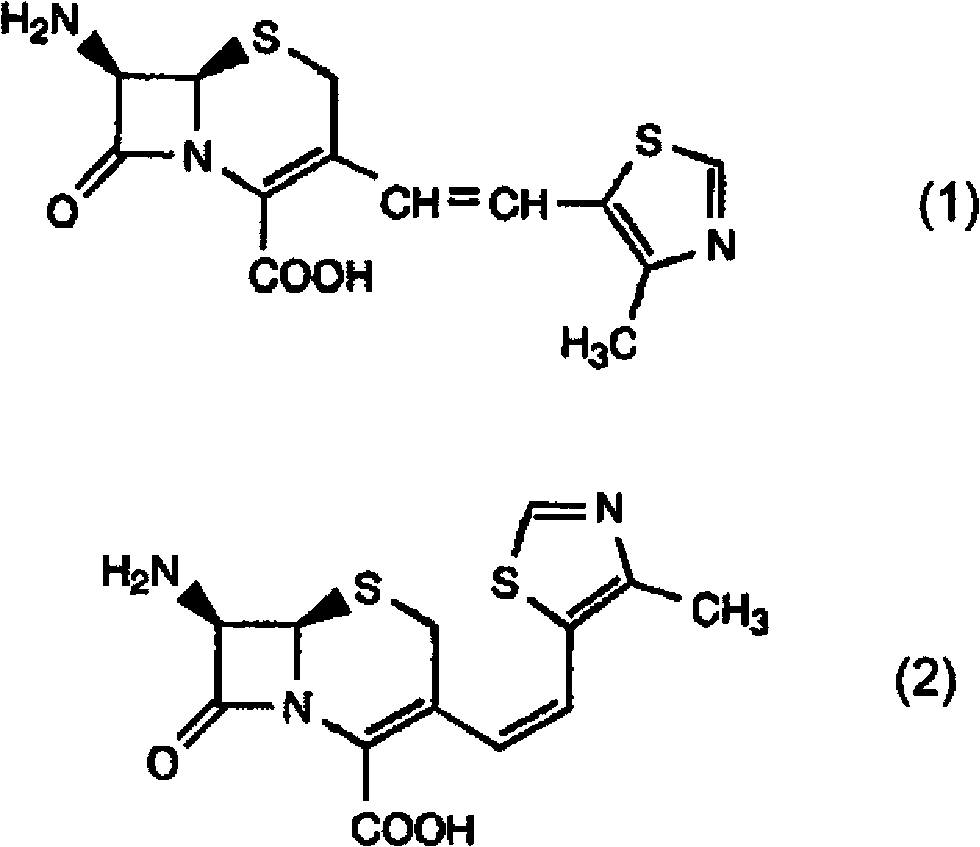
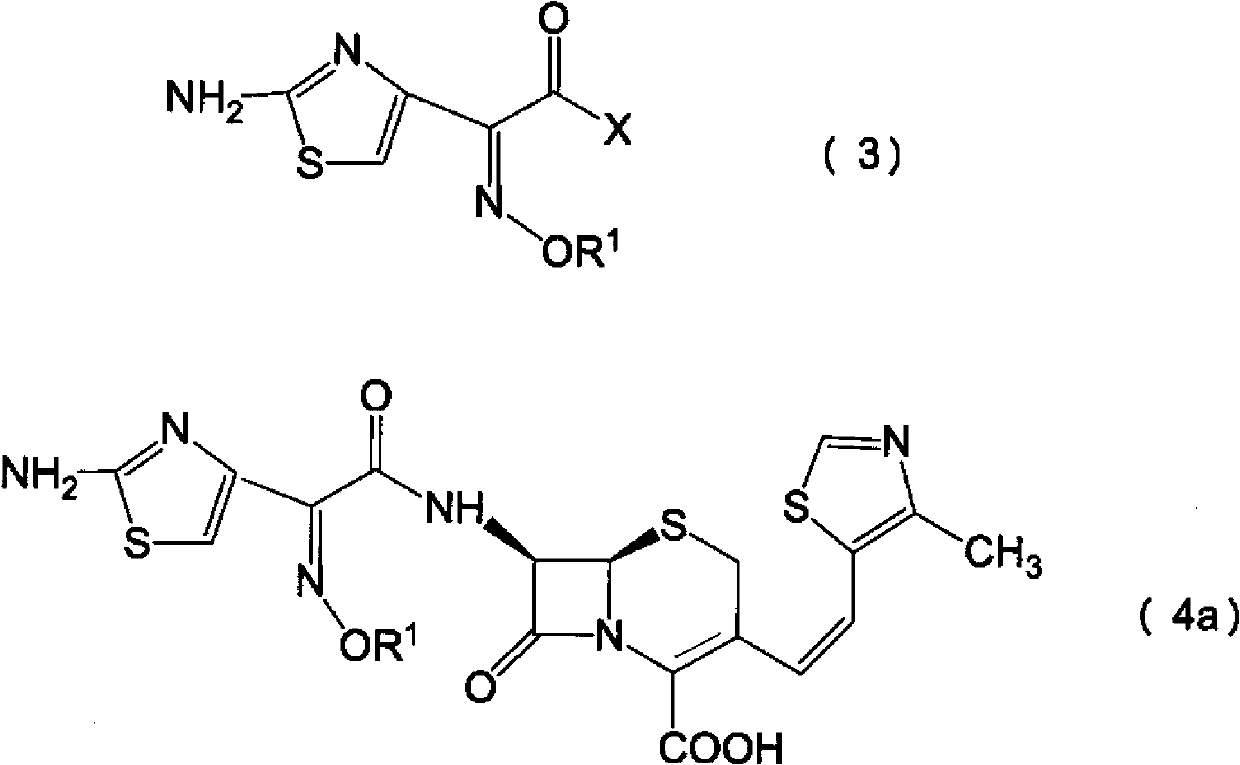
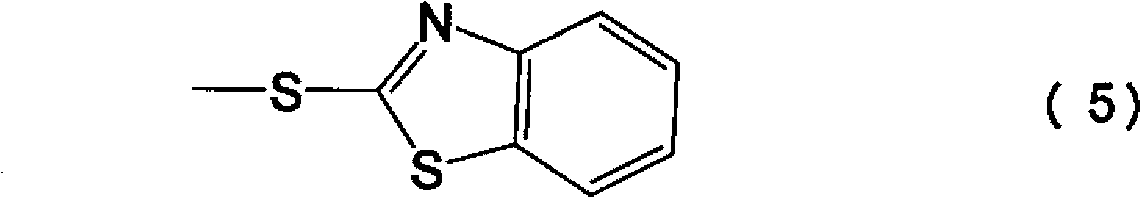
Process for preparation of cephalosporin derivative
A manufacturing method and technology of cephalosporins are applied in the directions of medical preparations containing active ingredients, antibacterial drugs, pharmaceutical formulas, etc., and can solve the problems of complicated procedures and unfavorable industries.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0129] (1) Deprotection reaction process of the 7-position amide bond
[0130] In a four-necked flask, 10.0 g of a compound represented by the following formula (10) (3.5% content of E body) was weighed, and 240 g of a 6% by weight aqueous sodium bicarbonate solution was added to prepare an aqueous sodium salt solution. 7.0 g of penicillin-G acylase (PGA-450, manufactured by Dallas Biotech Limited) was added to this aqueous solution. A 5% by weight aqueous solution of sodium carbonate at a liquid temperature of 25 to 35° C. was added to control the pH at 7.5 to 8.5, and the 7-position deprotection reaction of the sodium salt of the compound represented by the formula (10) was performed for 2 hours. After completion of the reaction, 7.0 g of a sodium salt of a compound represented by the following formula (11) containing 3.5% of the E body in terms of the E body content was contained in the aqueous solution. In addition, it contains 16.6% of phenylacetic acid in terms of pheny...
Embodiment 2
[0156] In the first step of Example 1, except that 2.8 g of CL-KP (trade name) manufactured by Ajinomoto Fine Chemicals Co., Ltd. was used as activated carbon, the carbon dioxide represented by the formula (11) was obtained in the same manner as in Example 1. Compound crystals. The iodine adsorption performance of the activated carbon is 1620mg / g, and the methylene blue adsorption performance is 280ml / g.
