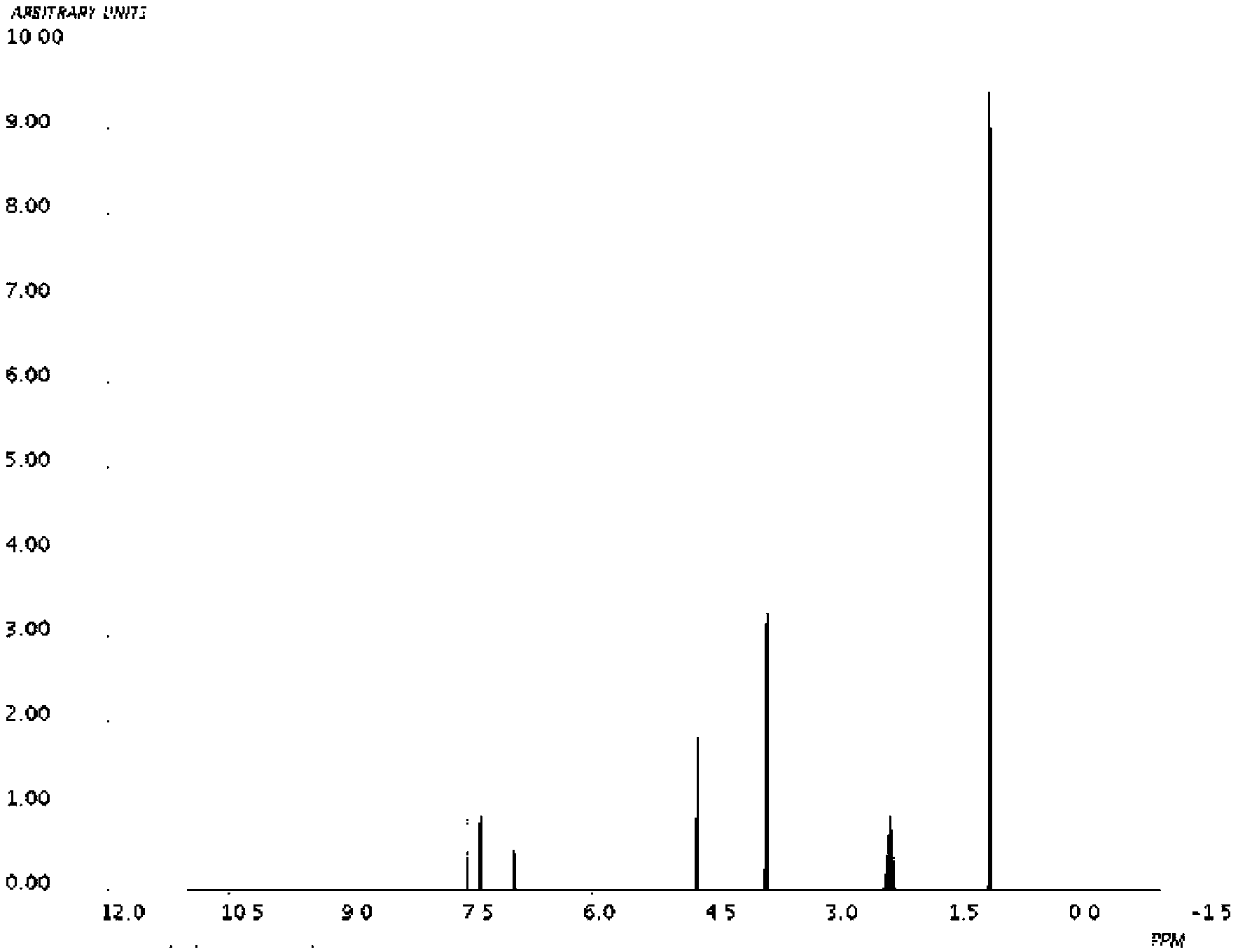
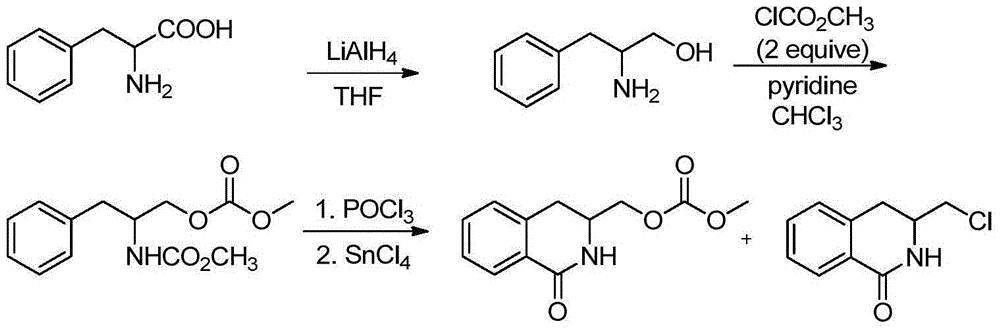
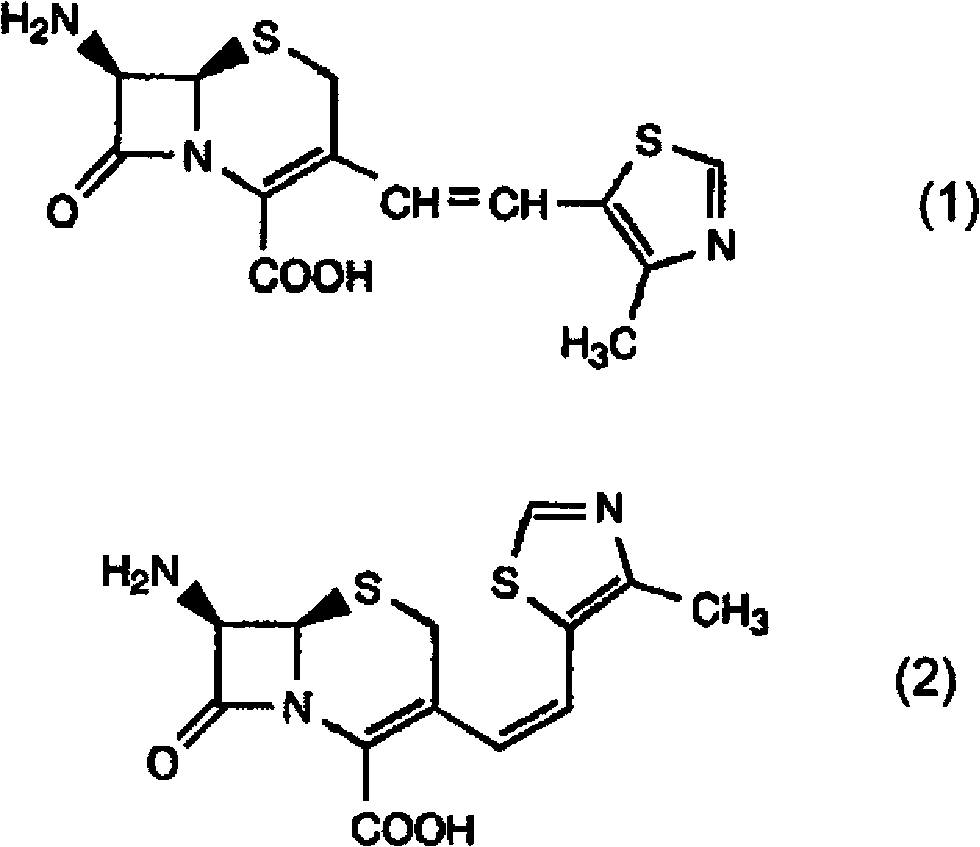
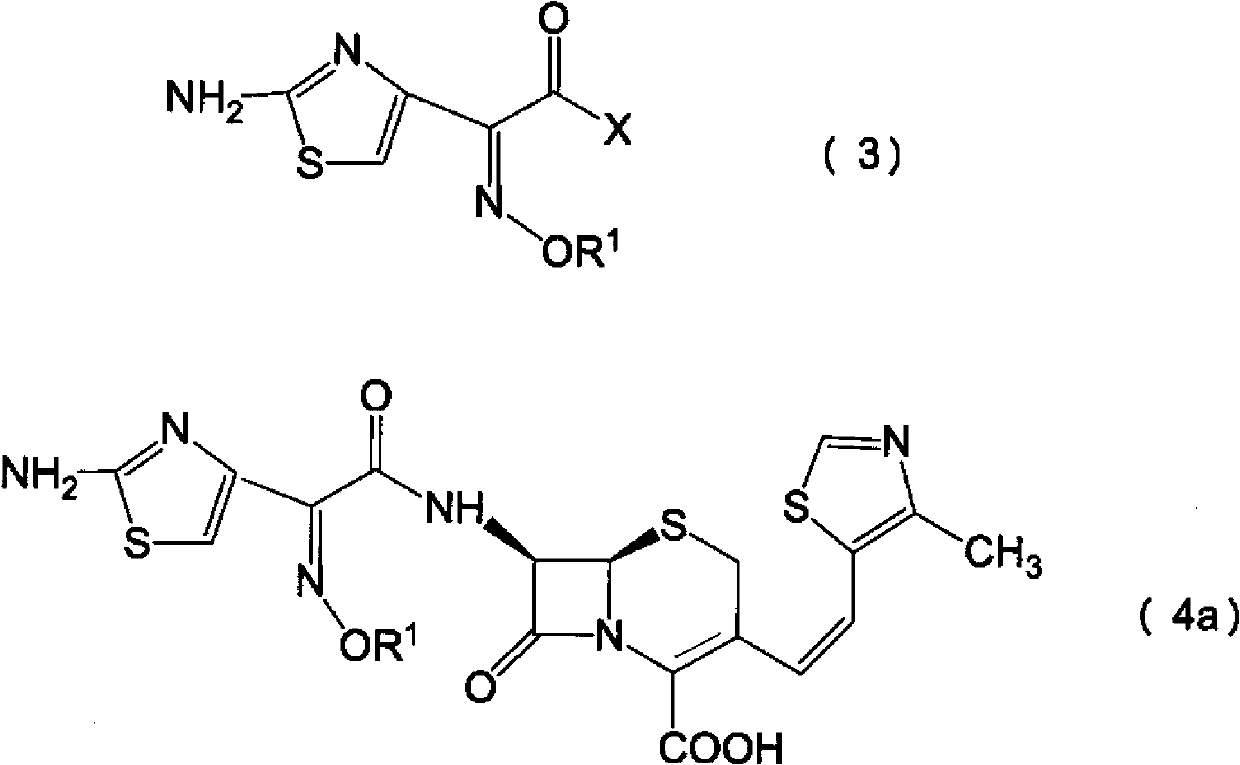
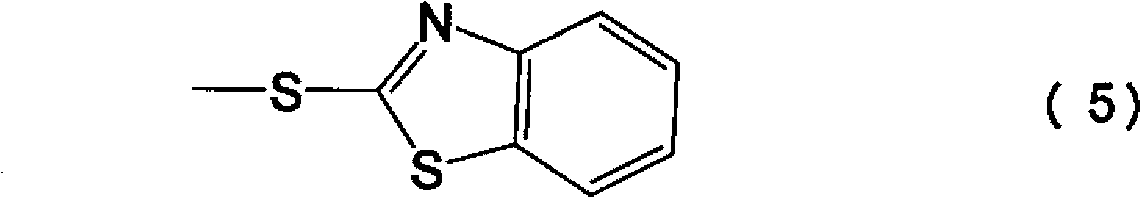
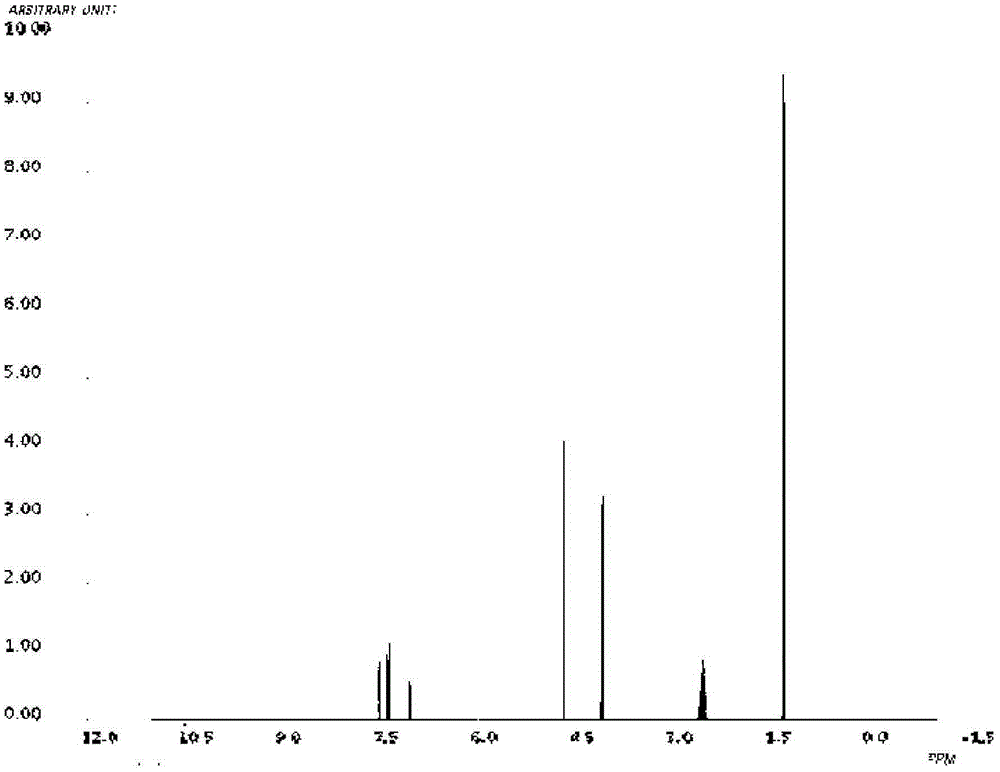
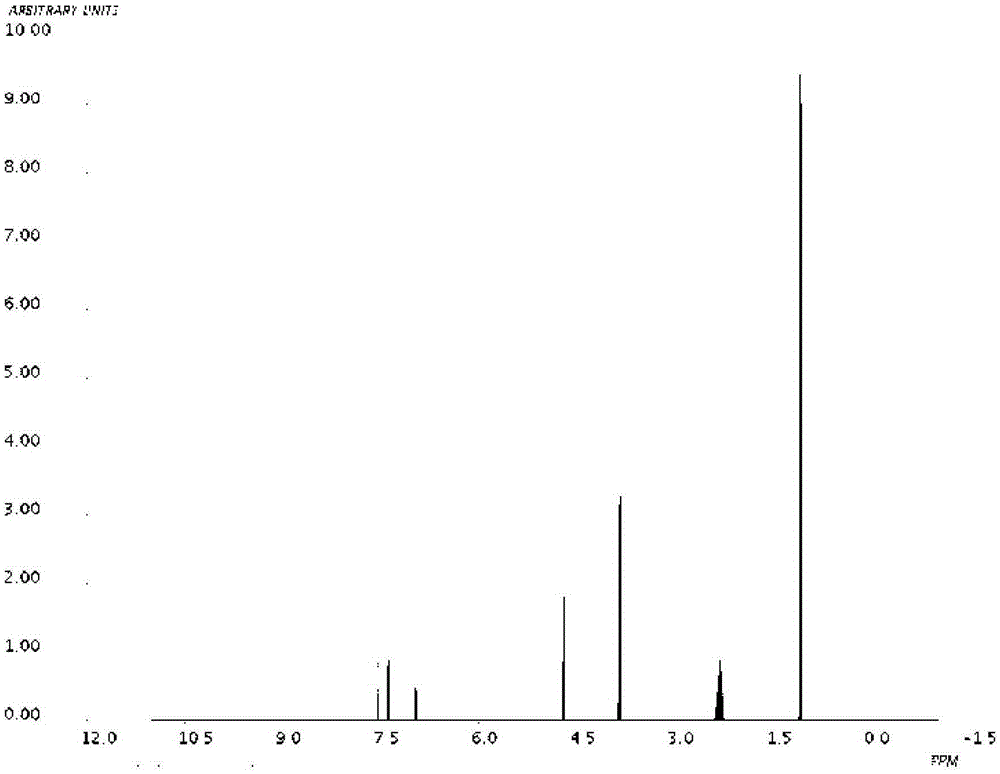
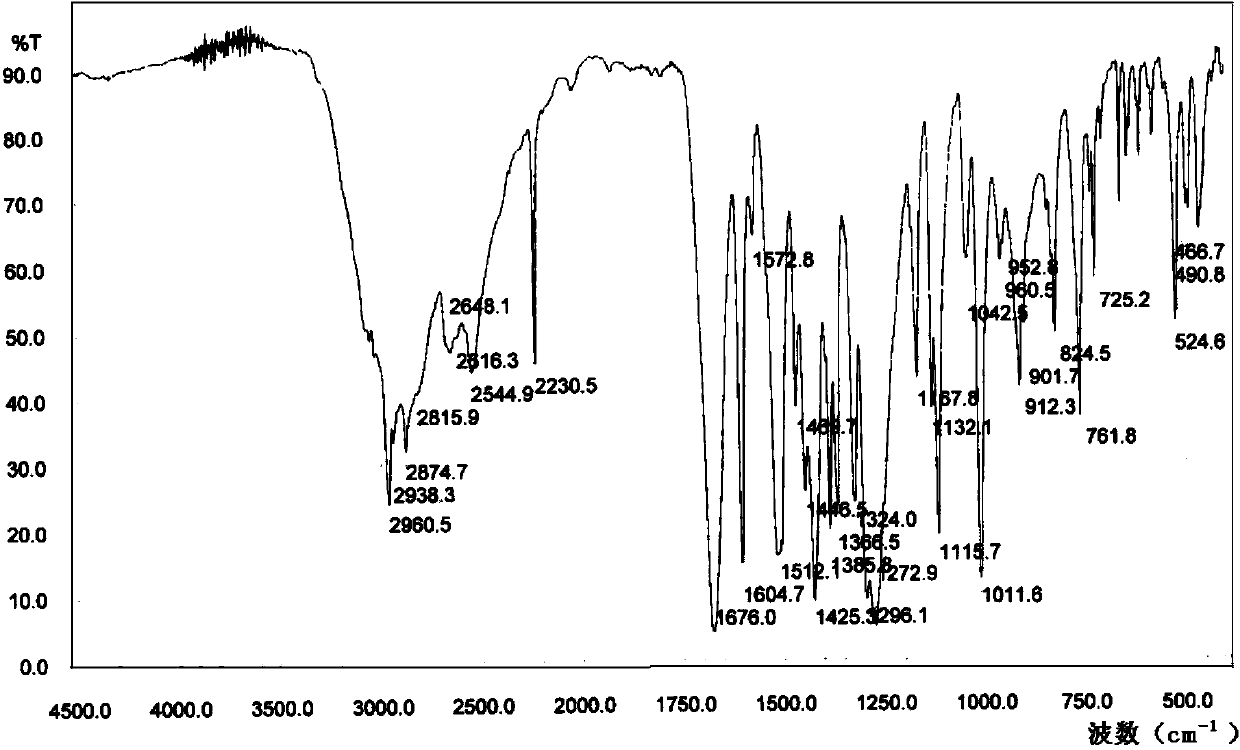
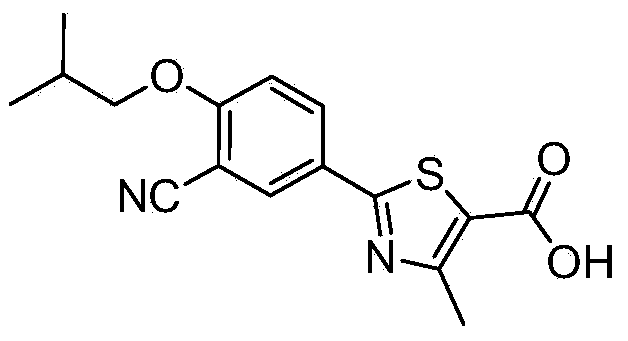
Novel derivative of 2-[3-cyano-4-isobutoxyphenyl]-4-methylthiazol-5-formic acid, preparation method for novel derivative and application of novel derivative
The invention provides a compound as shown in a formula (I), or a pharmaceutical salt of the compound or stereisomers of the compound. The formula (I) is as shown in the specification. The compound as shown in the formula (I), or the pharmaceutical salt of the compound or the stereisomers of the compound applied to preventing and treating hyperuricemia have more superior uric-acid-reducing effect and more excellent safety compared to those of febuxostat.
