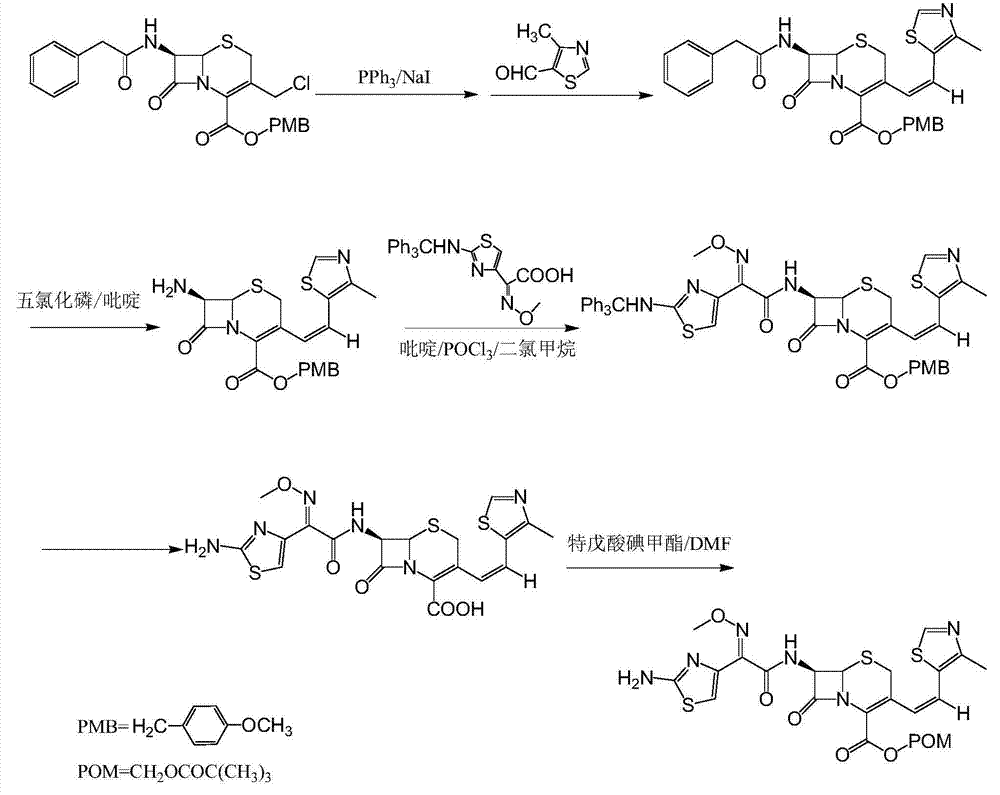
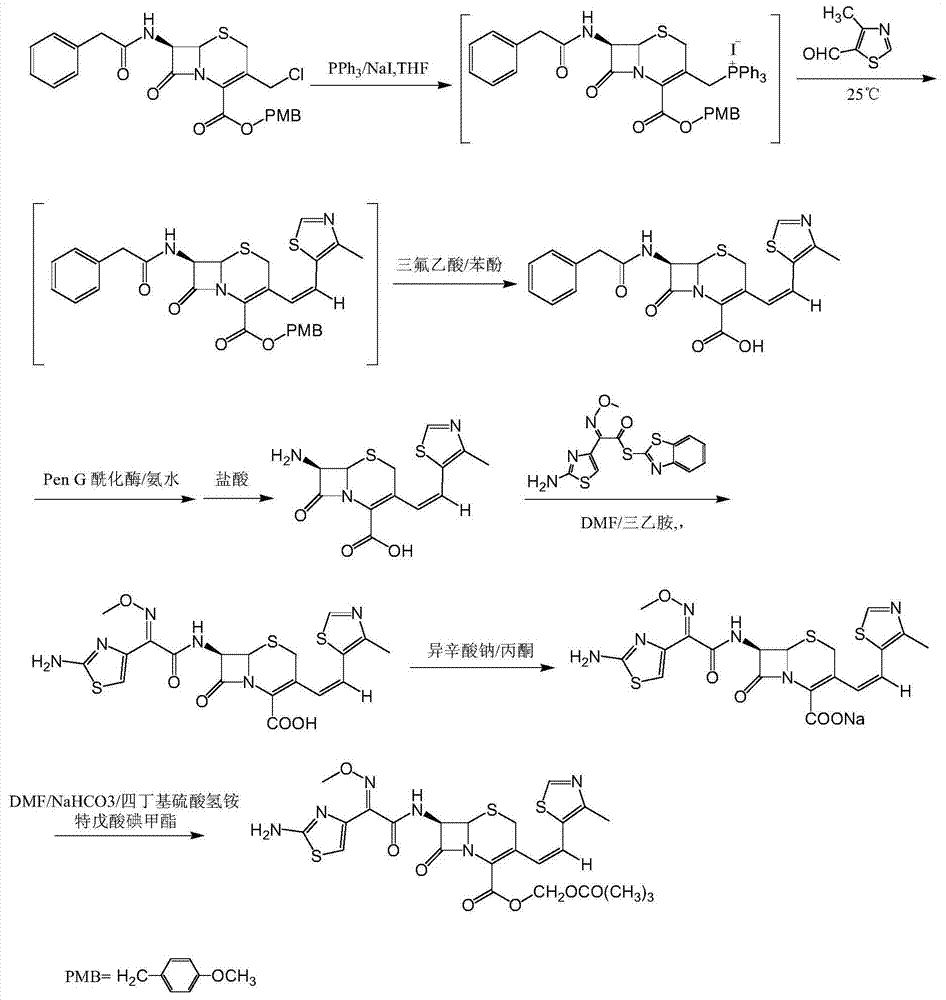
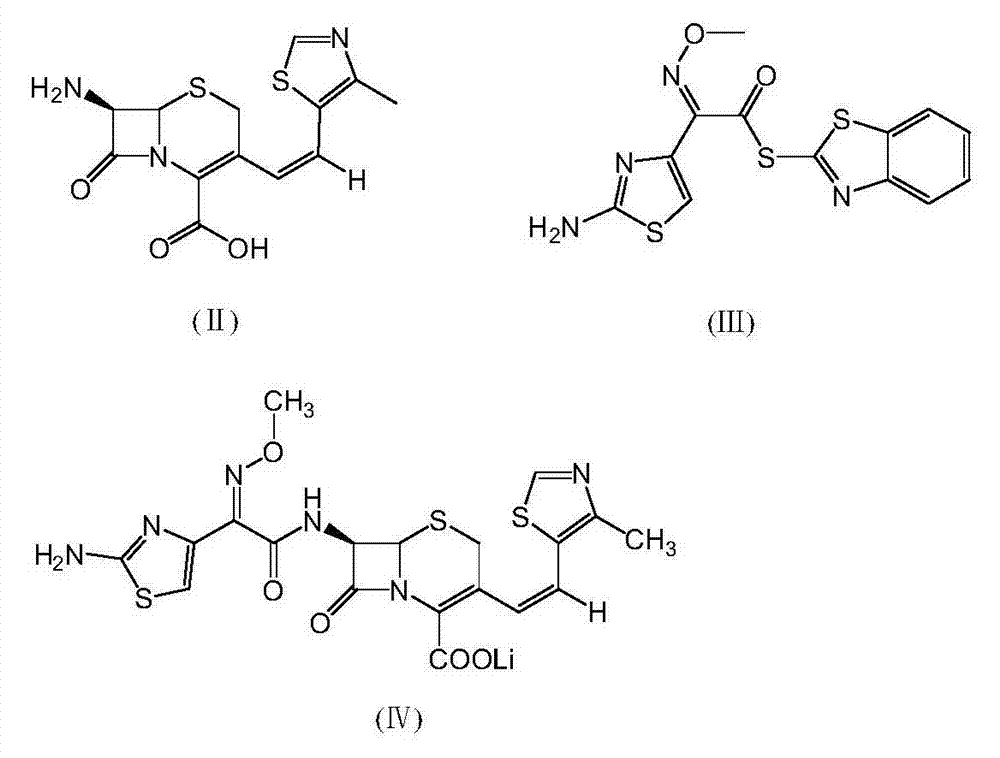
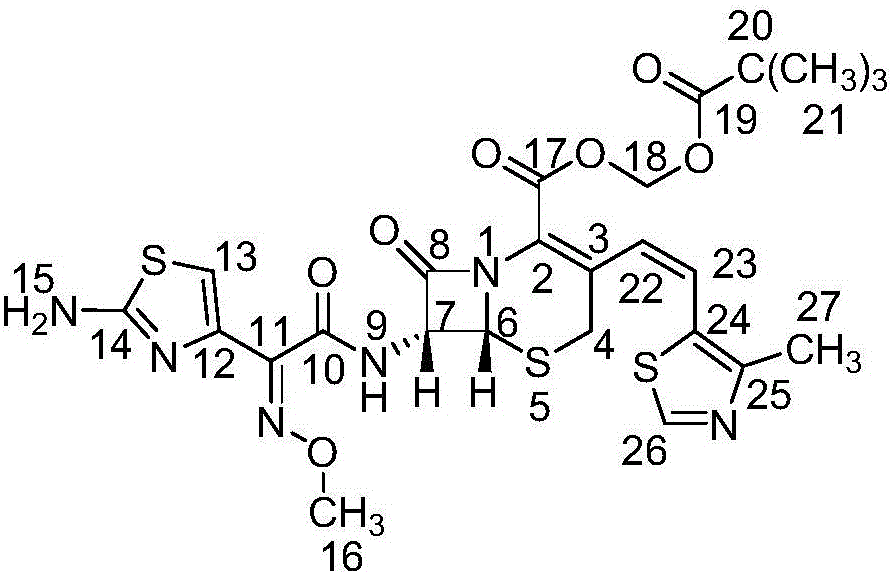
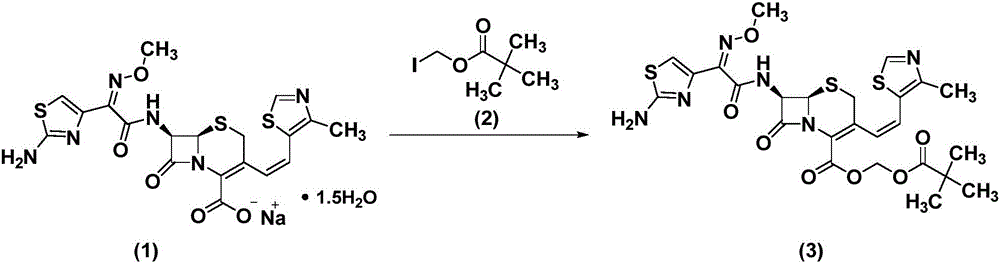
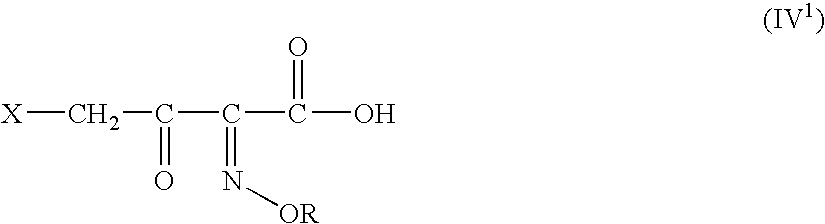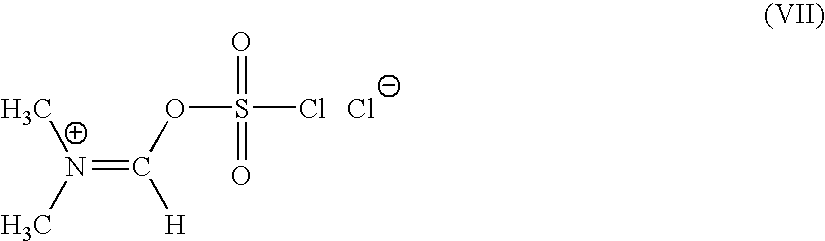Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
84 results about "Cefditoren Pivoxil" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
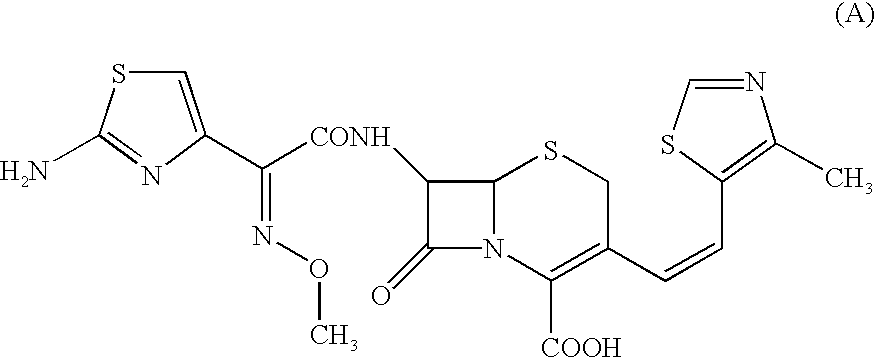