[0157] Next, after reacting in the same manner as in the second step of Example 1, 40 g of a 2.5% aqueous sodium bicarbonate solution was added, followed by washing with 80 g of dichloromethane twice, and the aqueous layer was concentrated. The sodium salt of the compound represented by formula (7) precipitates by concentration. Furthermore, 50 g of acetone was added and aged at 0 to 5° C. for 30 minutes, and the precipitated crystals were collected by filtration, washed with water and acetone, and dried. In addition, the various physical properties of the compound of form...
Embodiment 3
[0161] (1) Deprotection reaction process of the 7-position amide bond
[0162] Enzyme reaction was carried out under the same operation and conditions as in Example 1. After completion of the reaction, 7.0 g of the sodium salt of the compound represented by the formula (11) containing 3.5% of the E body content was contained in the aqueous solution. In addition, it contains 16.6% of phenylacetic acid in terms of phenylacetic acid content.
[0163] (2) Phenylacetic acid removal process (B process)
[0164] The enzyme (PGA-450) was filtered out from the aqueous solution obtained in the first step, the liquid temperature was kept at 10°C and adjusted to pH 4.2 with concentrated hydrochloric acid, and aged for 1 hour directly. The compound represented by the formula (1) is precipitated by this aging, and then filtered to recover the precipitate. In addition, the phenylacetic acid content of the obtained precipitate was 0.5%.
[0165] (3) The first process
[0166] 7.0 g of th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Iodine adsorption performance | aaaaa | aaaaa |
| Iodine adsorption performance | aaaaa | aaaaa |
| Iodine adsorption performance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com