Owner:XIANGBEI WELMAN PHARMA CO LTD
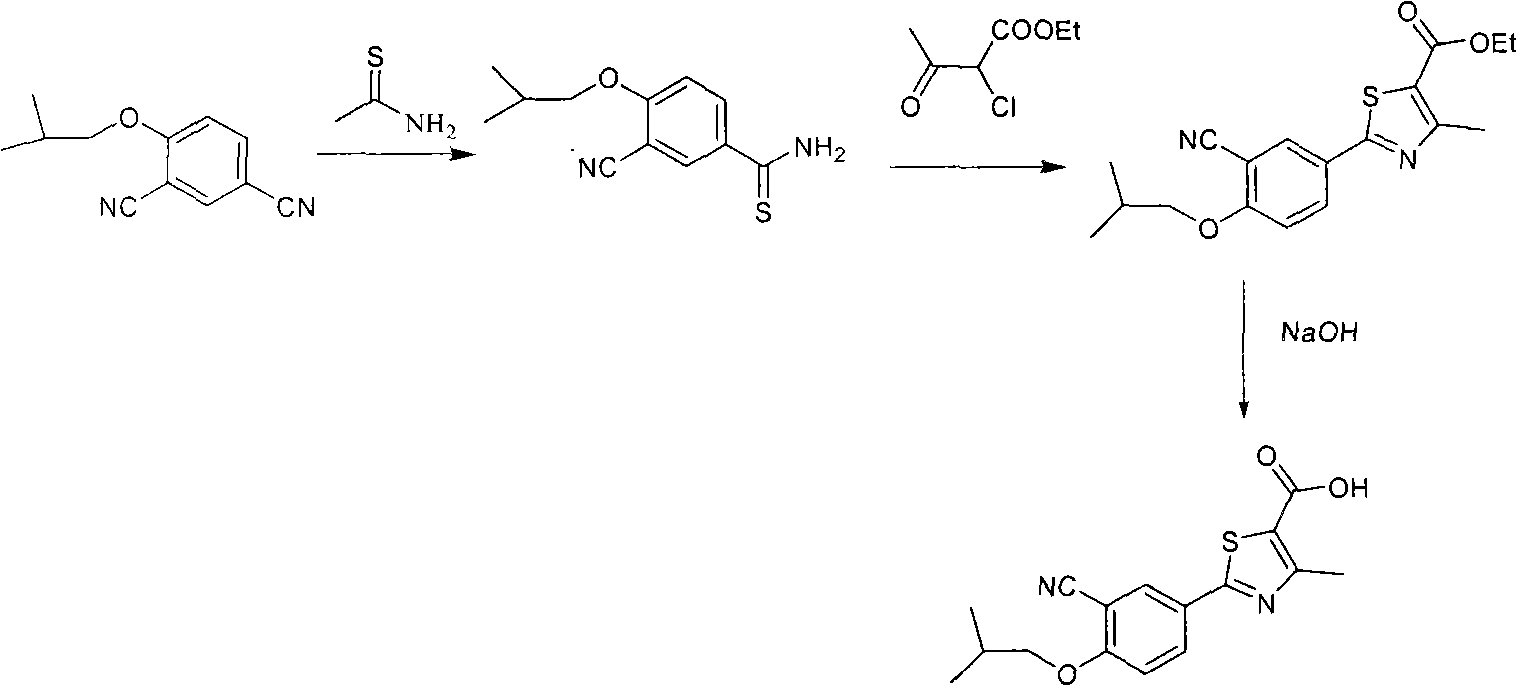
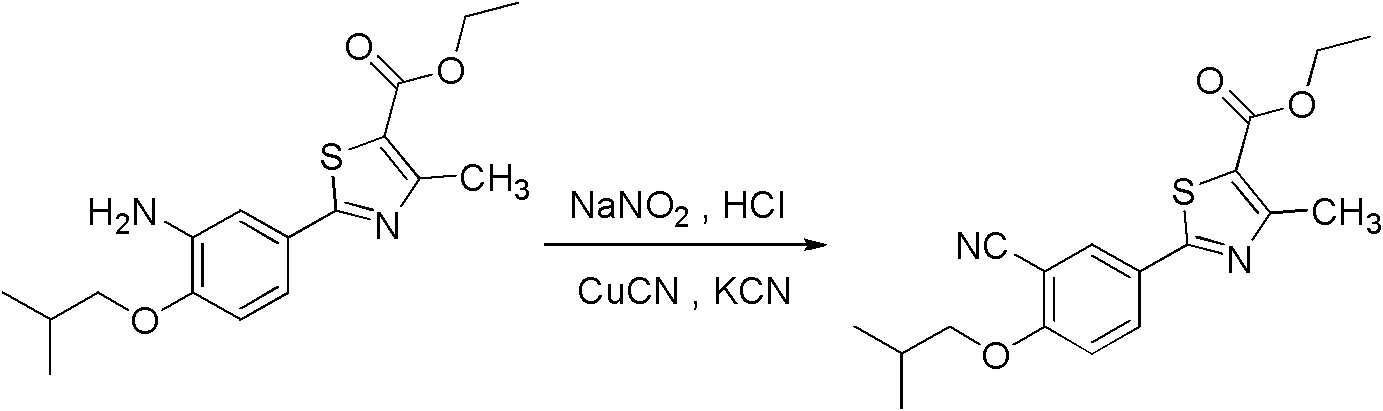
Method for preparing 2-(3-cyano-4-isobutyl methoxyphenyl)-4-methylthiazol-5-ethyl formate
InactiveCN102070559AOvercome yieldOvercome environmental problemsOrganic chemistryPhenyl EthersPhenol
The invention discloses a method for preparing a midbody of medicine febuxostat of 2-(3-cyano-4-isobutyl methoxyphenyl)-4-methylthiazol-5-ethyl formate, which comprises the following steps: using cyano phenol as raw materials, taking reaction to obtain 4-hydroxide thiobenzamide, carrying out cyclization to obtain 2-(4-hydroxy phenyl)-4-methylthiazol-5-ethyl formate, carrying out formylation to obtain 2-(3-carboxaldehyde-4-hydroxy phenyl)-4-methylthiazol-5-ethyl formate, carrying out isobutyl reaction to obtain 2-(3-carboxaldehyde-4-isobutyl phenyl ether)-4-methylthiazol-5-ethyl formate, and carrying out cyanation to obtain the 2-(3-cyano-4-isobutyl methoxyphenyl)-4-methylthiazol-5-ethyl formate. The raw materials used by the method disclosed by the invention can be easily obtained, the reaction conditions are mild, the cost is lower, and the method is applicable to industrialized production.
Owner:JIANGSU TOHOPE PHARMA
Method for synthesizing isoquinoline ketone compounds
The invention discloses a method for synthesizing isoquinoline ketone compounds. The method includes that dual-core salicylic acid copper complexes which are cheap and easy to synthesize are taken as catalyzers, oxygen which is green, environment-friendly and nontoxic is taken as an oxidizing agent, chlorine salt, 3-benzyl-5-(2-hydroxy ethyl) -4-methyl thiazole moiety or thiamine hydrochloride are taken as auxiliary catalizers, and 2-aryl-1,2,3,4-tetrahydroisoquinoline compounds are subjected to further reaction to obtain the isoquinoline ketone compounds. The method is simple in operation, the catalyzers are cheap and easy to obtain, reaction conditions are moderate, product yield is high, shortcomings that traditional raw material reagents are high in price, harsh in condition, complex in synthesizing step, low in overall yield and the like are overcome, and good application prospect is achieved.
Owner:SHAANXI NORMAL UNIV
Preparation method for 3-[(4-amino-5-pyrimidinyl)methyl]-5-(2-hydroxyethyl)-4-methylthiazole nitrate
The invention discloses a preparation method for 3-[(4-amino-5-pyrimidinyl)methyl]-5-(2-hydroxyethyl)-4-methylthiazole nitrate. The method comprises the following steps: contacting liquid sodium alkoxide with formamidine hydrochloride and then contacting an obtained substance with a compound represented by a formula (1) so as to obtain 4-amino-5-formylaminomethylpyrimidine; contacting 4-amino-5-formylaminomethylpyrimidine with an alkaline aqueous solution and then contacting an obtained substance with carbon disulfide and gamma-chloroacetyl propanol so as to obtain a compound represented by a formula (2); contacting an acidic aqueous solution with the compound represented by the formula (2) so as to obtain a compound represented by a formula (3); and contacting the compound represented by the formula (3) with hydrogen peroxide, then contacting an obtained substance with nitrate, carrying out neutralization with alkali and then carrying out solid-liquid separation so as to obtain 3-[(4-amino-5-pyrimidinyl)methyl]-5-(2-hydroxyethyl)-4-methylthiazole nitrate. With 3-[(4-amino-5-pyrimidinyl)methyl]-5-(2-hydroxyethyl)-4-methylthiazole nitrate prepared in the invention, accurate qualitative and quantitative analysis of demethylated thiamine can be realized.
Owner:江西天新药业股份有限公司
Method for synthesizing cefditoren pivoxil
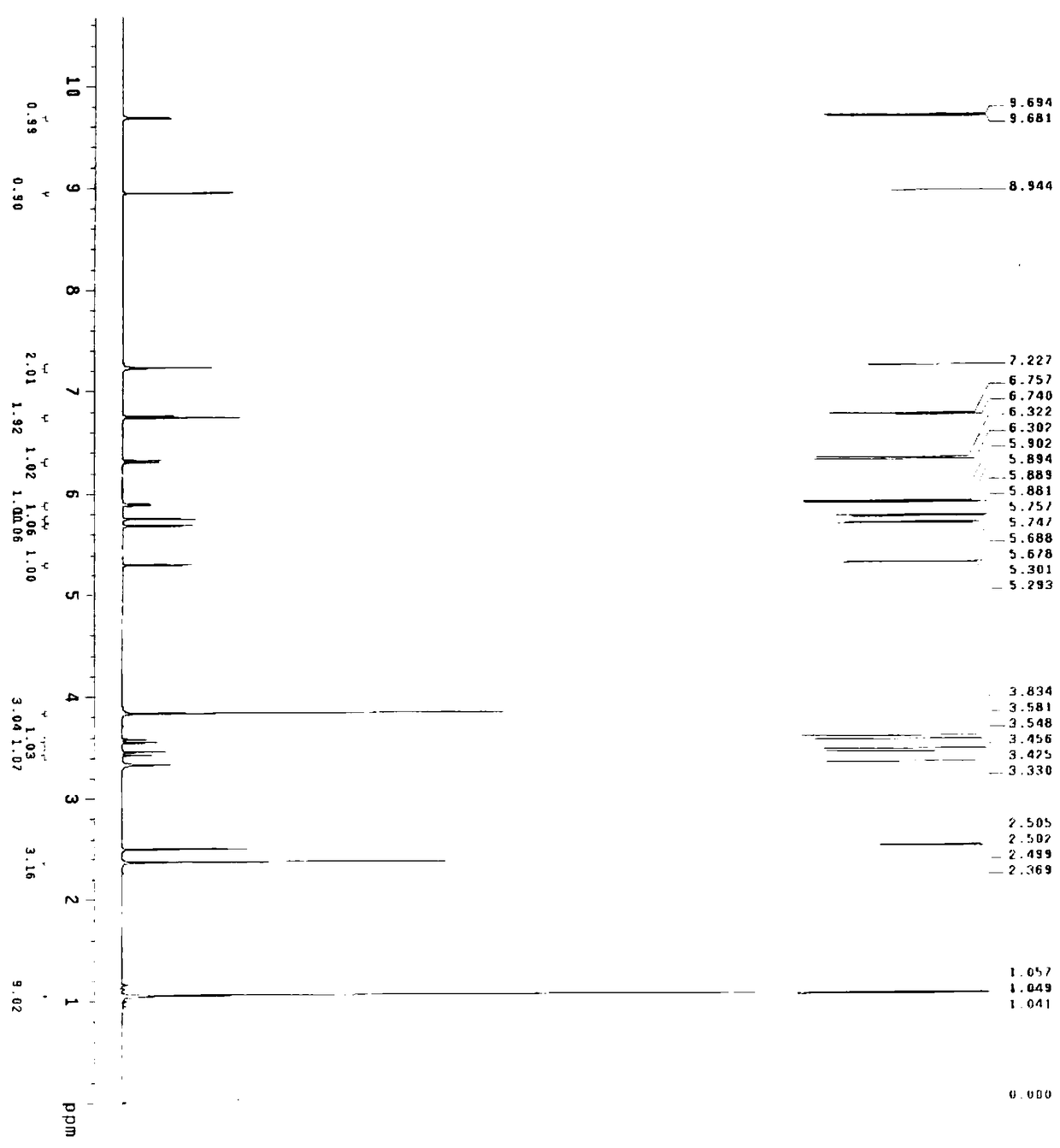
The invention relates to a method for synthesizing cefditoren pivoxil. The method comprises that D-7ACA reacts with an oxidizing reagent to produce a compound 1, the compound 1 is protected through silanization to produce a compound 2, 4-methylthiazole-5-methanol and NaI undergo an iodination reaction in the presence of a small amount of sulfuric acid for catalysis, triphenylphosphine is added into the reaction system and undergoes a reaction to produce a compound 3, the compound 3 is added into the compound 2 liquid and undergoes a reaction, the reaction product is concentrated, methanol anda small amount of concentrated hydrochloric acid are added into the concentrated product, the concentrated product is deprotected and crystallized to form cefditoren mother nucleuses, 7-ATCA and an AEactive ester undergo a reaction under alkaline conditions, the reaction product is crystallized to form a cefditoren sodium wet product, the cefditoren sodium wet product is added into iodomethyl pivalate and undergoes a reaction in the presence of a phase transfer catalyst and the product is crystallized to form a cefditoren pivoxil crude product. In preparation of the compound 1, cefditoren sodium and cefditoren pivoxil, single solvents are used and are easy to recover. The method has the advantages of simple operation, high product conversion rate, few impurities and low production cost and is suitable for industrial production of cefditoren pivoxil.
