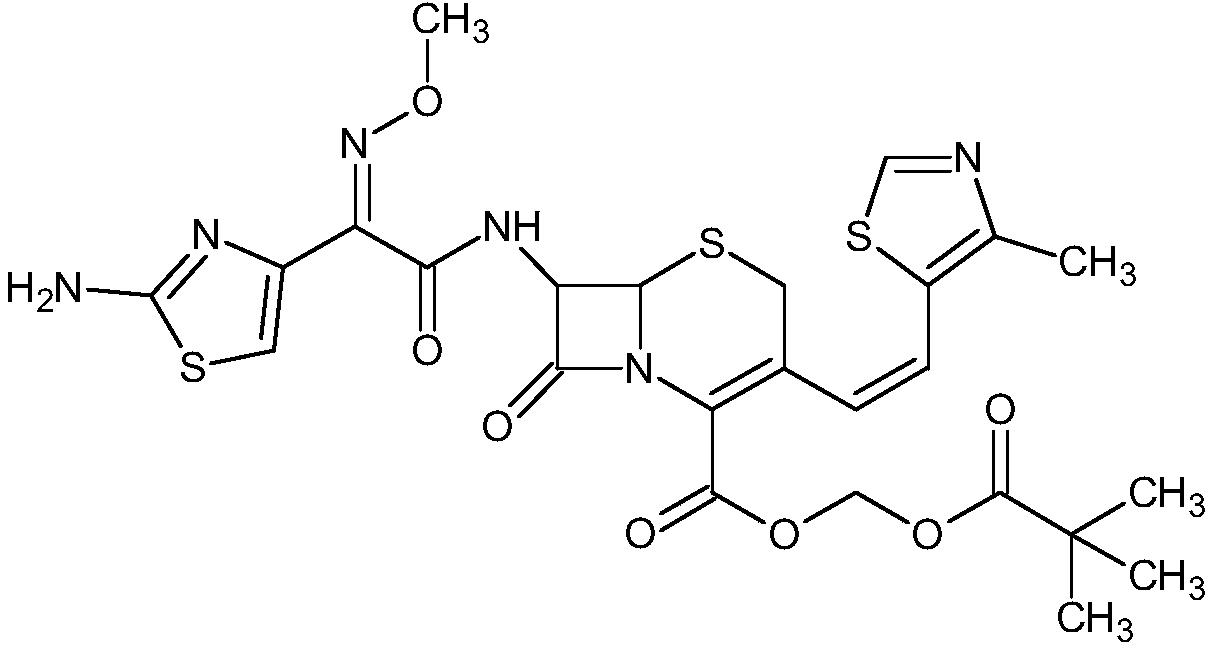
Method for synthesizing cefditoren pivoxil
A cefditoren pivoxil and synthetic method technology, applied in the direction of organic chemistry, etc., can solve problems such as high pressure on environmental protection, difficult recycling, complicated cefditoren pivoxil, etc., and achieve the goal of reducing production costs and environmental protection expenditures, and being easy to recycle and apply Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
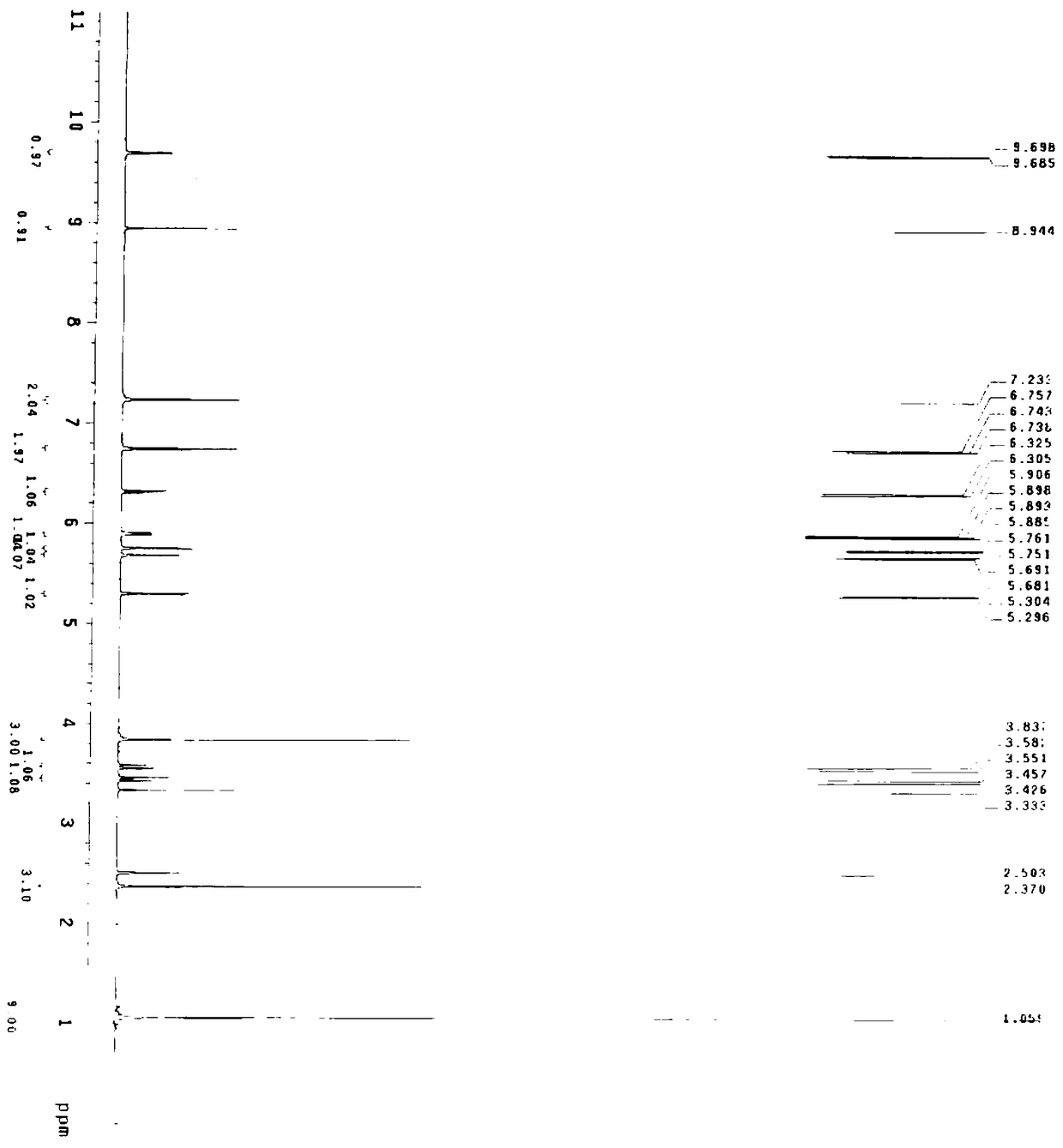
[0060] Embodiment 1 A kind of preparation method of cefditoren pivoxil, the preparation method comprises the following steps:
[0061] (1) Preparation of cefditoren mother nucleus: drop into 200ml of water in reaction bottle, temperature 0~10 ℃, add D-7ACA50g, add dropwise 7% sodium bicarbonate to adjust pH to dissolve clear, add sodium bromide 10g, 2,2,6,6-tetramethylpiperidine nitrogen oxide 2g, 50ml of 10% sodium hypochlorite was added dropwise, reacted for 2-3 hours, and 3N hydrochloric acid was added dropwise to adjust the pH to 3.5 to obtain about 47.08g of compound 1.