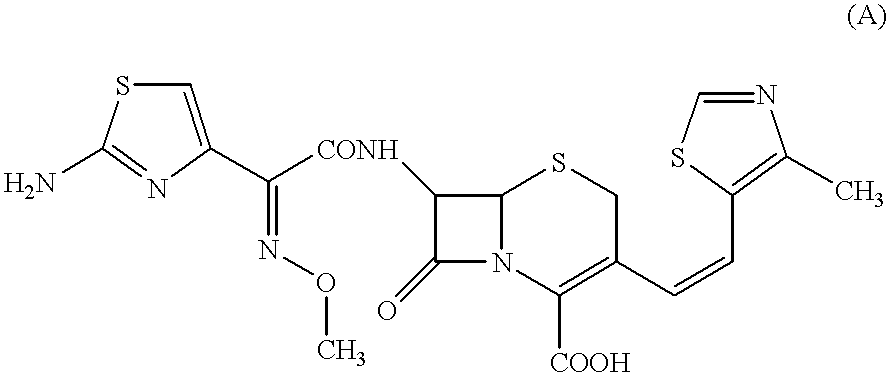
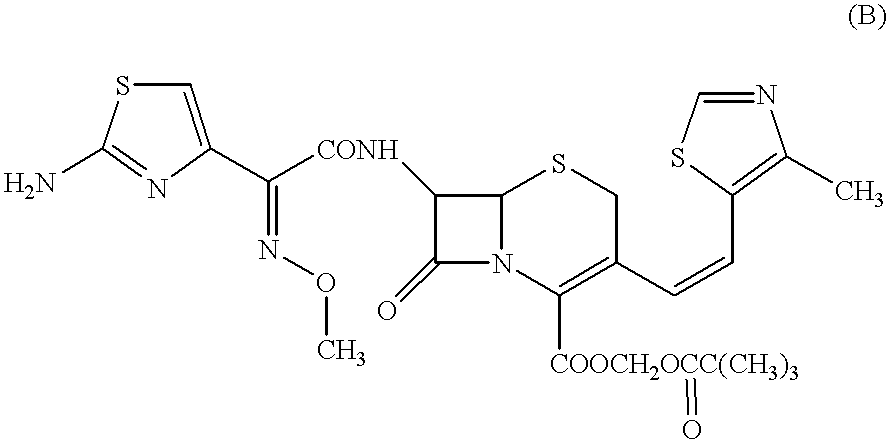
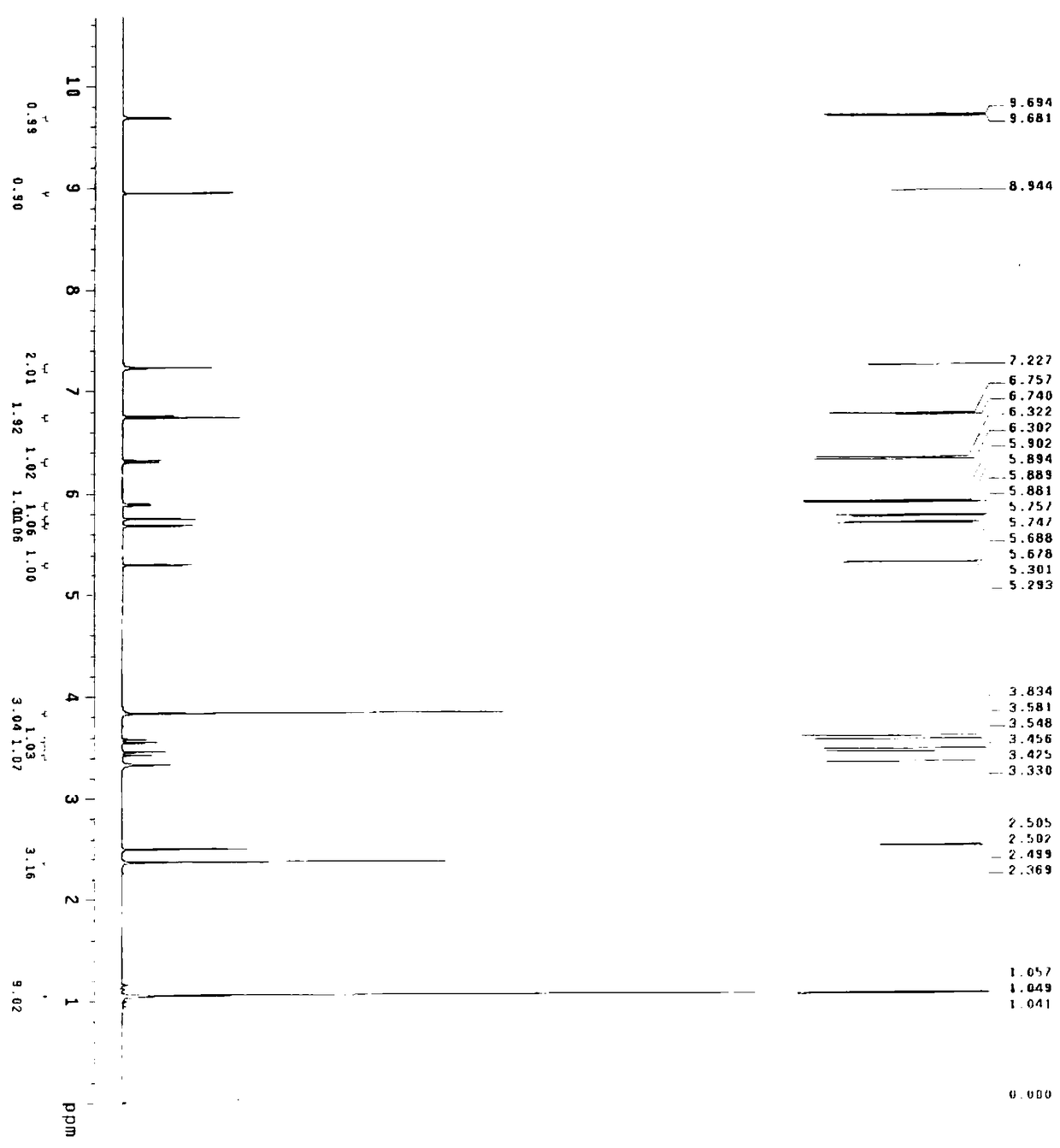
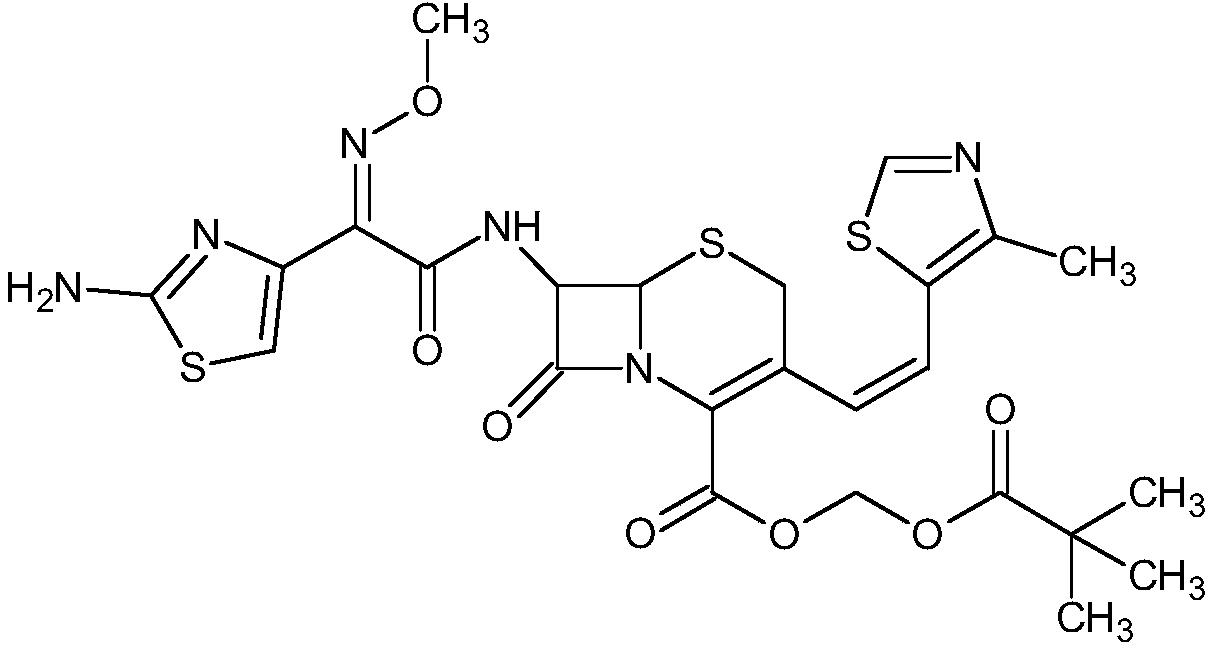
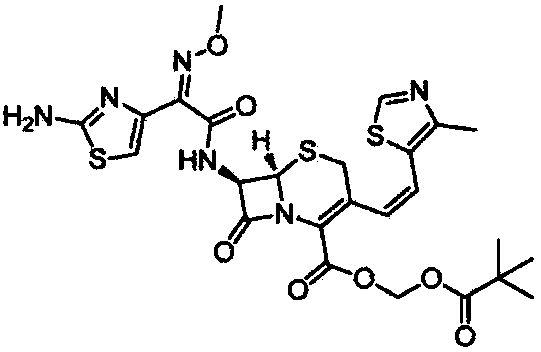
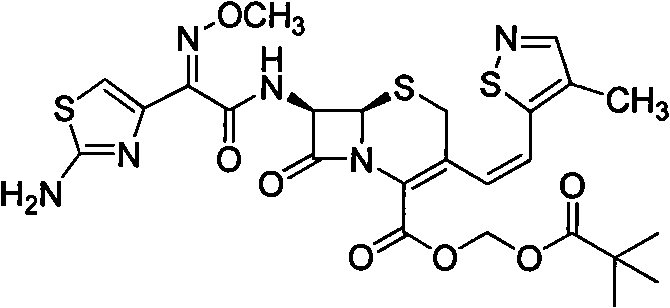
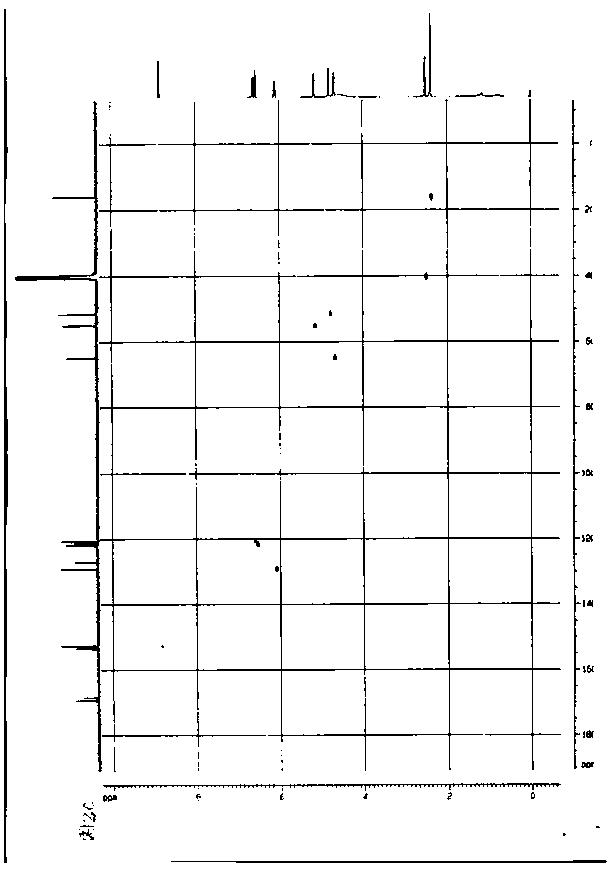
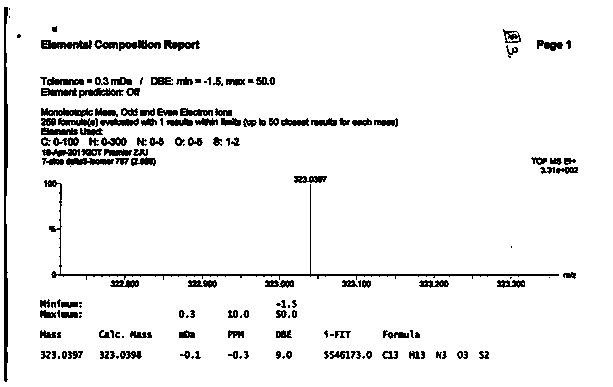
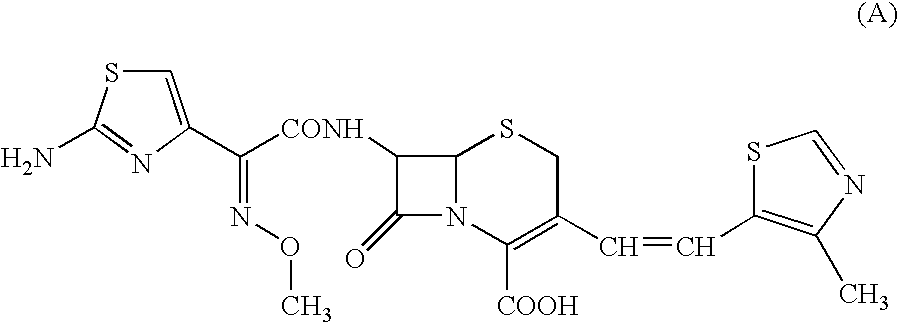
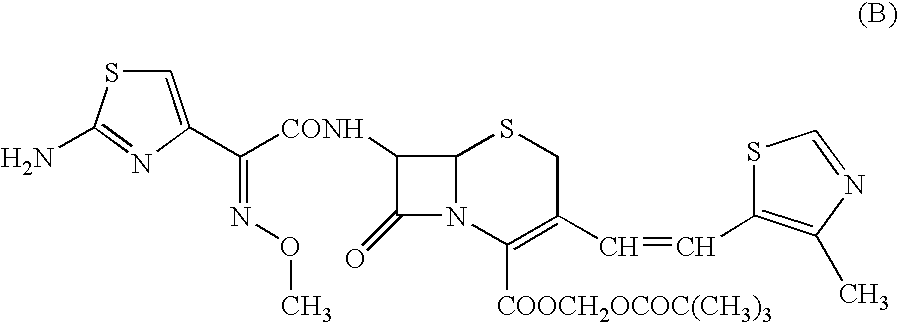
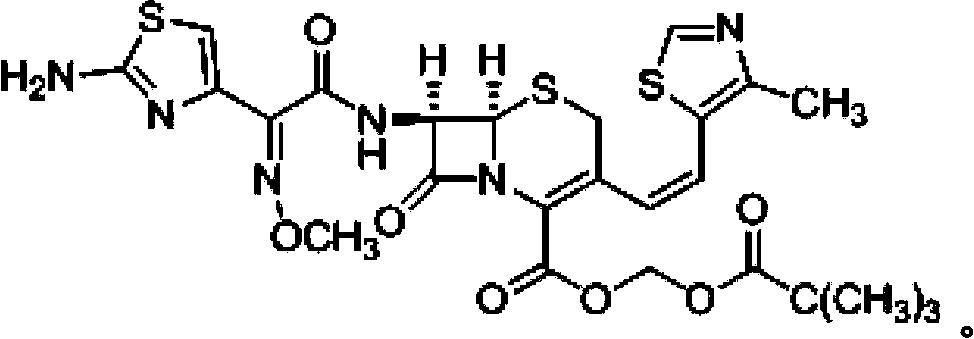
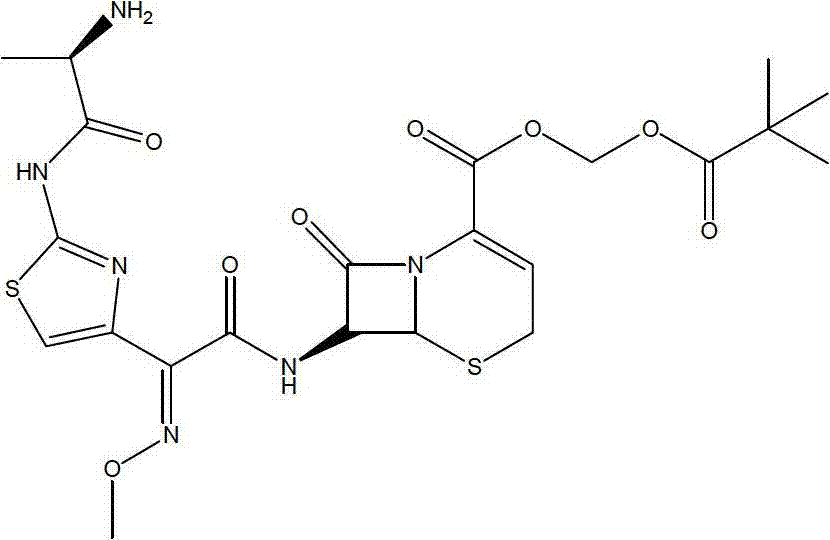