Owner:QILU ANTIBIOTICS PHARMA
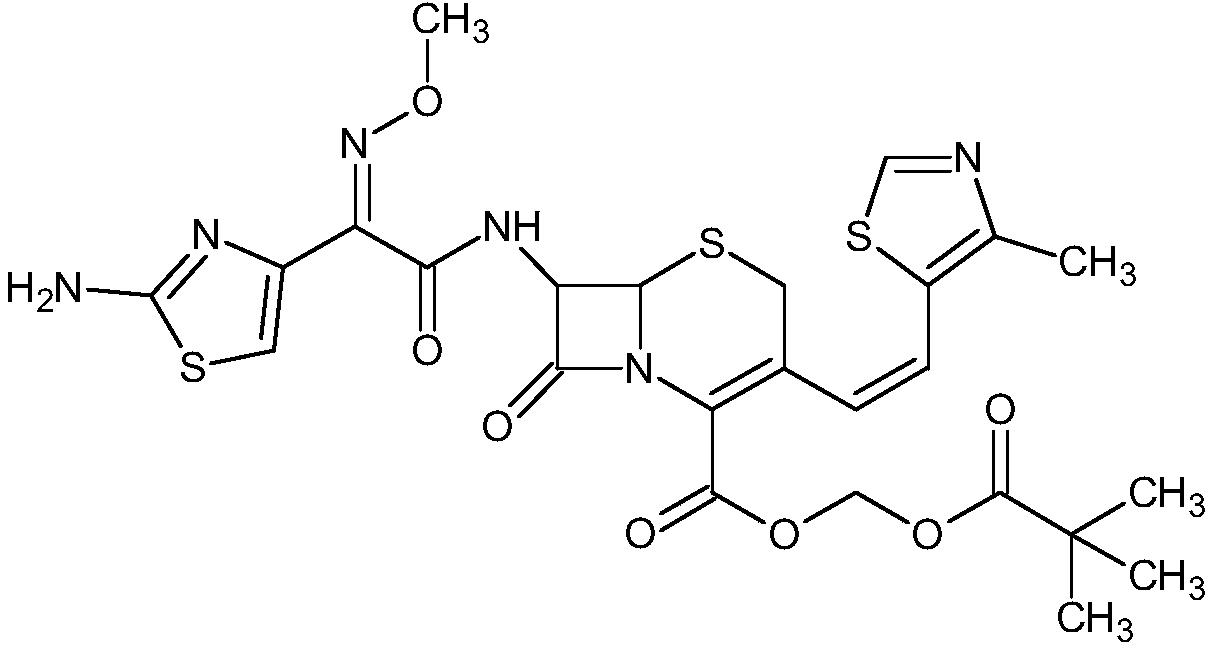
Process for preparation of cephalosporin derivative
InactiveCN102725297AIncrease contentAntibacterial agentsOrganic active ingredientsCarboxylic acidPhotochemistry
Provided is a process by which an objective cephalosporin derivative having a high Z-isomer content or an alkali metal salt thereof can be prepared via simple steps with industrial advantages. A process for the preparation of a cephalosporin derivative (4a) or an alkali metal salt thereof, characterized by comprising the first step of bringing an aqueous solution of 7-amino-3-[(E / Z)-2-(4-methylthiazol-5-yl)vinyl]-3-cephem-4-carboxylic acid (1) or an alkali salt thereof into contact with an active carbon that has an iodine adsorptivity of 1200mg / g or more as determined by JIS K-1474 and a methylene blue adsorptivity of 250ml / g or more as determined thereby to prepare the carboxylic acid (1) having an enhanced content of Z-isomer (2) or an alkali metal salt thereof, and the second step of subjecting the carboxylic acid (1); which has been prepared in the first step and has an enhanced content of Z-isomer (2) to reaction with a compound (3).
Owner:NIPPON CHECMICAL IND CO LTD
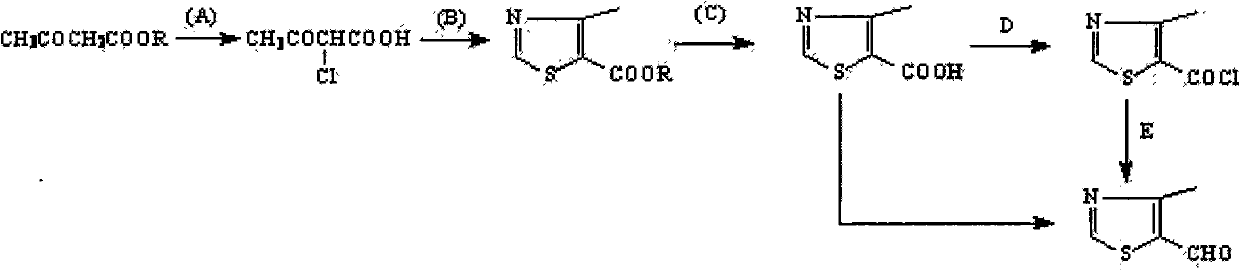
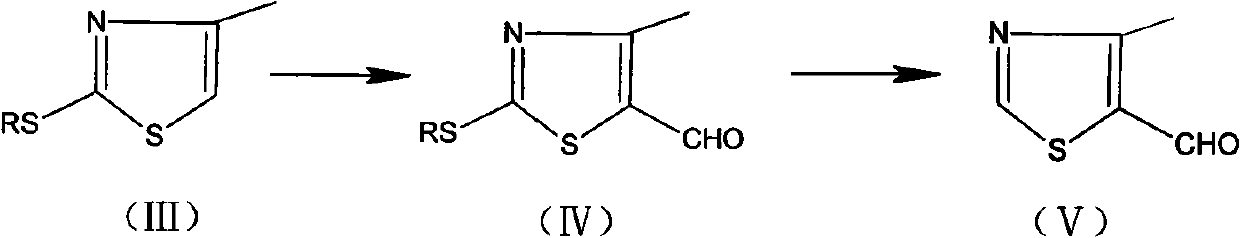
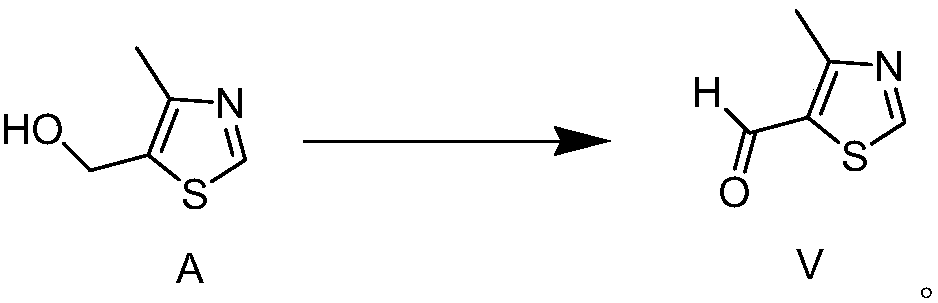
Preparation method of 4-methylthiazolaldehyde-5
The invention relates to a preparation method of 4-methylthiazolaldehyde-5. The method comprises the following steps of: carrying out a Vilsmeier reaction to obtain 2-thiol-4-methylthiazolaldehyde-5 (IV) by using 2-thiol-4-methylthiazol (III) as a raw material; and then in the presence of a temperature not exceeding 50 DEG C, a gage pressure of 0.06-0.2 MPa and a hydrogenation catalyst which is 0.5-7.5 times of the weight of the raw material, removing thiol groups through hydrogenation reaction to obtain the 4-methylthiazolaldehyde-5 (V) as a target product. The method has the advantages of high reaction yields in all steps, good product purity, easy obtaining of chemical raw materials, no harsh environmental protection requirement and suitability for industrial scale production. The reaction flow of the method is disclosed in the specification, wherein R in the formula is a methyl group.
Owner:CHENGDU KAOENSI SCI & TECH
Preparation method of compound 2-(3-formyl-4-isobutoxyphenyl)-4-methylthiazole-5-carboxylic acid ethyl ester and febuxostat
The invention provides a preparation method of 2-(3-formyl-4-isobutoxy phenyl)-4-methyl thiazole-5-ethyl formate which is obtained by using 4-isobutoxy cyanophenyl as an initial raw material and through a series of reactions. The invention also provides a preparation method of febuxostat, which comprises the following steps: reacting 2-(3-formyl-4-isobutoxy phenyl)-4-methyl thiazole-5-ethyl formate with hydroxylamine hydrochloride under the action of a catalyst to obtain a compound with a structure as shown in formula (VIII); hydrolyzing the compound with the structure as shown in formula (VIII) under an alkaline condition, and performing acidification to obtain febuxostat. The preparation method of the invention prepares febuxostat without using cyanides, and is high in safety. The preparation methods of the invention are simple in operation and high in yield. Experiment results show that the yield of step (A) is up to 90%, the yield of step (B) is up to 85%, the yield of step (C) is up to 90%, the yield of step (D) is up to 90%, and the yield of step (E) is up to 97%.
Owner:ZHEJIANG AUSUN PHARMA
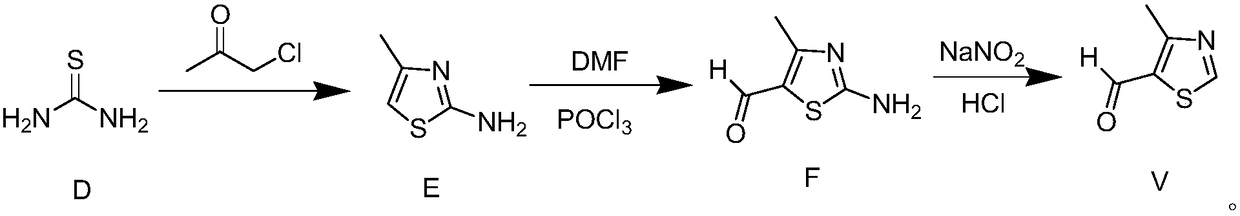
New method for preparing thiabendazole
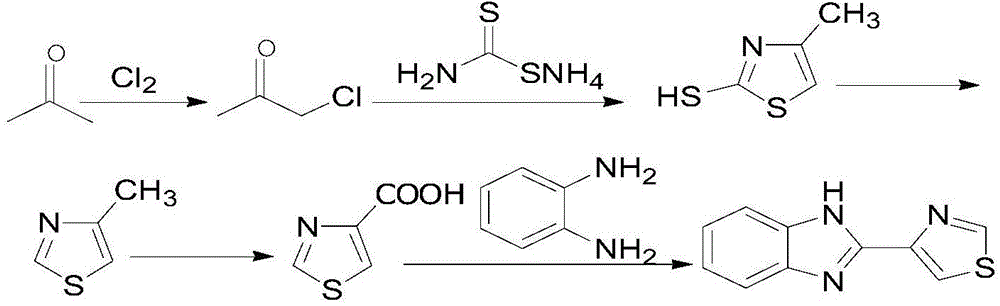
InactiveCN104557902ASolve problems that are not suitable for large-scale mass productionEasy to produceOrganic chemistryDisinfectantThiourea
The invention relates to a new method for synthesizing thiabendazole. Acetone and chlorine are taken as starting materials to synthesize chloroacetone, chloroacetone not subjected to separation can directly react with thiocarbamide to obtain 2-Amino-4-methylthiazole which is subjected to diazotization to obtain 4-methylthiazole, and 4-methylthiazole is oxidized to produce 4-thiazolecarboxylic acid, and finally, 4-thiazolecarboxylic acid reacts with o-phenylenediamine to obtain the target object thiabendazole. The thiabendazole is a broad-spectrum anthelmintic, can repel roundworms, hookworms, whipworms, pinworms, strongyloises stercoralis and trichinization, is also a broad-spectrum efficient disinfectant and is widely used as a fruit fresh-keeping agent and a bactericidal mildew inhibitor in various fields over the past decade in China.