[0062] A: Put 100ml of dichloromethane into the reaction bottle, add about 23.55g of compound 1, control the temperature at 0-20°C, add hexamethyldisilazane, and react under reflux for about 5 hours to obtain the feed solution of compound 2, keep it warm 0~10℃ for later use.
[0063] B: Put 100ml of dichloromethane into the reaction bottle, add 14.4g (112mmol) of 4-methylthiazole-5-methanol, 17.7g (118m...
Embodiment 2
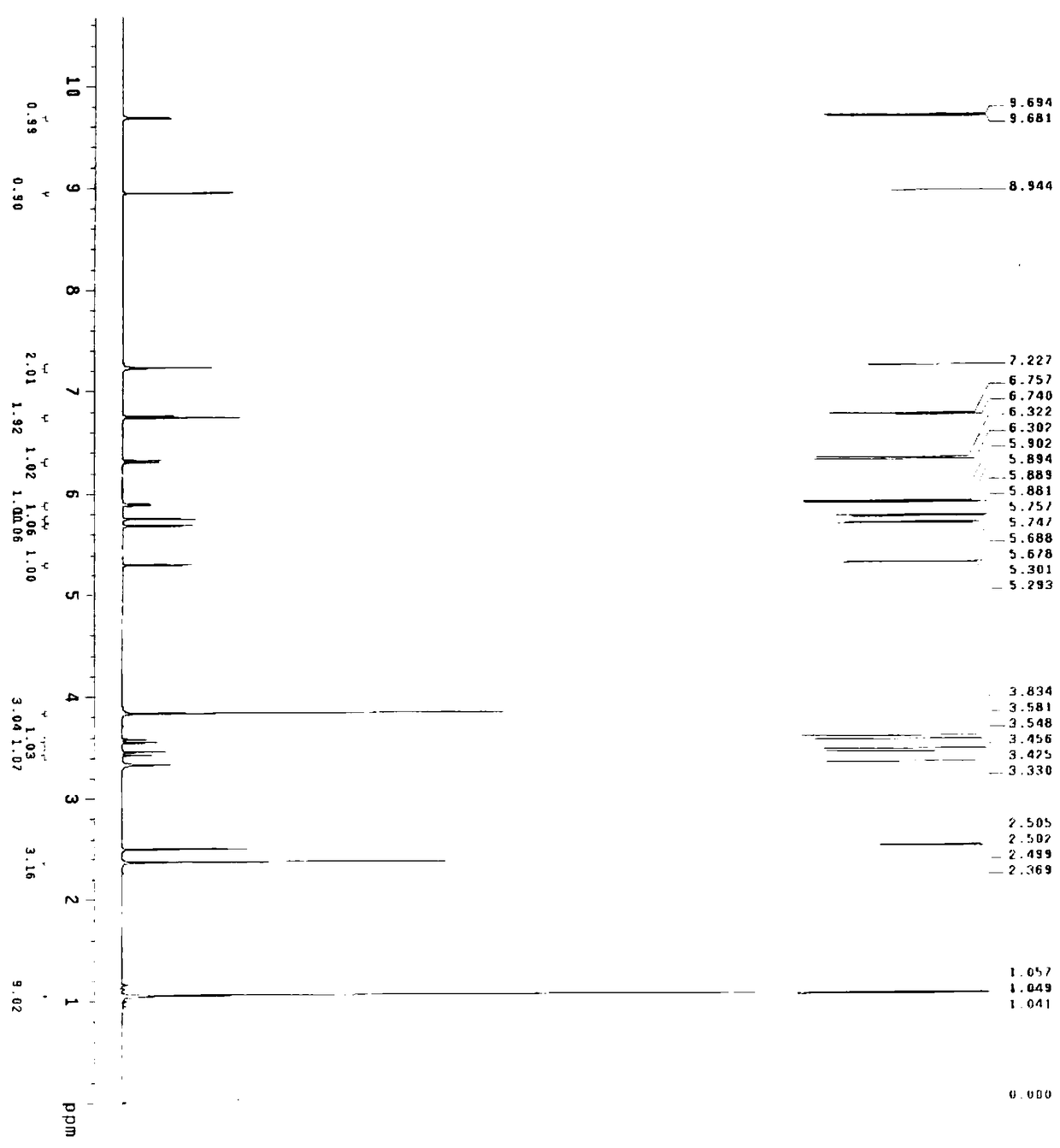
[0069] Embodiment 2 A kind of preparation method of cefditoren pivoxil, the preparation method comprises the following steps:
[0070] (1) Preparation of cefditoren mother nucleus: drop into 200ml of water in reaction bottle, temperature 0~10 ℃, add D-7ACA50g, add dropwise 7% sodium bicarbonate to adjust pH to dissolve clear, add sodium bromide 10g, 2,2,6,6-tetramethylpiperidine nitrogen oxide 2g, 50ml of 10% sodium hypochlorite was added dropwise, reacted for 2-3 hours, and 3N hydrochloric acid was added dropwise to adjust the pH to 3.5 to obtain about 47.08g of compound 1.
[0071] A: Put 100ml of dichloromethane into the reaction bottle, add about 23.55g of compound 1, control the temperature at 0-20°C, add hexamethyldisilazane, and react under reflux for about 5 hours to obtain the feed solution of compound 2, keep it warm 0~10℃ for later use.