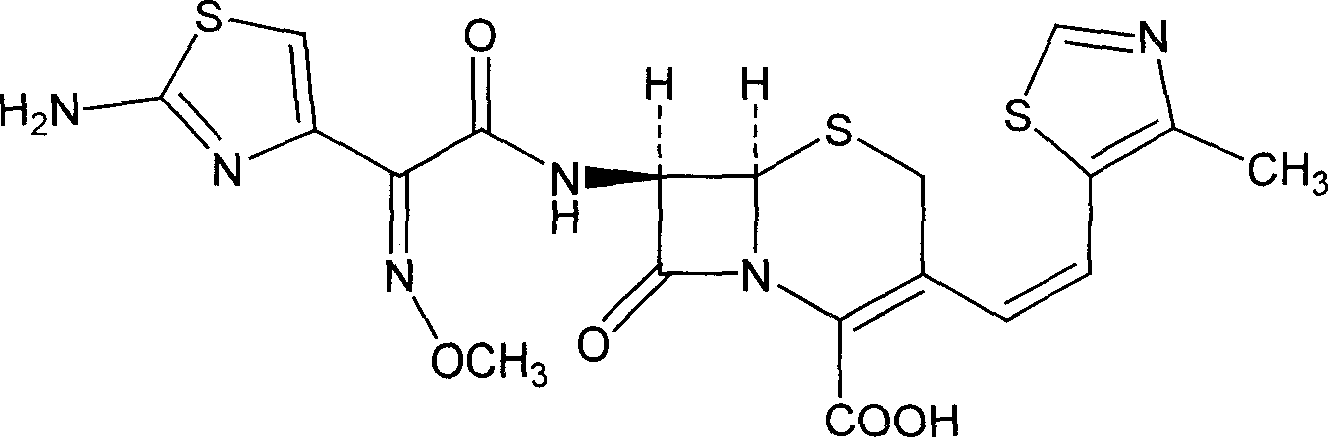
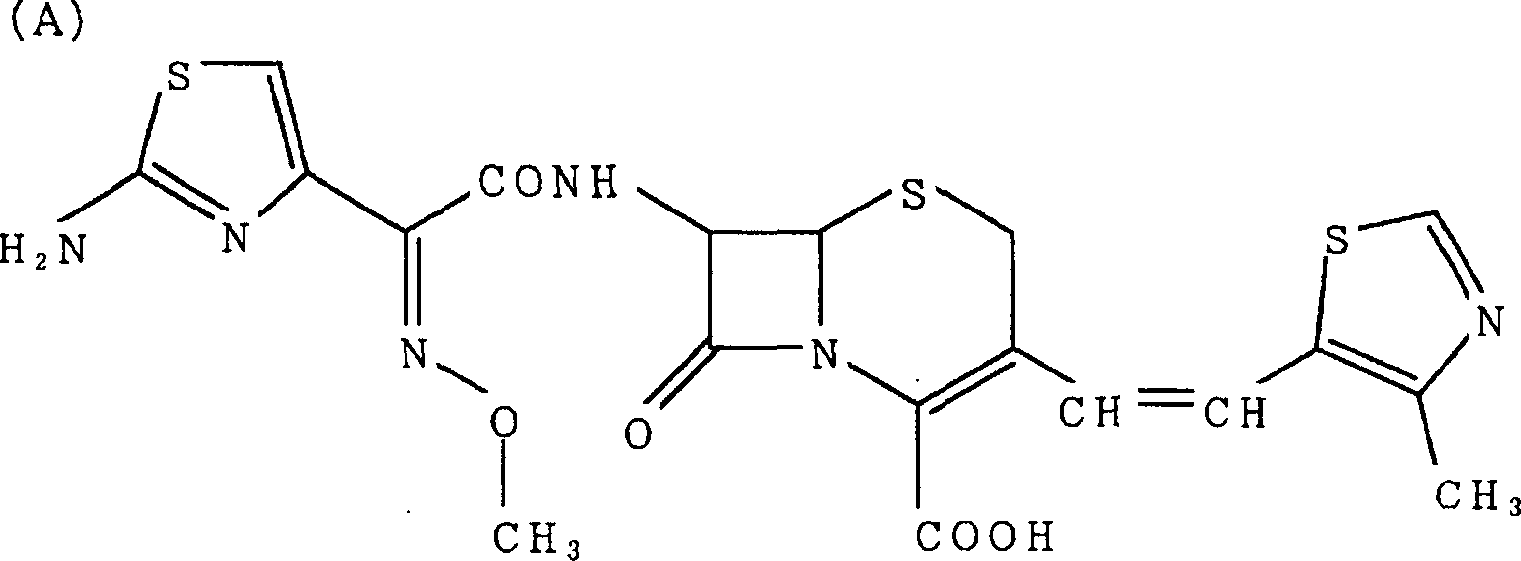
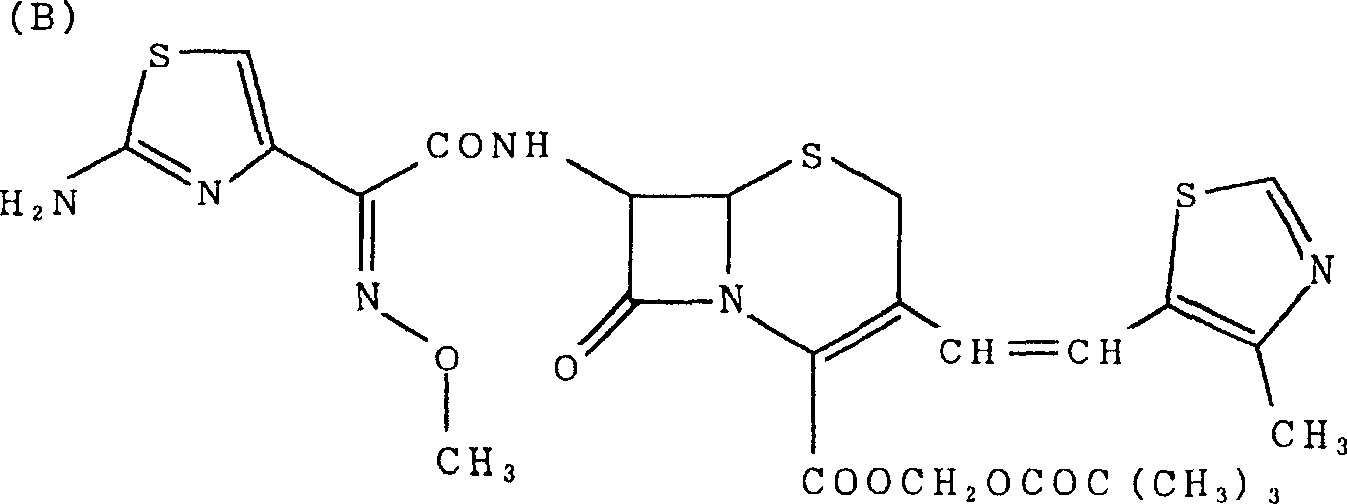
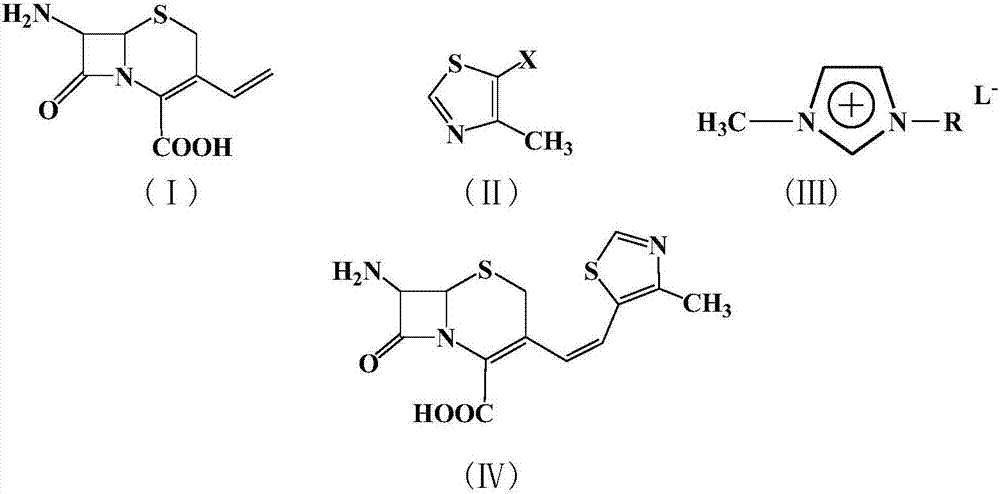
A semi-synthetic, broad-spectrum, beta-lactamase resistant, third-generation cephalosporin antibiotic with bactericidal activity. Cefditoren pivoxil is a prodrug that is rapidly hydrolyzed by intestinal esterases during absorption to the microbiologically active cefditoren, an active aminothiazolyl cephalosporin. Cefditoren inactivates penicillin binding proteins (PBPs) thereby interfering with peptidoglycan synthesis and inhibiting bacterial cell wall synthesis. Another consequence of beta-lactam exposure results in the loss of lipoteichoic acids from the cell wall. Lipoteichoic acids inhibit murein hydrolase activity and their absence from the cell wall triggers uncontrolled autolytic activity rendering bacterial cells susceptible to osmotic shock. This results in a reduction of cell wall stability and causes cell lysis.