Owner:YANTAI BESTENPHARM TECH CO LTD
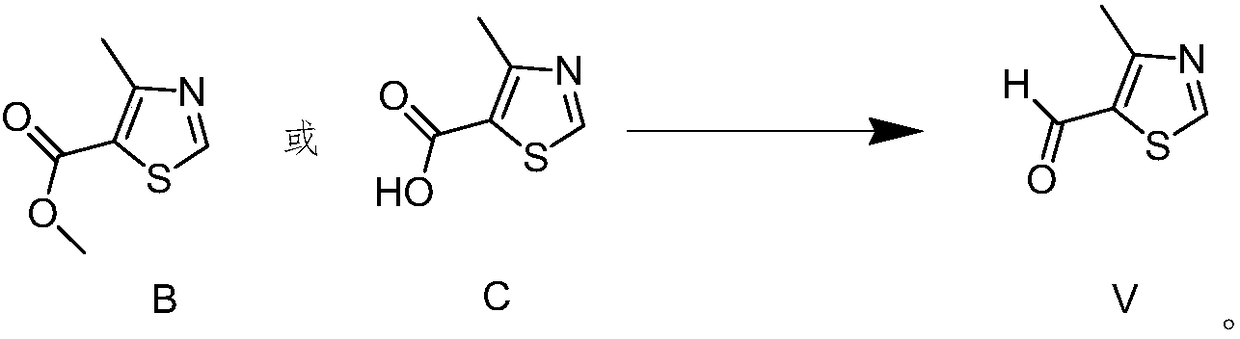
Novel preparation method for 4-methylthiazole-5-carboxaldehyde
The invention discloses a novel preparation method of 4-methylthiazole-5-carboxaldehyde. The novel preparation method includes the steps of subjecting 2-amino-4-methylthiazole to amino methylation toobtain 2-methylamino-4-methylthiazole, subjecting the 2-methylamino-4-methylthiazole to Vilsmeier reaction to obtain 2-methylamino-4-methyl-5-thiazolecarboxaldehyde, and hydrogenating the 2-methylamino-4-methyl-5-thiazolecarboxaldehyde to remove methylamino to obtain the 4-methylthiazole-5-carboxaldehyde. The preparation method has the advantages of moderate reaction condition, high yield, less generated waste and suitability for mass industrial production.
Owner:JINAN ENLIGHTEN BIOTECH CO LTD
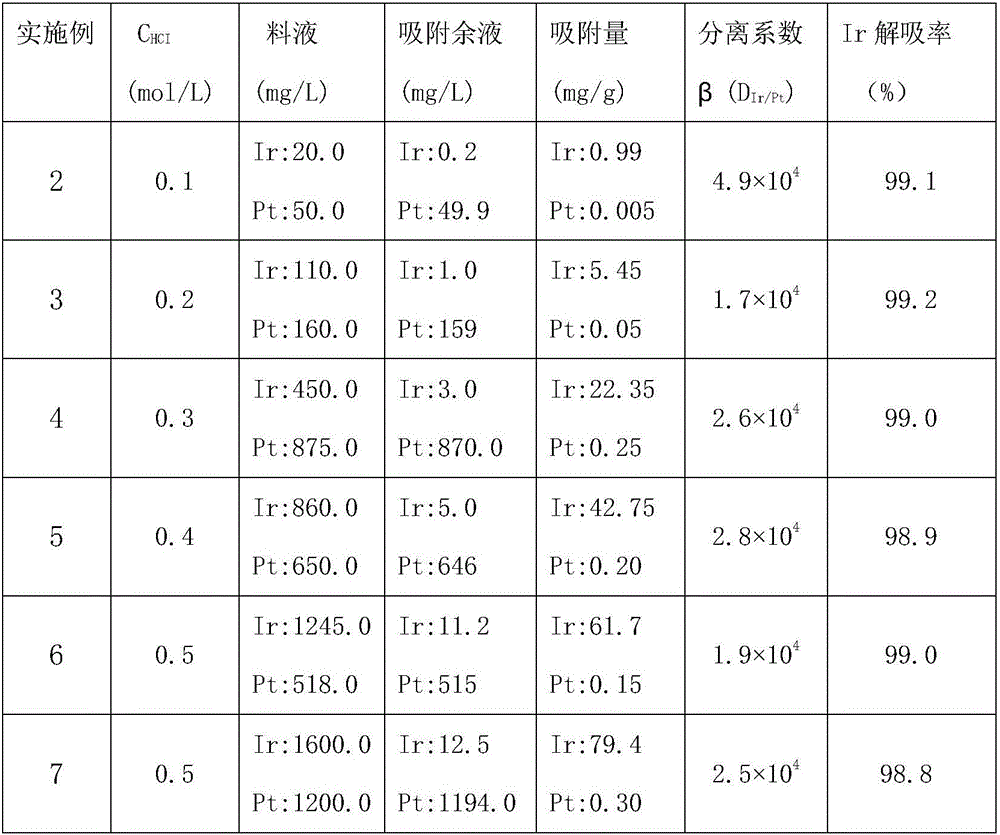
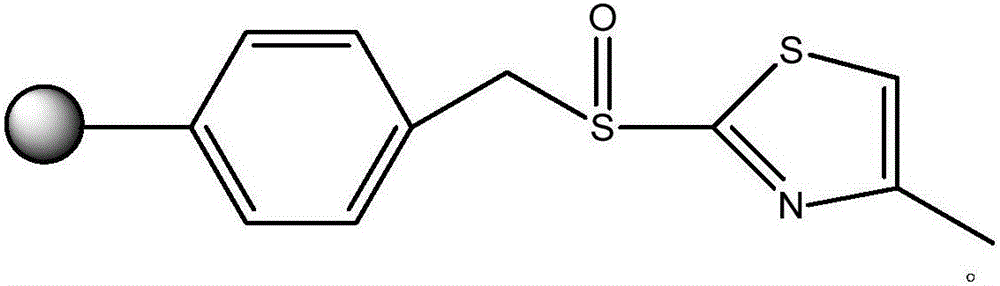
Method for separating iridium and platinum through polystyrene-4-methylthiazole sulfoxide resin
InactiveCN106086450AHigh separation factorHighly selective separationProcess efficiency improvementIridiumSorbent
The invention discloses a method for separating iridium and platinum through polystyrene-4-methylthiazole sulfoxide resin, and belongs to the technical field of platinum group metal separation. The method comprises the steps that the polystyrene-4-methylthiazole sulfoxide resin (PS-4-MTS for short) is used as an adsorbent, Ir(IV) in a 0.1-0.5 mol / L hydrochloric acid medium is adsorbed according to the solid-liquid ratio of 1:50(g / mL), the PS-4-MTS adsorbs Ir in an Ir and Pt mixed solution, Pt is reserved in a water phase, and Ir on the resin is desorbed through an 0.5%-1% NaOH solution. Ir and Pt are separated through the adsorbability difference of the PS-4-MTS adsorbent for Ir and Pt, the operation process is simple and environmentally friendly, the Ir and Pt separation coefficient is high, and the PS-4-MTS adsorbent is good in stability and capable of being reused.
Owner:YUNNAN UNIV
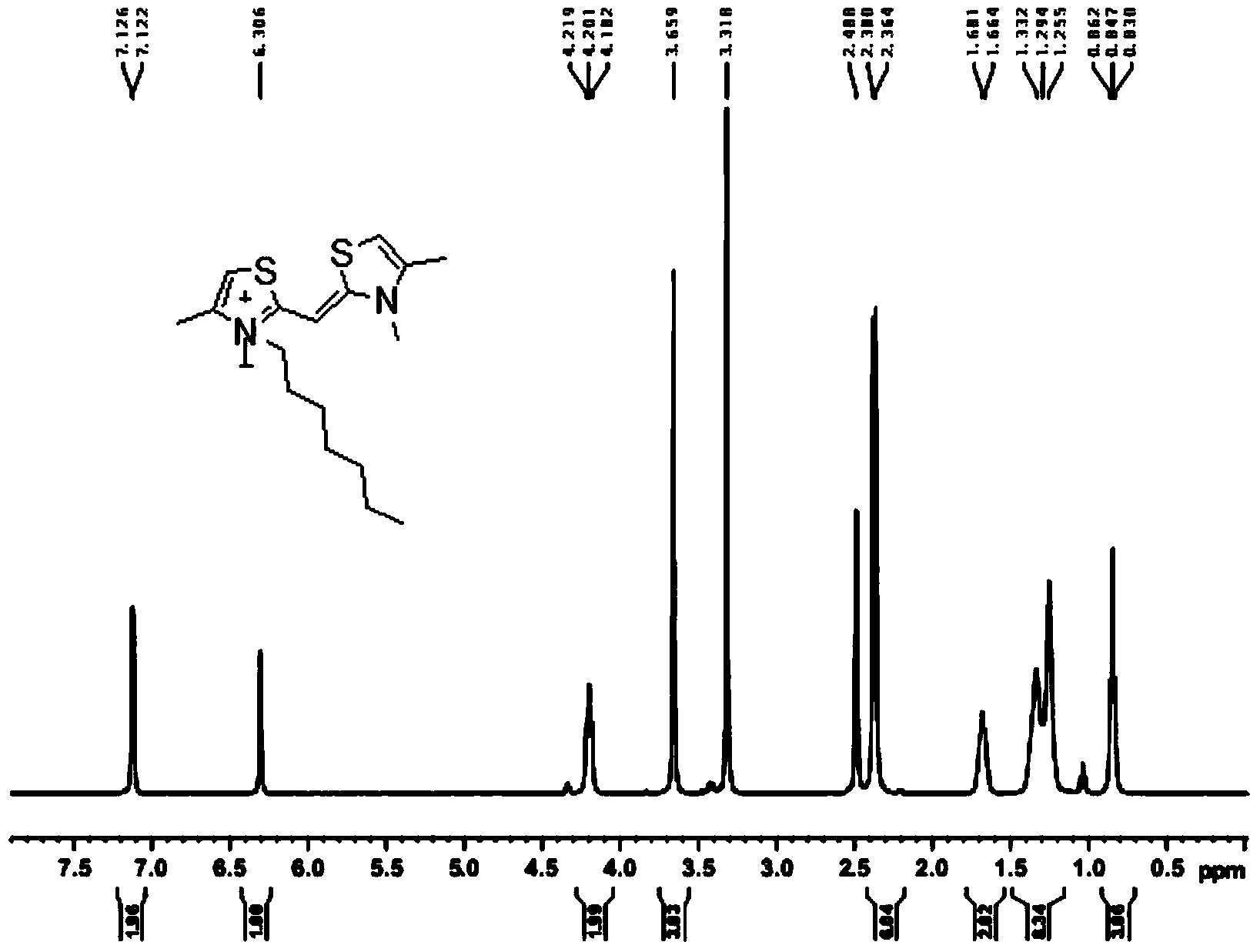
Synthesis method of dithiazole quaternary ammonium salt
The invention provides a synthesis method of a dithiazole quaternary ammonium salt, which comprises the following steps of: when R is equal to R', step one, adding iodo n-heptane in 2,4-dimethylthiazole for reaction to obtain 2,4-dimethyl-3-heptyl thiazole-3-onium iodo salt, step two, carrying out reaction on 4-methylthiazole-2-mercaptan and iodo n-heptane to obtain a product 3-heptyl-4-methylthiazole-2(3H)-sulfoketone, step three, carrying out reaction on the product in the step two and iodo n-heptane to obtain an intermediate 3-heptyl-2-heptyl sulfenyl-4-methylthiazole-3-onium iodo salt, and step four, carrying out reaction on the products in the steps three and one in the presence of organic alkali to obtain yellow dithiazole quaternary ammonium salt; and when R is not equal to R', replacing the alkyl substituent group R' on N in the step two by R' different from the step one. According to the synthesis method, the generation of by-products is avoided, the final product is easy to separate, and the purity and the yield are high, so that the synthesis method is easy for large-scale industrial production and application.