[0072] B: Put 100ml of dichloromethane into the reaction bottle, add 14.4g (112mmol) of 4-methylthiazole-5-methanol, 20.1g (134m...
Embodiment 3
[0076] Embodiment 3 A kind of preparation method of cefditoren pivoxil, the preparation method comprises the following steps:
[0077] (1) Preparation of cefditoren mother nucleus: drop into 200ml of water in reaction bottle, temperature 0~10 ℃, add D-7ACA50g, add dropwise 7% sodium bicarbonate to adjust pH to dissolve clear, add sodium bromide 10g, 2,2,6,6-tetramethylpiperidine nitrogen oxide 2g, 50ml of 10% sodium hypochlorite was added dropwise, reacted for 2-3 hours, and 3N hydrochloric acid was added dropwise to adjust the pH to 3.5 to obtain about 47.08g of compound 1.
[0078] A: Put 100ml of dichloromethane into the reaction bottle, add about 23.55g of compound 1, control the temperature at 0-20°C, add hexamethyldisilazane, and react under reflux for about 5 hours to obtain the feed solution of compound 2, keep it warm 0~10℃ for later use.
[0079] B: Put 100ml of dichloromethane into the reaction bottle, add 14.4g (112mmol) of 4-methylthiazole-5-methanol, 20.1g (134m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com