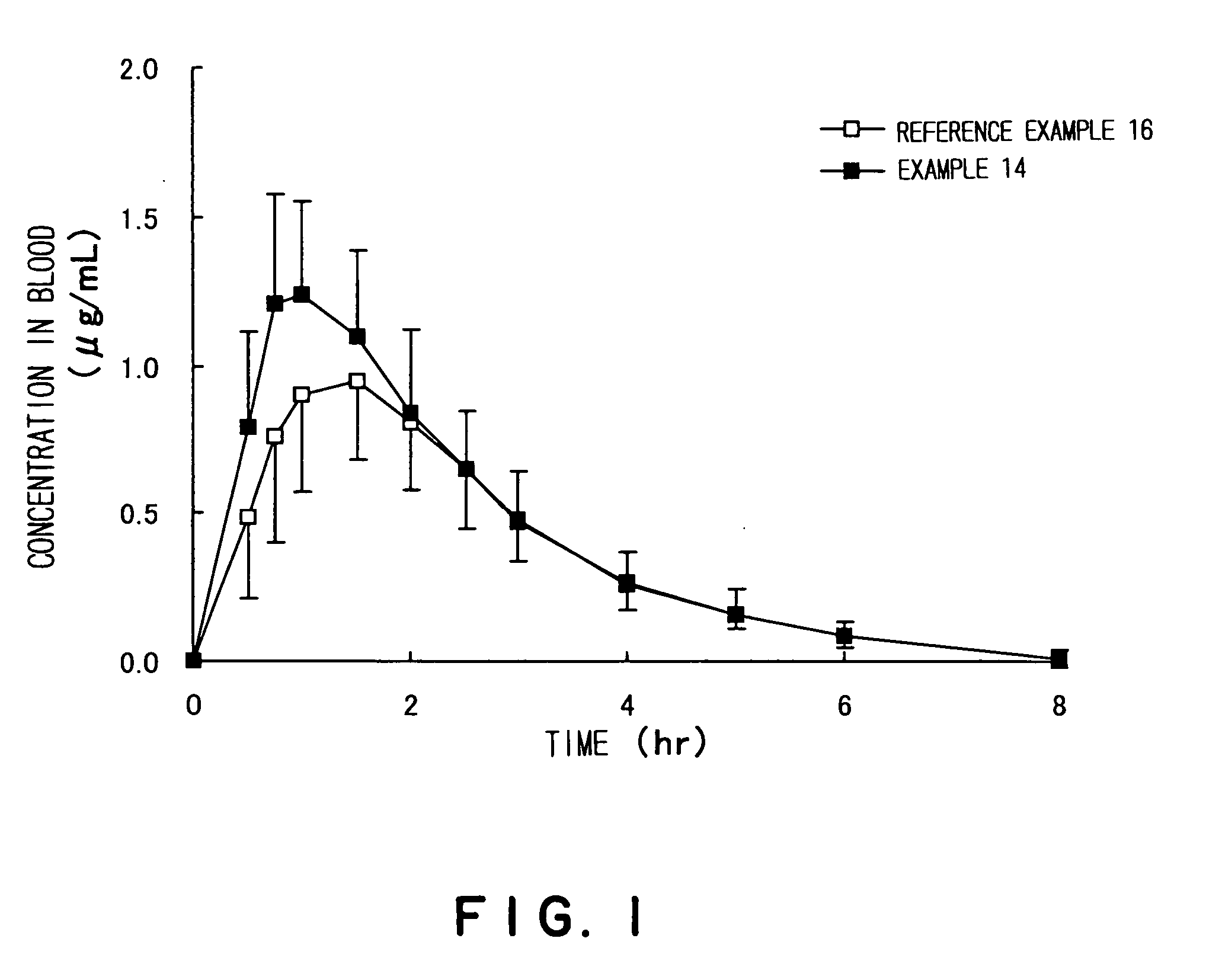
Enhancement of oral bioavailability of non-emulsified formulations of prodrug esters with lecithin
InactiveUS20050113337A1Prevent degradationImprove efficiencyBiocideDispersion deliveryAntibiotic YLiposome
A method for enhancing the oral bioavailability of a prodrug ester by formulating the ester as a non-emulsified formulation with lecithin; as well as a pharmaceutical composition of at least one antibiotic and lecithin in a non-emulsified formulation; a method of treating infections with the non-emulsified formulation, and a method for preparing tablets by direct compression of blends of drugs with lecithin are disclosed. Non-emulsified formulations include solids, tablets, capsules, lozenges, suspensions, elixirs and solutions, and exclude emulsions, liposomes, lipid matrix systems and micro-emulsions. A suitable prodrug ester is a cephalosporin β-lactam antibiotic such as cefditoren pivoxil, and a suitable non-emulsified formulation is a solid formulation.
Owner:TAP PHARM PROD INC
Composition comprising a crystallographically stable, amorphous cephalosporin and processes for the preparation thereof
InactiveUS6486149B2Good water solubilityImprove solubilityAntibacterial agentsOrganic active ingredientsWater solubleAqueous solution
Owner:MEIJI SEIKA KAISHA LTD
Novel intermediates for synthesis of cephalosporins and process for preparation of such intermediates
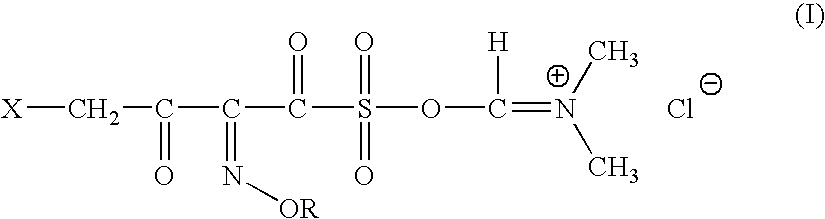
InactiveUS20060135761A1Easily hydrolysableSulfuric acid esters preparationBulk chemical productionCefmenoximeAntibiotic Y
A novel 4-halo-2-oxyimino-3-oxo butyric acid-N,N-dimethyl formiminium chloride chlorosulfate of formula (I) useful in the preparation of cephalosporin antibiotics wherein X is chlorine or bromine; R is hydrogen, C1-4 alkyl group, an easily removable hydroxyl protective group, —CH2COOR5, or —C(CH3)2COOR5, wherein R5 is hydrogen or an easily hydrolysable ester group. The compound of formula (I) is prepared by reacting 4-halo-2-oxyimino-3-oxobutyric acid of formula (IV1), wherein X, R and R5 are as defined above, with N,N-dimethylformiminium chloride chlorosulphate of formula (VII) in an organic solvent at a temperature ranging from −30° C. to −15° C. The cephalosporins that may be prepared from the intermediate include cefdinir, cefditoren pivoxil, cefepime, cefetamet pivoxil, cefixime, cefmenoxime, cefodizime, cefoselis, cefotaxime, cefpirome, cefpodoxime proxetil, cefquinome, ceftazidime, cefteram pivoxil, ceftiofur, ceftizoxime, ceftriaxone and cefuzonam.