Owner:SHANGHAI LUGUAN BIOTECH
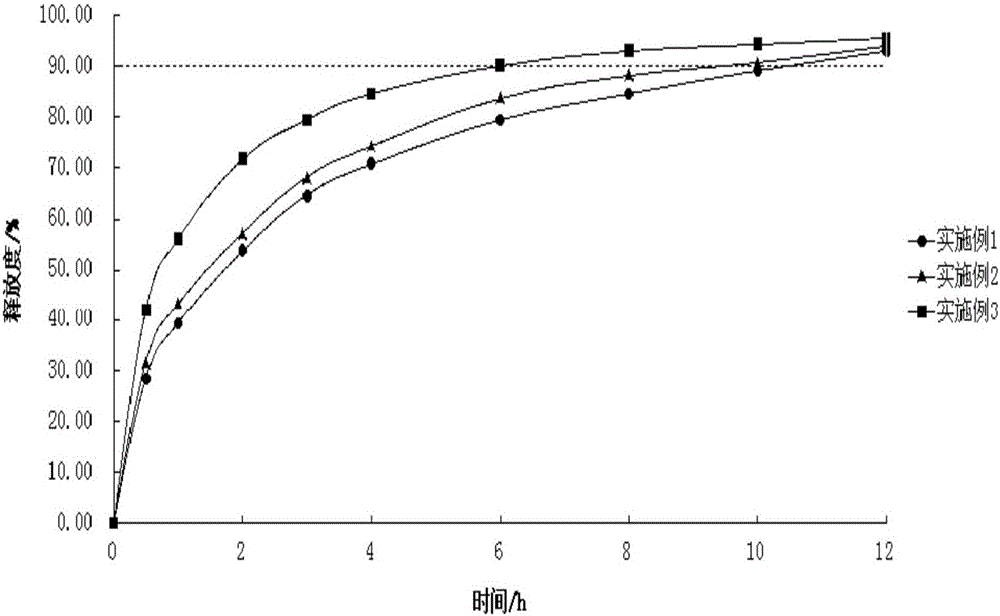
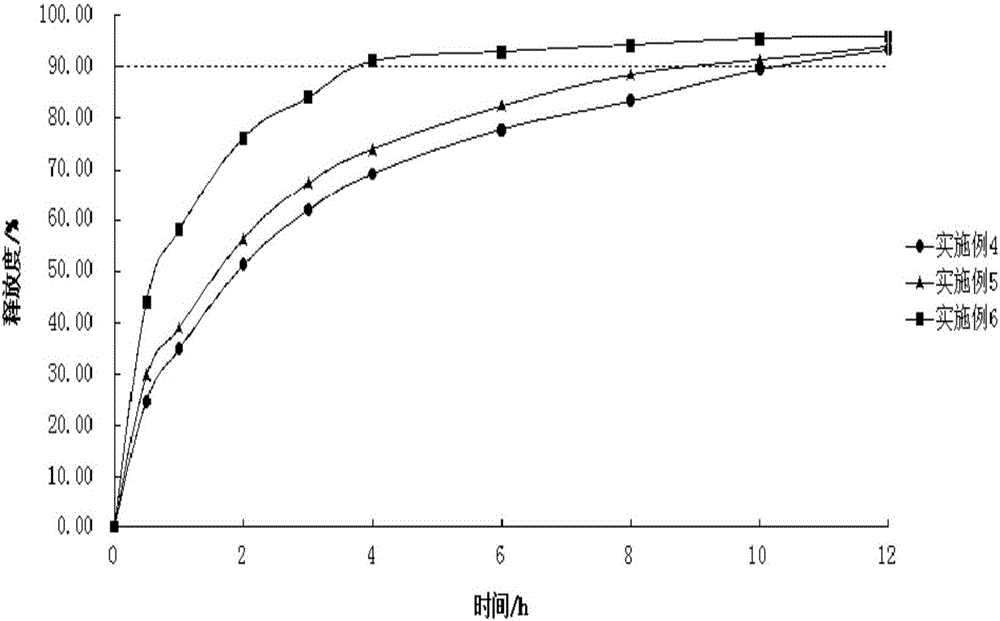
Sustained release preparation used for treating alzheimer's disease, and preparation method thereof
InactiveCN106166142AIn vitro cumulative optimizationIn vitro cumulative release optimizationOrganic active ingredientsNervous disorderDiseaseSide effect
The invention relates to a sustained release preparation, and a preparation method thereof. The sustained release preparation contains 0.1 to 0.5% of nitric acid-2-(4-methylthiazole-5-yl) ethyl ester hydrochloride or nitric acid-2-(4-methylthiazole-5-yl) ethyl ester maleate as the main drug; and a main sustained-release material is prepared from hydroxypropyl methyl cellulose, a filling agent, a lubricating agent, and a binding agent or a wetting agent. The nitric acid-2-(4-methylthiazole-5-yl) ethyl ester hydrochloride or nitric acid-2-(4-methylthiazole-5-yl) ethyl ester maleate sustained release preparation is prepared via wet granulation; influences of gastrointestinal peristalsis speed on the release behavior of the sustained release preparation are few; sustained release is realized; relatively stable blood concentration can be maintained; action time is relatively long; less toxic and side effect is caused; and the sustained release preparation is convenient to use. The sustained release preparation is used for preventing and / or treating alzheimer's disease.
Owner:INST OF MATERIA MEDICA CHINESE ACAD OF MEDICAL SCI
Method for synthesizing ethyl 4-methylthiazole-5-formate employing single step
The invention discloses a method for synthesizing ethyl 4-methylthiazole-5-formate employing a single step. The method comprises the following steps: reacting at 100-180 DEG C for 2-6 hours after mixing 2-chloroacetoacetic acid ethyl ester and ammonium thiocyanate; adding water to dissolve generated ammonium chloride after reaction is finished, and then filtering and drying, so as to obtain ethyl 4-methylthiazole-5-formate, wherein the molar ratio of 2-chloroacetoacetic acid ethyl ester to ammonium thiocyanate is 1:1.01 to 1:1.5. Compared with the prior art, the method has the characteristics as follows: 1, no solvent is used in a reaction process, 2 the method adopts single step synthesis, and is fewer in steps and simple and convenient to operate, and 3, the poisonous and harmful or flammable and combustible raw materials are not used, and the pressure for environmental protection is greatly reduced.
Owner:SHANDONG HUIHAI PHARMA & CHEM
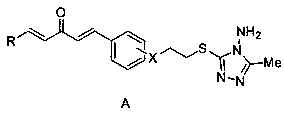
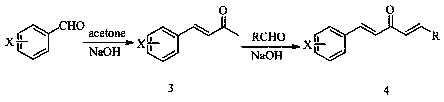
1, 4-pentadiene-3-one derivative containing thioether triazole, preparation method and application
The invention discloses a 1, 4-pentadiene-3-one derivative containing thioether triazole, a preparation method and application. The structural general formula is shown as the specification, wherein Xis 2-O or 4-O, R is phenyl, substituted phenyl, heterocyclyl or 4-methylthiazole. The 1, 4-pentadiene-3-one derivative containing thioether triazole provided by the invention has good control effect on tobacco mosaic virus, ralstonia solanacearum and Xanthomonas oryzae pv. oryzae.
Owner:GUIZHOU UNIV
Juicy peach essence for oil-based ink and preparation method of juicy peach essence
InactiveCN105154233AImprove fragranceGood oil solubilityInksEssential-oils/perfumesSolubilityBenzaldehyde
The invention discloses juicy peach essence for oil-based ink. The juicy peach essence for oil-based ink is composed of juicy peach essence, maltodextrin and starch sodium octenylsuccinate. The juicy peach essence is composed of ethyl acetate, ethyl butyrate, butyl butyrate, ethyl 2-methyl-butanoate, leaf alcohol, ethyl acetoacetate, benzaldehyde, 2-isopropyl-4-methyl thiazole, benzyl alcohol, linalool oxide, linalool, ethyl benzoate, gamma-octanoic lactone, linalyl acetate, gamma-decalactone, delta-decalactone, benzyl benzoate, benzyl formate, benzyl butyrate, 8-mercaptomenthone, damacscone beta, Brazil sweet orange oil, raspberry ketone and glycerol triacetate. The invention further provides a preparation method of the juicy peach essence for oil-based ink. The juicy peach essence, maltodextrin, starch sodium octenylsuccinate and deionized water are evenly mixed and subjected to homogeneous emulsification, spraying and drying are then performed, the granulated juicy peach essence is formed, and therefore the aroma retaining effect and the oil solubility of the essence are improved, and the juicy peach essence for oil-based ink is suitable for adding the aroma of the oil-based link.
Owner:SHANGHAI INST OF TECH
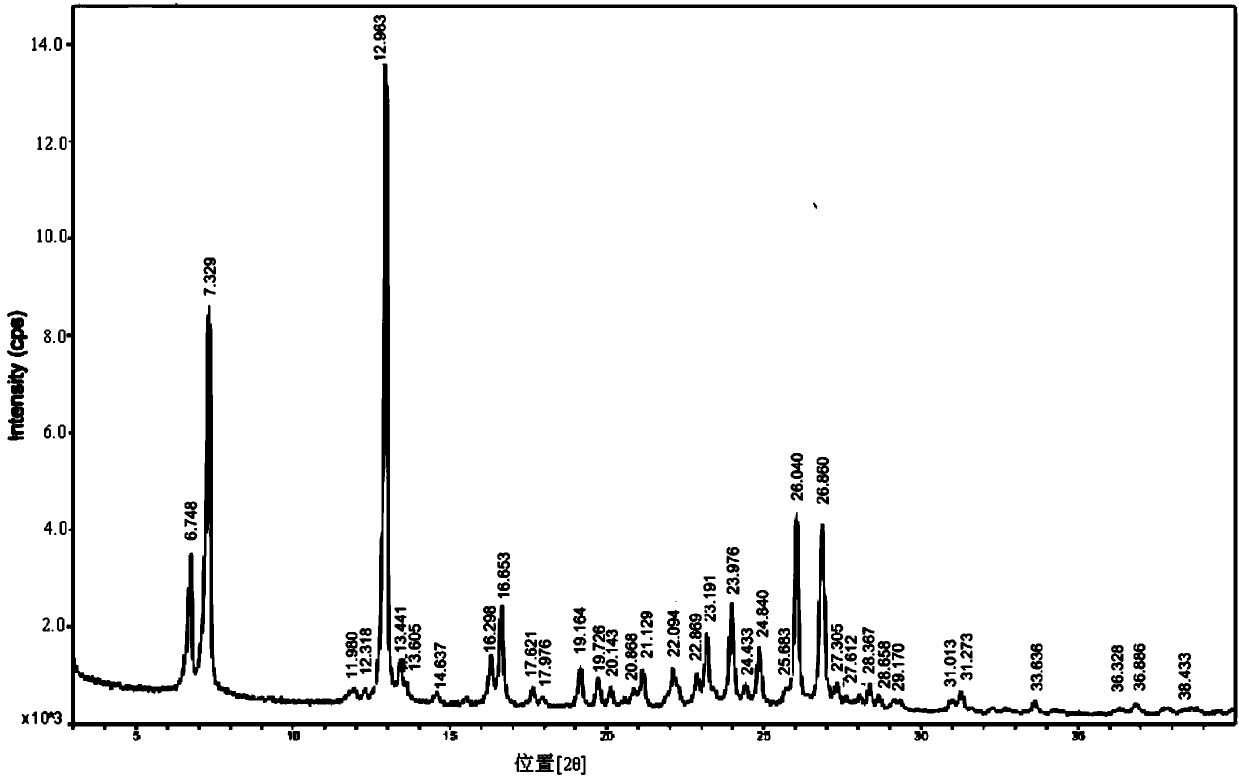
Preparation method of 2-(3-cyan-4-isobutoxyphenyl)-4-methylthiazole-5-formic acid A crystal
The invention discloses a preparation method of 2-(3-cyan-4-isobutoxyphenyl)-4-methylthiazole-5-formic acid A crystal. The preparation method comprises the following steps: mixing 2-(3-cyan-4-isobutoxyphenyl)-4-methylthiazole-5-formic acid with a solvent I and a solvent II, heating until the compound is completely dissolved, and cooling the solution to crystallize so as to obtain a febuxostat crystal; wherein the solvent I is one or more components selected from ethanol, 1-propanol, 2-propanol, 1-butanol, 2-butanol, acetone, and ethyl acetate; and the solvent II is one or more components selected from n-propyl acetate, isopropyl acetate, n-butyl acetate, isobutyl acetate, and methyl tertbutyl ether. The preparation method has the advantages that: a febuxostat crystal can be prepared through the solvent system provided by the invention, and does not contain any impurity namely febuxostat acetate; moreover the solvents used in the system are all third-kind solvents with small toxicity, and thus the preparation method is environment-friendly.
Owner:ZHEJIANG APELOA KANGYU PHARMA +2
Preparation method of 4-methylthiazol
InactiveCN103232404AEasy to recycle and reuseAvoid pollutionOrganic chemistryChemical industryChloroacetone
The invention relates to a preparation method of 4-methylthiazol. The invention aims at solving the problem that in the existing method for preparing the methylthiazol, the reaction route is long, the reaction is complicated and not easy to control, the production cost of the used catalyst is high and the yield is high. The preparation method comprises the following steps of A, mixing formamide, anhydrous glycol dimethyl ether and phosphorus pentasulfide, heating the mixture to be reacted at the temperature of 45 to 50 DEG C, and filtering the mixture to obtain thioformamide; B, adding chloroacetone into the thioformamide to be reacted at the temperature of 50 to 60 DEG C, adjusting the pH value to 6 to 7, and cooling, filtering, washing and dehydrating the mixture to obtain the 4-methylthiazol. Raw materials are cheap and easy to obtain, the reaction condition is moderate, the synthesis step is simple, no catalyst is used, no pollution exists, the yield is high, operation is simple and convenient, and the solvent can be recycled. The preparation method is applied to the technical field of chemical industry.
Owner:HEILONGJIANG UNIV
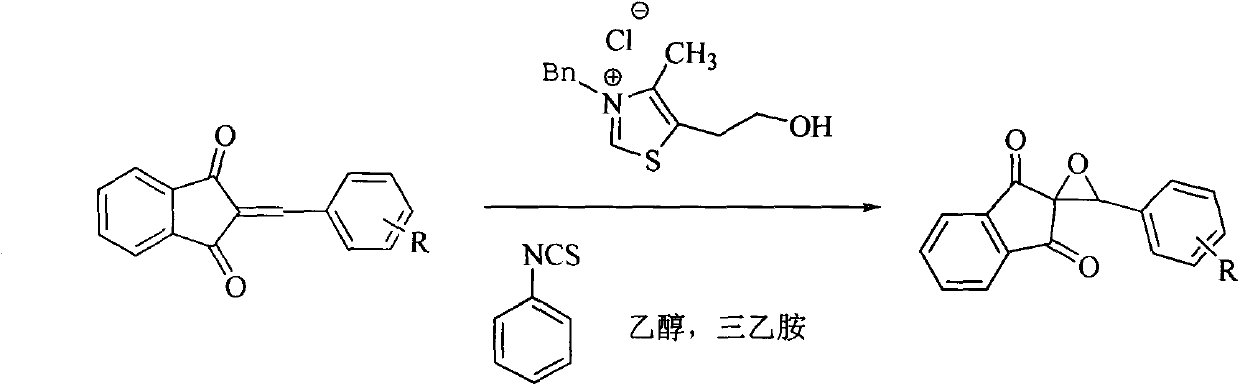
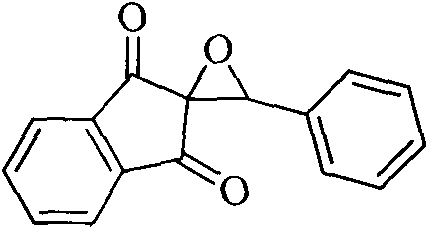
New epoxidation method of 2-benzylidene-1,3-indan diketone double bond
The invention relates to a new epoxidation method of 2-benzylidene-1,3-indan diketone double bond. The method comprises the following steps of: adding 2-benzylidene-1,3-indan diketone, phenyl isothiocyanate, 3-benzyl-5-(2-ethoxy)-4-methyl thiazole chlorate and triethylamine in a molar ratio of 1:0.25:0.25:0.25 into a reaction container containing a certain amount of ethanol, stirring at room temperature until the reaction is ended, steaming and removing the ethanol, and carrying out thin layer chromatography separation on residues to obtain the epoxidized product of 2-benzylidene-1,3-indan diketone double bond. By adopting the method, the defects that substrates are destroyed due to strong oxidation property of hydrogen peroxide in the double-bond epoxidation method by using hydrogen peroxide as the epoxidation reagent and epoxidation products cannot be synthesized from highly conjugated double bonds by the method are overcome. The method is simple to operate, low in energy consumption and high in productivity, and heating is not required.
Owner:YANGZHOU UNIV
Process for producing 4-thiazolylmethyl derivative
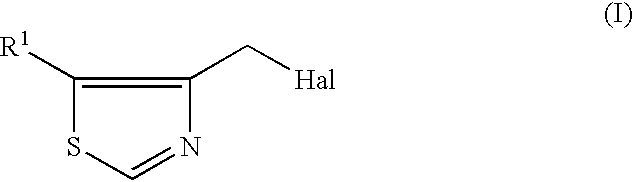
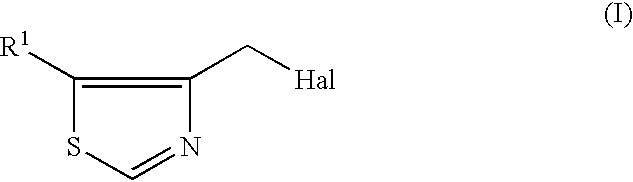
InactiveUS6506903B1Simple and economical productionEasy to purifyPeptide/protein ingredientsPeptidesHalogenHydrogen
A process for the production of 4-thiazolylmethyl derivatives are provided. A process for the production of the compound represented by the formula (I):wherein R1 is hydrogen or halogen and Hal is halogen, which comprises reacting 4-methylthiazole with N-halosuccinimide in a solvent in the presence of a radical initiator.
Owner:SHIONOGI & CO LTD
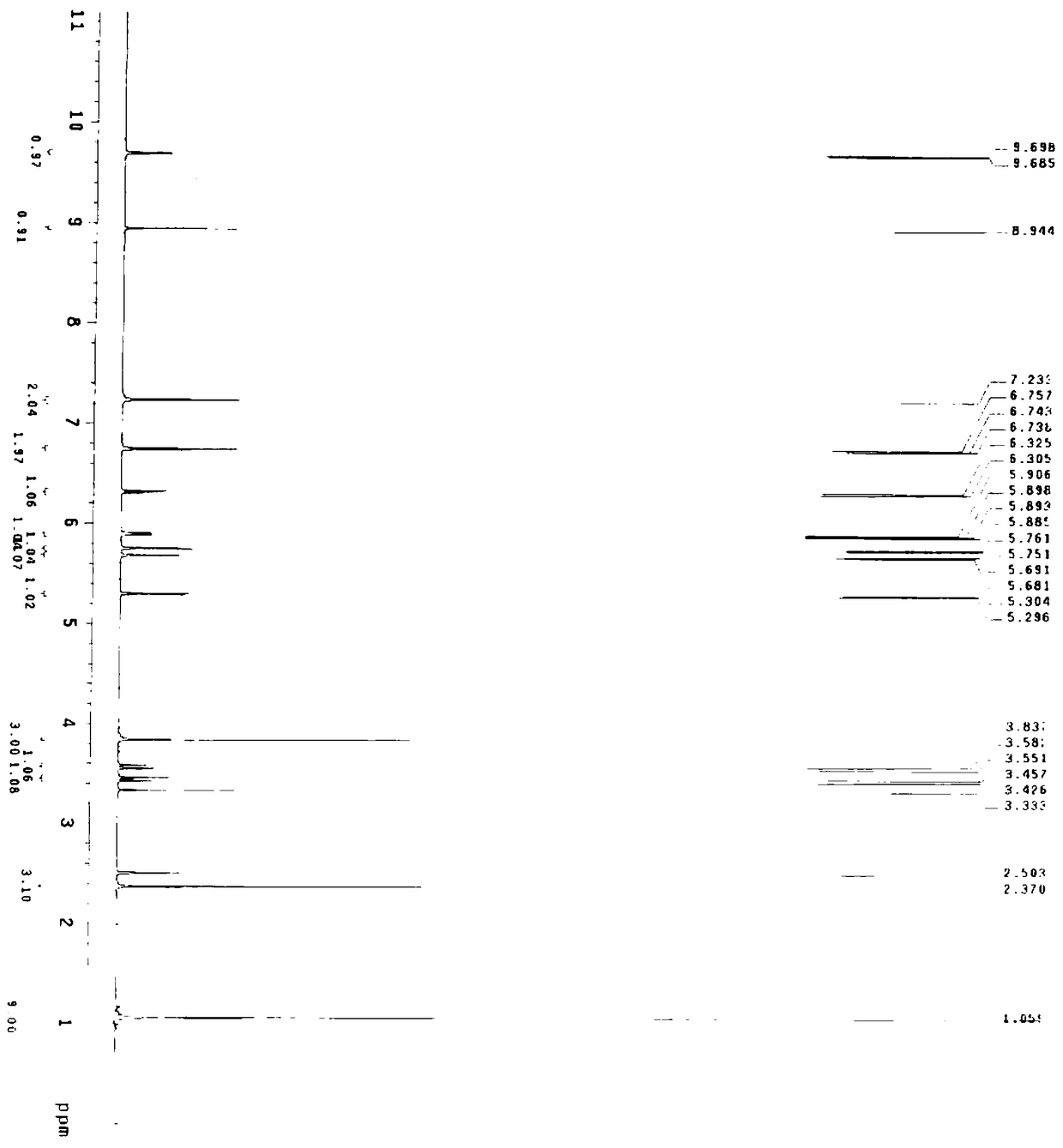
Preparation method of 3-[(4-amino-2-methyl-5-pyrimidinyl) methyl]-5-(2-sulfonyl oxy ethyl)-4-methylthiazole
ActiveCN102558166AEasy to separate and purifyEasy to prepareOrganic chemistryOrganic solventEsterification reaction
The invention discloses a preparation method of 3-[(4-amino-2-methyl-5-pyrimidinyl) methyl]-5-(2-sulfonyl oxy ethyl)-4-methylthiazole. The preparation method comprises the step that vitamin B1 is in contact with a sulfonating agent in the existence of an organic solvent under the condition of a sulfuric acid esterification reaction. By adopting the method disclosed by the invention, products with higher purity can be obtained; and besides, the preparation method is simple, reaction raw materials are easy to obtain, the reaction condition is wild and the reaction products are easy to separate and purify. The preparation method of the 3-[(4-amino-2-methyl-5-pyrimidinyl) methyl]-5-(2-sulfonyl oxy ethyl)-4-methylthiazole, which is provided by the invention, has the advantages that a guarantee is provided for the identification of important related substances used during the production of the vitamin B1, and an evidence is provided for the improvement on the quality of the vitamin B1 in the production process at the same time.