Owner:LUPIN LTD
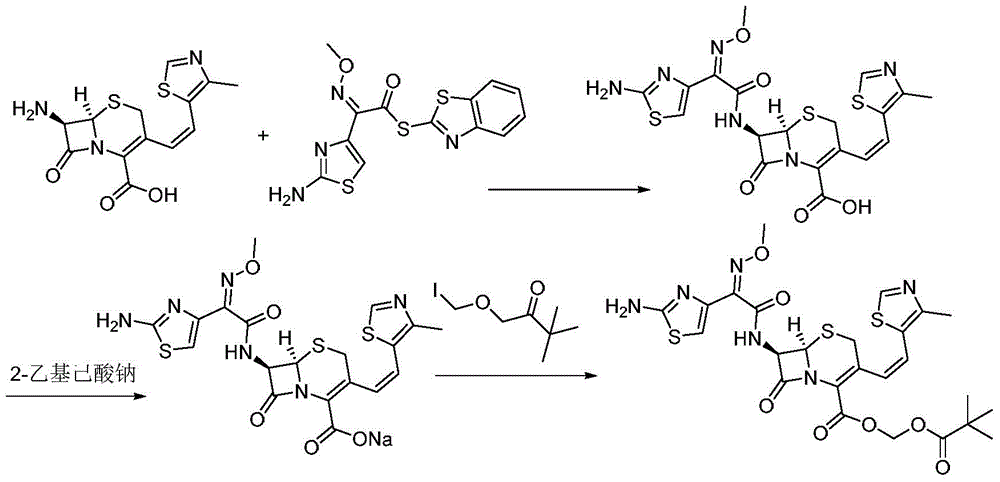
Preparation method of cefditoren pivoxil
ActiveCN104513256ASources are cheap and readily availableEasy to operateOrganic chemistrySodium bicarbonateCefditoren
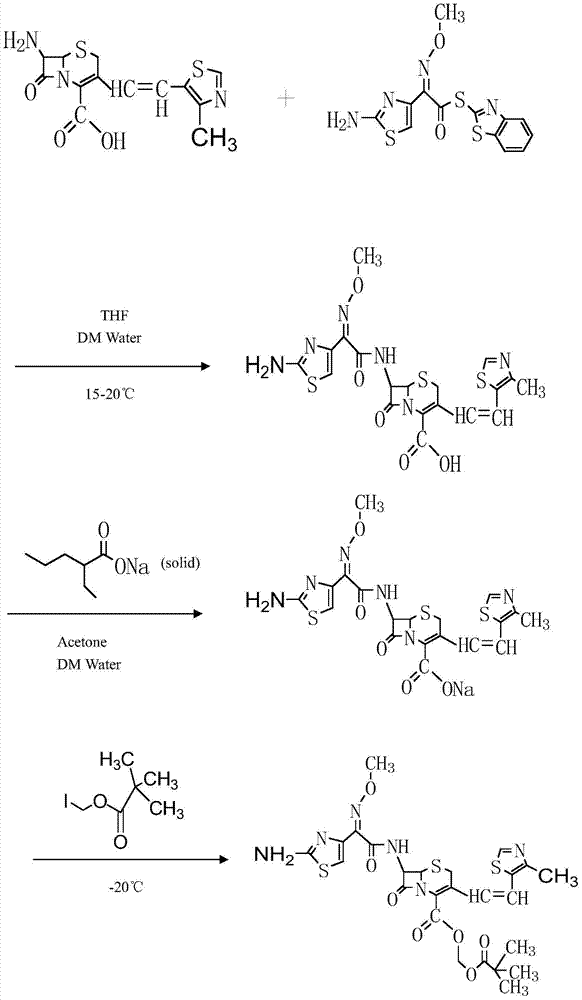
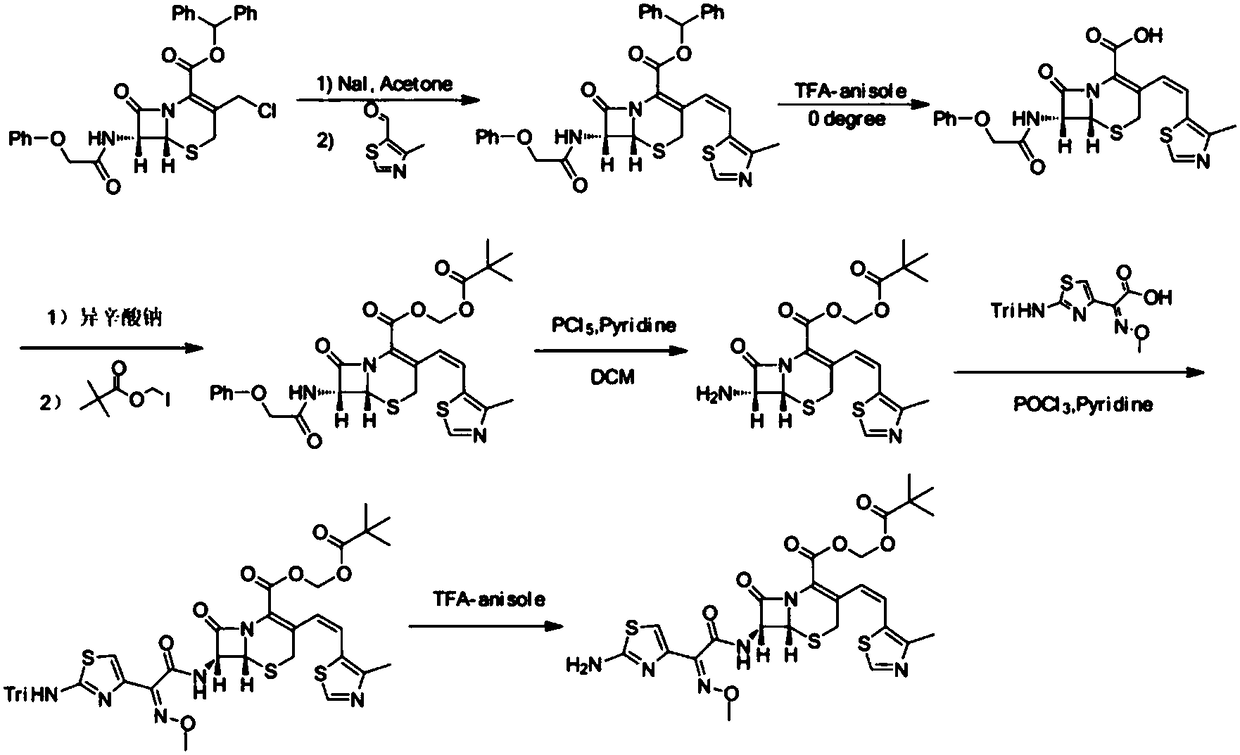
The invention belongs to the technical field of medicines and particularly relates to a preparation method of cefditoren pivoxil. The preparation method particularly includes following steps: (1) carrying out a reaction between cefditoren mother nucleus 7ATCA and AE-activated ester with dichloromethane as a solvent under an alkaline condition at 0-5 DEG C; (2) performing extraction with pure water and adding a sodium iso-octoate / acetone solution to obtain cefditoren sodium; (3) carrying out a reaction between the cefditoren sodium and iodomethyl pivalate under the alkaline condition at -40 DEG C to obtain a cefditoren pivoxil solution; (4) adding pure water to separate out a crystal to obtain a crude product of the cefditoren pivoxil. The technical scheme also comprises steps of dissolving the crude product of the cefditoren pivoxil in a mixed solution including dichloromethane and anhydrous ethanol, washing the material solution with a 1% sodium bicarbonate solution and pure water, collecting an organic phase, and performing a pressure-reducing evaporate-drying process to obtain the cefditoren pivoxil being higher than 99% in purity and less in impurities. The preparation method is simple in operation, is easy to control, is high in yield, allows the raw material to be obtained easily and is suitable for industrialized large-scale production.
Owner:LUNAN BETTER PHARMA
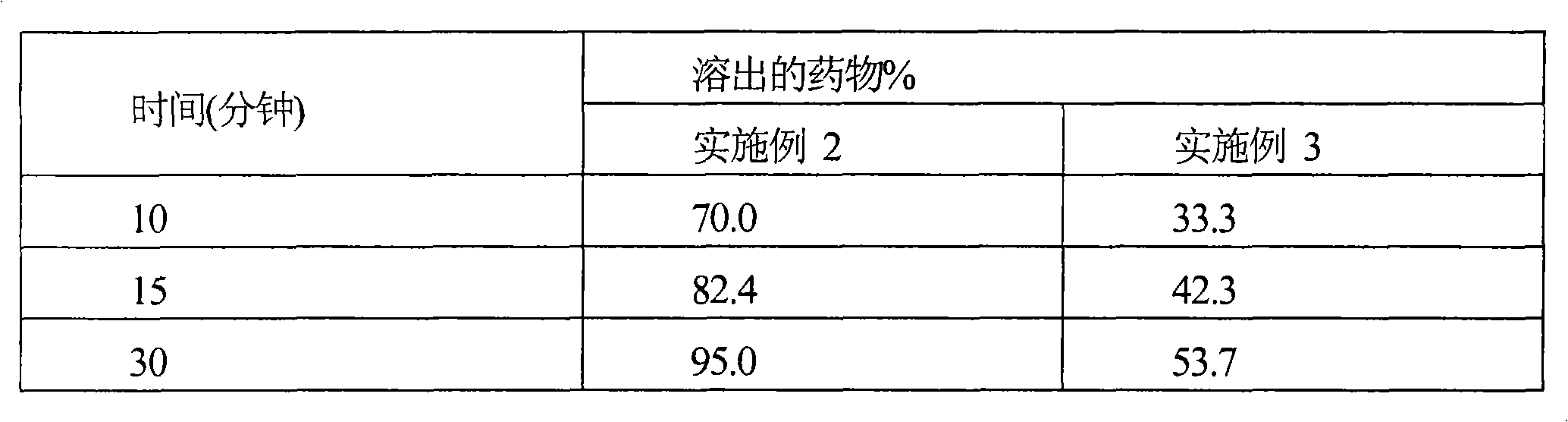
Cefditoren pivoxil tablet and its preparation method
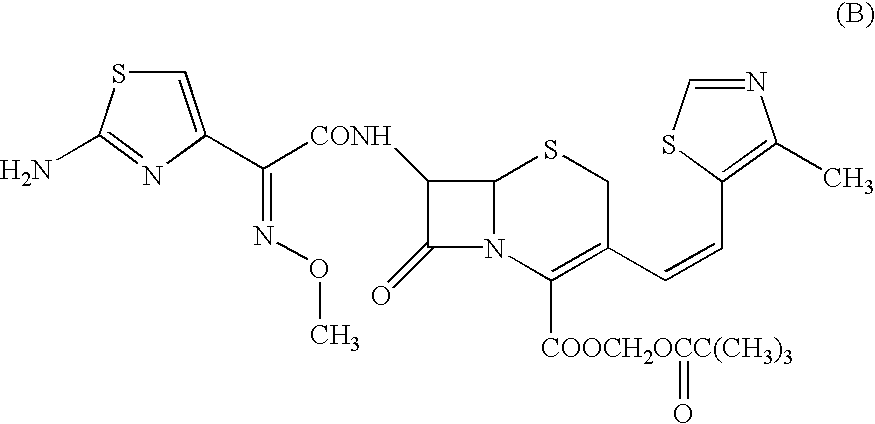
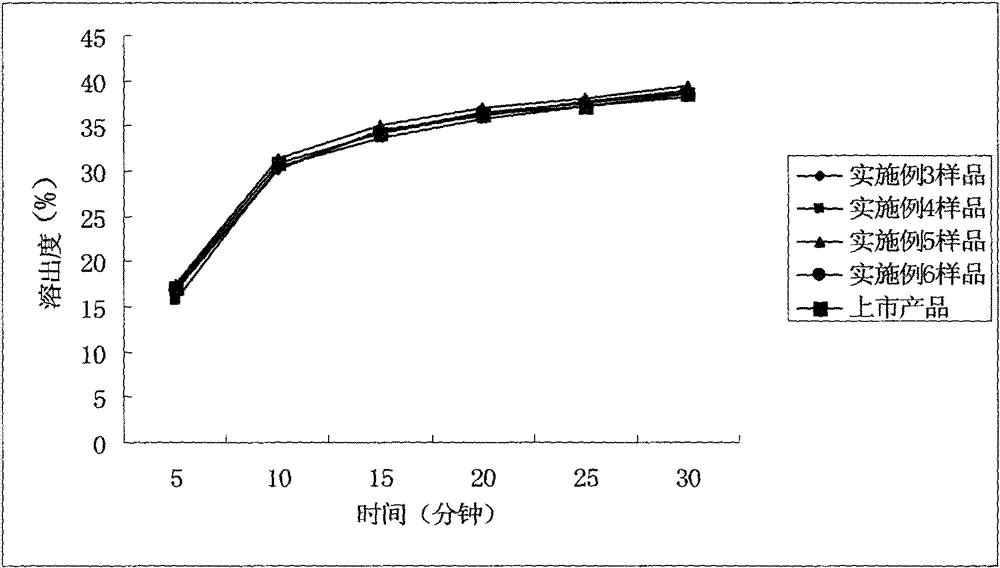