Owner:江西天新药业股份有限公司
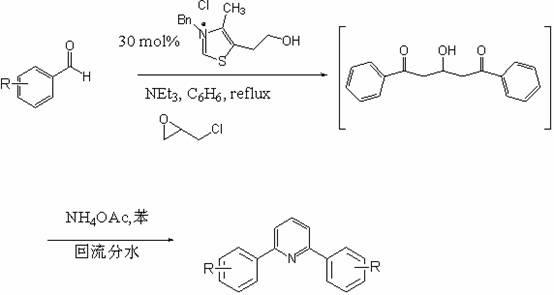
Synthetic method of diarylpyridines
The invention discloses a method for synthesizing diarylpyridine medicines. The method comprises the following steps of: 1) adding 1 to 2 weight parts of carbene catalyst, namely 3-benzyl-5-(2-hydroxyethyl)-4-methylthiazolium chloride, 1 to 2 weight parts of triethylamine, 100 to 200 weight parts of epichlorohydrin and a solvent into a container and stirring to react at normal temperature; 2) adding 200 to 400 weight parts of substituted aldehyde, heating and stirring until the temperature of mixed liquor is 50 to 100 DEG C, and keeping the temperature for 20 to 40 minutes or radiating for 5 to 15 minutes under the microwave with the frequency of between 150 and 500Hz; 3) adding 100 to 200 weight parts of ammonium acetate and stirring uniformly; 4) heating and stirring until the temperature of mixed liquor is 50 to 100 DEG C, and keeping the temperature for 20 to 40 minutes or radiating for 5 to 15 minutes under the microwave with the frequency of between 150 and 500Hz; and 5) evaporating the solvent to dryness after the reaction is finished, and recrystallizing a solid by using ethanol to obtain the diarylpyridine medicines. By the method, the reaction time is shortened, the energy consumption is reduced, and the synthetic efficiency is improved. The catalyst which is nontoxic and harmless to the environment is adopted.
Owner:孙光辉
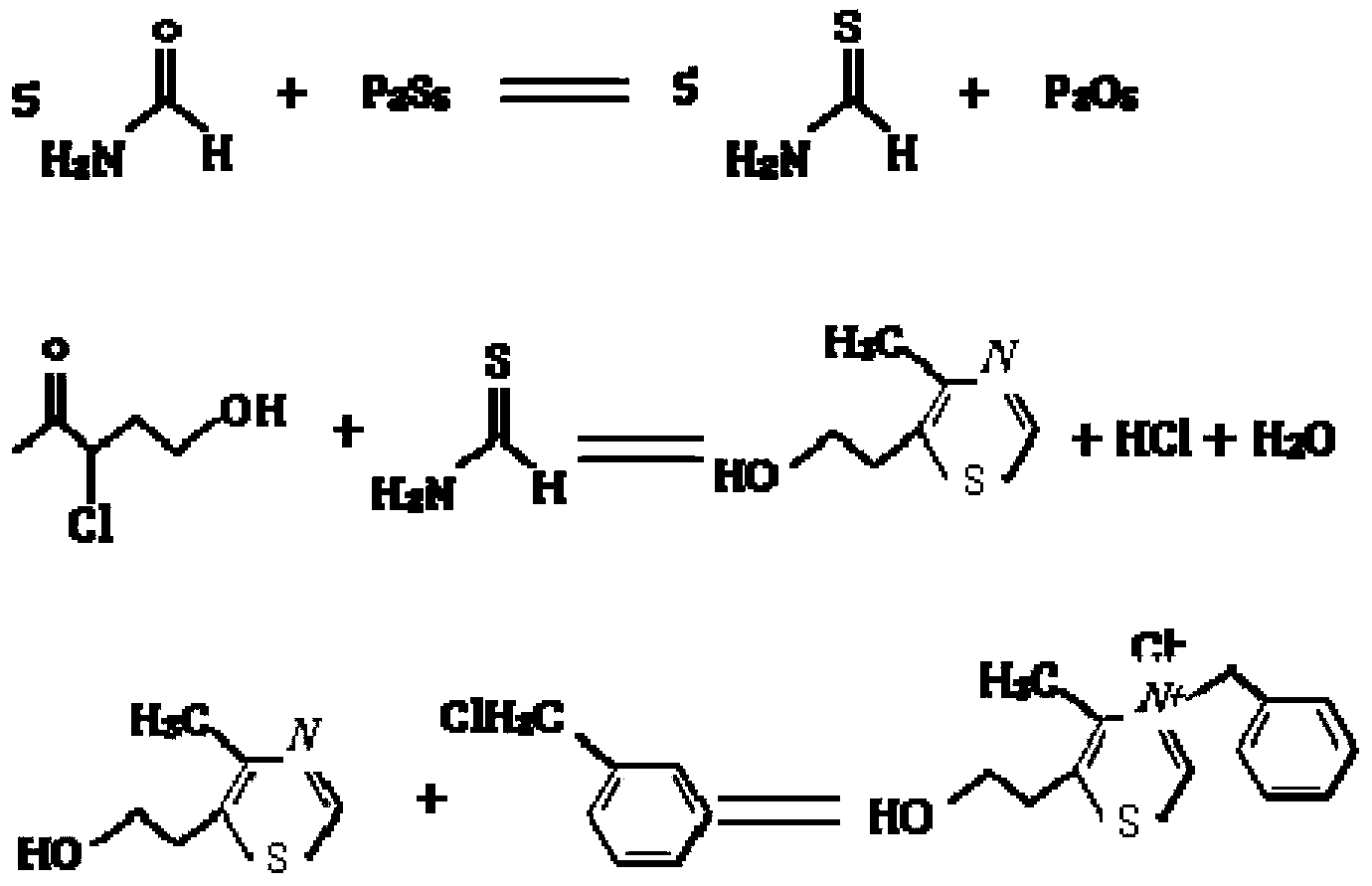
Synthetic method of 3-(phenylmethyl)-5-(2-hydroxyethyl)-4-methyl-thiazoline chloride
InactiveCN103351359AFew reaction stepsMild reaction conditionsOrganic chemistryBenzyl chloridePhosphorus pentasulphide
The invention provides a synthetic method of 3-(phenylmethyl)-5-(2-hydroxyethyl)-4-methyl-thiazoline chloride. In the method, 3-chloro-3-acetyl propanol, formamide, phosphorus pentasulfide and benzyl chloride is used as a raw material and acetonitrile is used as a solvent. The synthetic method comprises the following processes: preparing methanethioamide, preparing 4-methyl-5-(2-hydroxyethyl)-thiazole, synthesizing 3-phenylmethyl-5-(2 -hydroxyethyl)-4-methyl-thiazoline chloride, refining and drying. The preparation method provided by the invention has less reaction steps, a mild reaction condition, and high product yield.
Owner:BENGBU COLLEGE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com





![Novel derivative of 2-[3-cyano-4-isobutoxyphenyl]-4-methylthiazol-5-formic acid, preparation method for novel derivative and application of novel derivative Novel derivative of 2-[3-cyano-4-isobutoxyphenyl]-4-methylthiazol-5-formic acid, preparation method for novel derivative and application of novel derivative](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/d18dd353-e098-425d-ae7a-f472a364c0b9/BDA0000525228040000061.PNG)
![Novel derivative of 2-[3-cyano-4-isobutoxyphenyl]-4-methylthiazol-5-formic acid, preparation method for novel derivative and application of novel derivative Novel derivative of 2-[3-cyano-4-isobutoxyphenyl]-4-methylthiazol-5-formic acid, preparation method for novel derivative and application of novel derivative](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/d18dd353-e098-425d-ae7a-f472a364c0b9/BDA0000525228040000101.PNG)
![Novel derivative of 2-[3-cyano-4-isobutoxyphenyl]-4-methylthiazol-5-formic acid, preparation method for novel derivative and application of novel derivative Novel derivative of 2-[3-cyano-4-isobutoxyphenyl]-4-methylthiazol-5-formic acid, preparation method for novel derivative and application of novel derivative](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/d18dd353-e098-425d-ae7a-f472a364c0b9/BDA0000525228040000111.PNG)

![Preparation method for 3-[(4-amino-5-pyrimidinyl)methyl]-5-(2-hydroxyethyl)-4-methylthiazole nitrate Preparation method for 3-[(4-amino-5-pyrimidinyl)methyl]-5-(2-hydroxyethyl)-4-methylthiazole nitrate](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/0155700f-3441-4fcd-a8bd-98585c7e7a95/HDA00002661029300011.PNG)
![Preparation method for 3-[(4-amino-5-pyrimidinyl)methyl]-5-(2-hydroxyethyl)-4-methylthiazole nitrate Preparation method for 3-[(4-amino-5-pyrimidinyl)methyl]-5-(2-hydroxyethyl)-4-methylthiazole nitrate](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/0155700f-3441-4fcd-a8bd-98585c7e7a95/HDA00002661029300021.PNG)
![Preparation method for 3-[(4-amino-5-pyrimidinyl)methyl]-5-(2-hydroxyethyl)-4-methylthiazole nitrate Preparation method for 3-[(4-amino-5-pyrimidinyl)methyl]-5-(2-hydroxyethyl)-4-methylthiazole nitrate](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/0155700f-3441-4fcd-a8bd-98585c7e7a95/HDA00002661029300031.PNG)
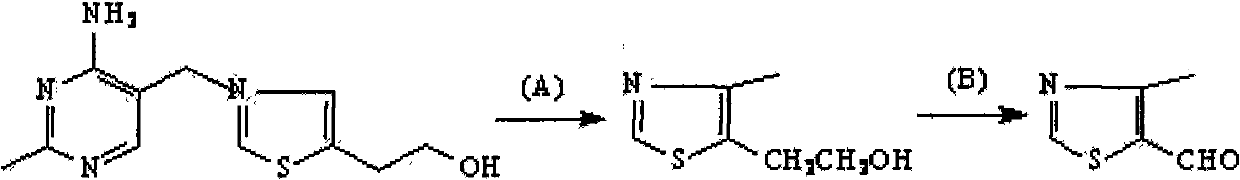



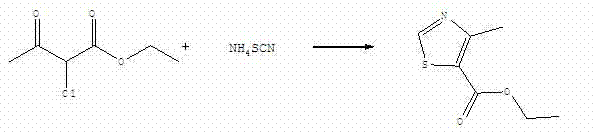
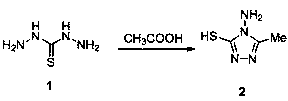







![Preparation method of 3-[(4-amino-2-methyl-5-pyrimidinyl) methyl]-5-(2-sulfonyl oxy ethyl)-4-methylthiazole Preparation method of 3-[(4-amino-2-methyl-5-pyrimidinyl) methyl]-5-(2-sulfonyl oxy ethyl)-4-methylthiazole](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/701ca328-3b47-41ac-9a0d-aa380f7f4efb/HDA0000123702150000011.png)
![Preparation method of 3-[(4-amino-2-methyl-5-pyrimidinyl) methyl]-5-(2-sulfonyl oxy ethyl)-4-methylthiazole Preparation method of 3-[(4-amino-2-methyl-5-pyrimidinyl) methyl]-5-(2-sulfonyl oxy ethyl)-4-methylthiazole](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/701ca328-3b47-41ac-9a0d-aa380f7f4efb/HDA0000123702150000021.png)
![Preparation method of 3-[(4-amino-2-methyl-5-pyrimidinyl) methyl]-5-(2-sulfonyl oxy ethyl)-4-methylthiazole Preparation method of 3-[(4-amino-2-methyl-5-pyrimidinyl) methyl]-5-(2-sulfonyl oxy ethyl)-4-methylthiazole](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/701ca328-3b47-41ac-9a0d-aa380f7f4efb/HDA0000123702150000031.png)