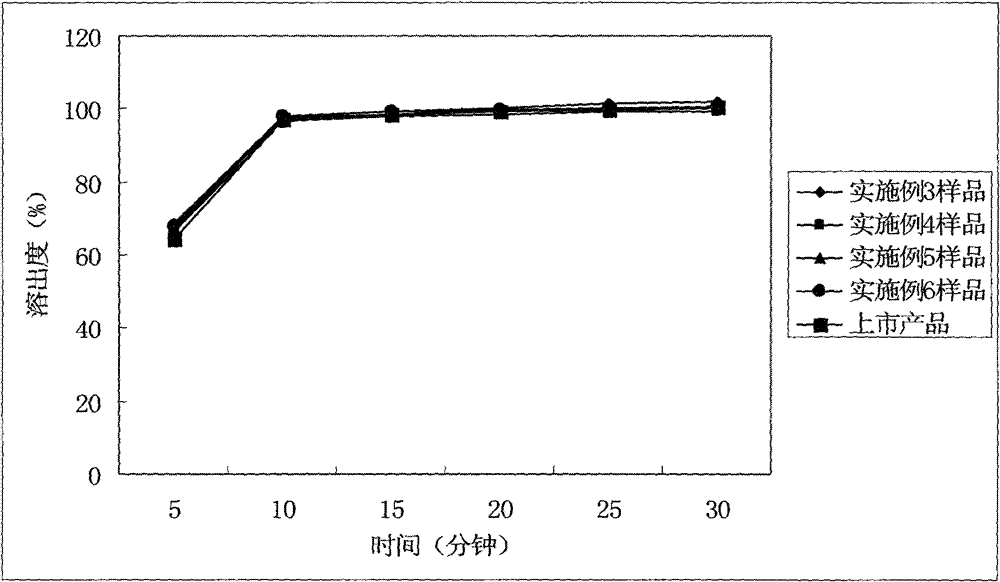
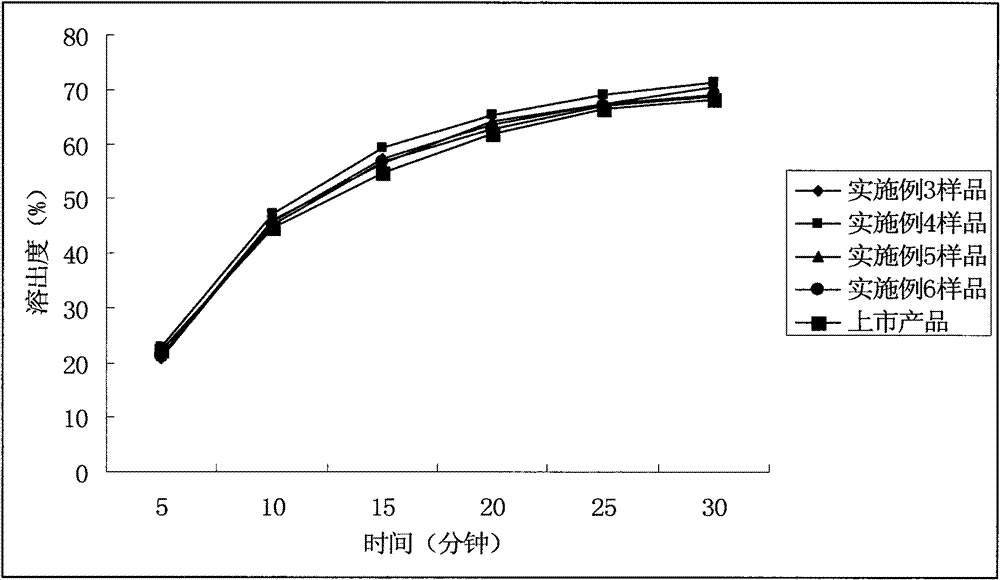
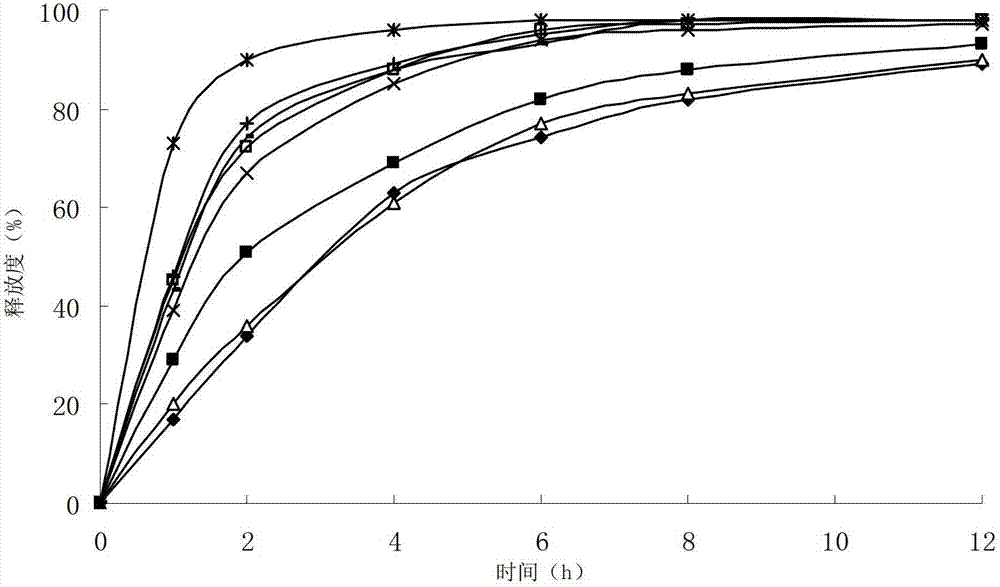
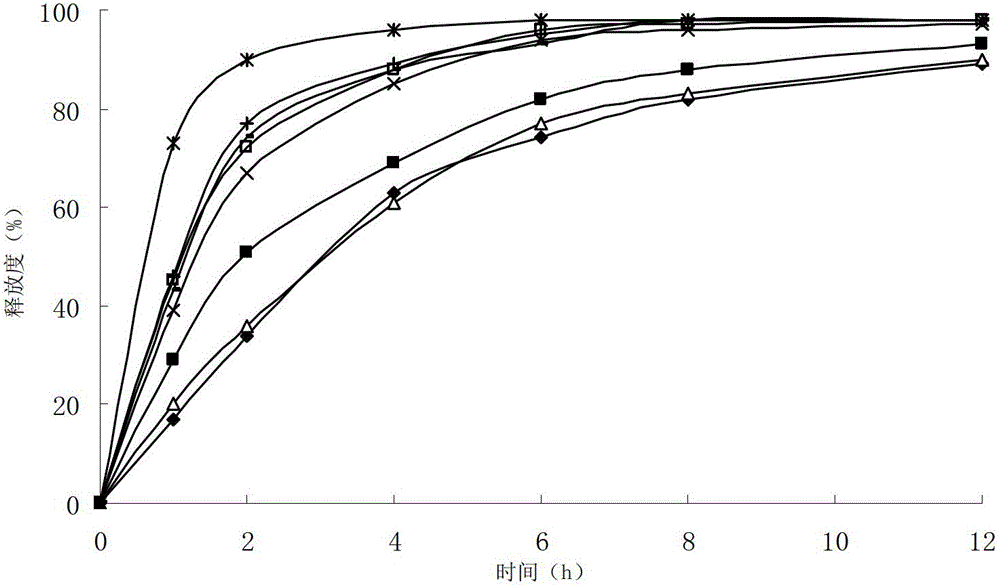
ActiveCN102949359AHigh dissolution rateImprove bioavailabilityAntibacterial agentsOrganic active ingredientsMedicineLow-substituted hydroxypropylcellulose
The invention relates to a cefditoren pivoxil tablet. The tablet comprises a tablet core and a coating layer, wherein the tablet core contains amorphous cefditoren pivoxil, a filler, a disintegrating agent, a solubilizer and a lubricant, and the filler is a mixture of D-mannitol and beta-cyclodextrin and / or low-substituted hydroxy propyl cellulose. The cefditoren pivoxil tablet avoids the conversion of amorphous cefditoren pivoxil to crystals and has good disintegration and dissolve-out properties under four dissolve-out conditions, and the dissolve-out curve of the cefditoren pivoxil tablet is consistent with the dissolve-out curve of a listed product Meiact.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD
Crystallographically stable amorphous cephalosporin compositions and process for producing the same
InactiveUS6342493B1Good water solubilityImprove solubilityAntibacterial agentsPowder deliveryOral medicationCefditoren
Processes are provided for the preparation of orally administrable, yellow and powdery compositions essentially consisting of particles composed of a homogeneous mixture of an amorphous Cefditoren pivoxil substance with a water soluble high-molecular additive. These compositions can be produced by dissolving crystalline Cefditoren pivoxil substance and the water-soluble high-molecular additive in an aqueous solution of an acid, then neutralizing the resultant solution, to co-precipitate the product, and drying the thus precipitated product, followed by recovering the product in the form of the above-mentioned particles.
Owner:MEIJI SEIKA KAISHA LTD
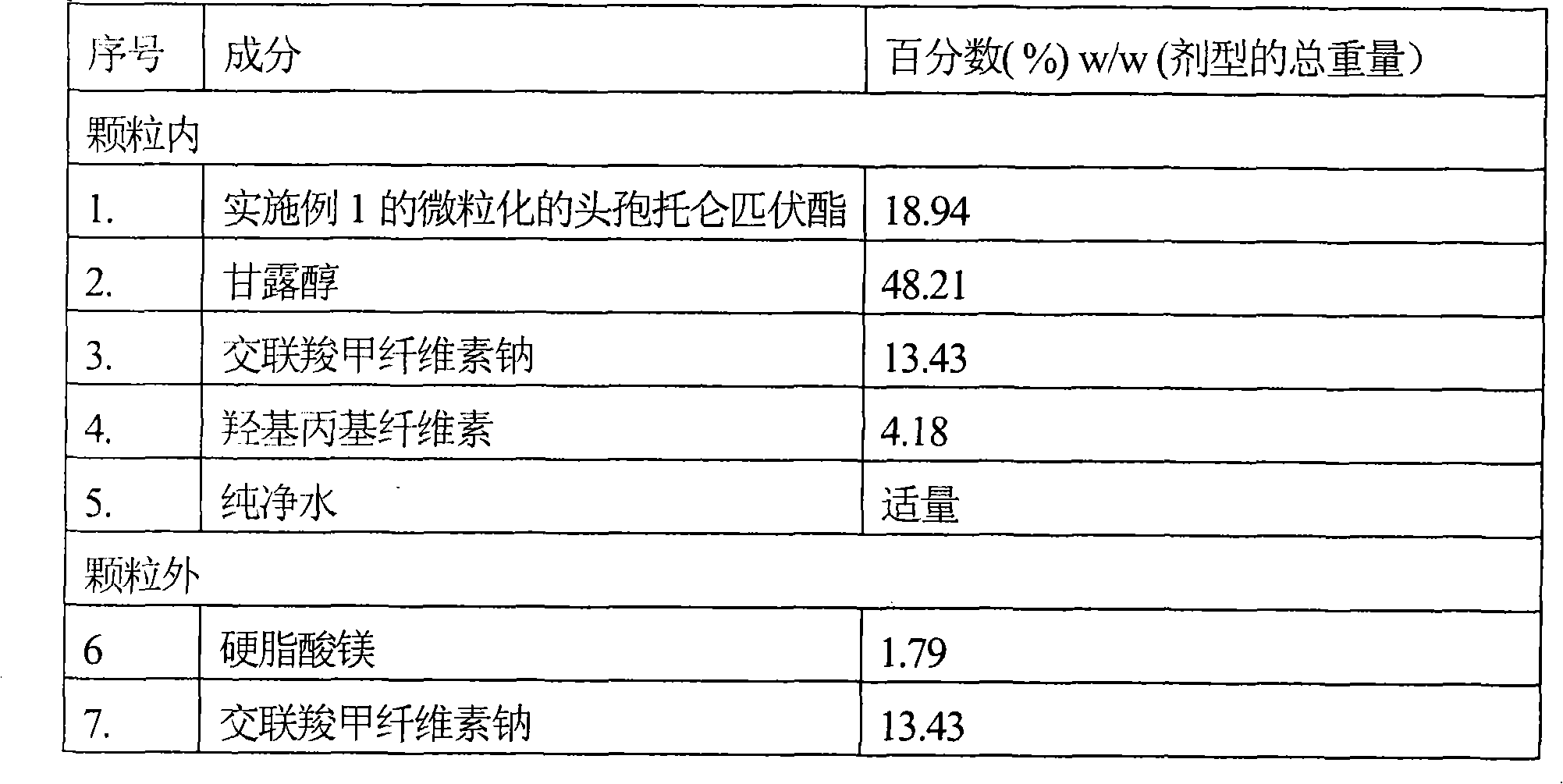
Stable solid preparation containing amorphous cefditoren pivoxil and preparation method thereof
The present invention relates to a stable drug compound of solid preparation containing amorphous cefditoren pivoxil, and its preparing method. The solid preparation can be prepared by drying method, and can be packaged with one or more layers water dispersoid which contains one or more kind film-forming agents. The drug compound can be used for antimicrobial.
Owner:RANBAXY LAB LTD
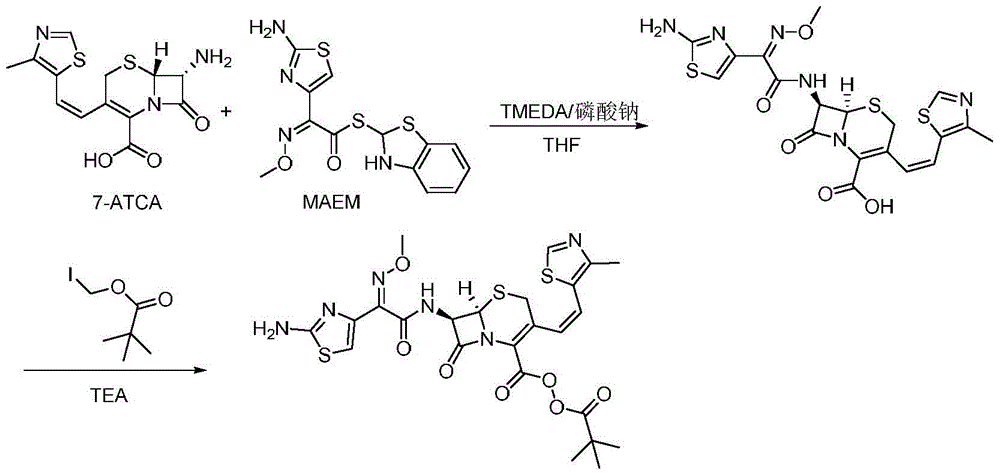
Method for preparing cefditoren pivoxil cephalosporins
The invention discloses a method for preparing cefditoren pivoxil cephalosporins.The method comprises the following steps that 1, on the presence of TMEDA and sodium phosphate, 7-ATCA and MAEM are subjected to a contact reaction in THF, and a mixture containing cefditore is obtained, and the contact reaction temperature ranges from 0 to 25 DEG C; 2, the temperature is kept, TEA is added to the mixture containing cefditore obtained in the step 1, then iodomethyl pivalate is added to the mixture to be stirred for reacting, the product is poured into water after the reaction is finished, a saturated ammonium chloride solution is added, the pH is adjusted to be 5 to 5.3, filtering is carried out, and a filter cake obtained through filtering is recrystallized in methyl alcohol to obtain cefditoren pivoxil cephalosporins.According to the method, separation treatment is not needed in the intermediate steps, one-pot operation is easy, cost is reduced, the yield is high, the number of by-products is small, aftertreatment is easy, and the method is especially suitable for industrial popularization.
Owner:湖北凌晟药业股份有限公司
Stable solid dosage form containing amorphous cefditoren pivoxil and process for preparation thereof
InactiveUS20080069879A1Antibacterial agentsOrganic active ingredientsFilm-forming agentSolid Dose Form
The present invention relates to stable solid dosage form and a dry process for preparing amorphous cefditoren pivoxil solid dosage forms and coating the solid dosage form with one or more layers of aqueous dispersion of film forming agents.
Owner:RANBAXY LAB LTD
Preparation method of cefditoren pivoxil
ActiveCN103665002AHigh yieldSuitable for commercial mass productionOrganic chemistryCarboxylic acidEsterification reaction
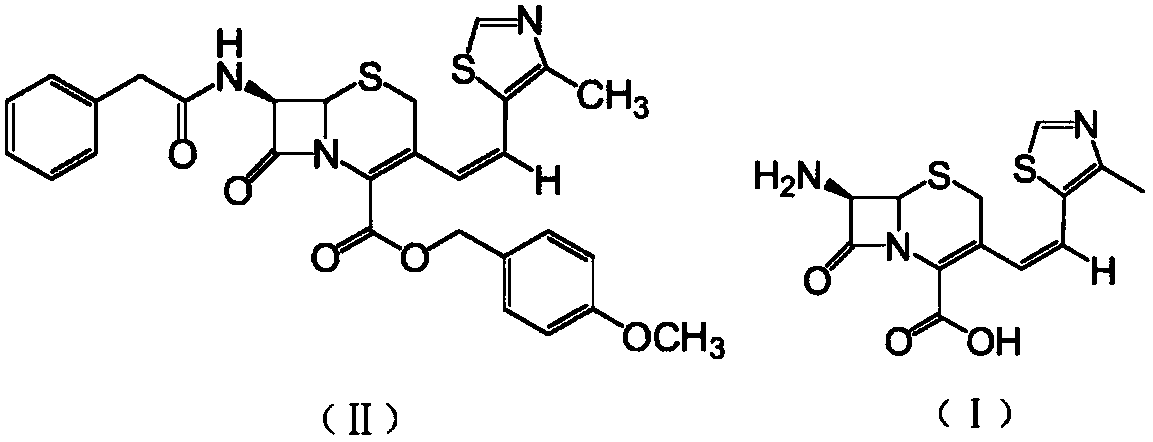
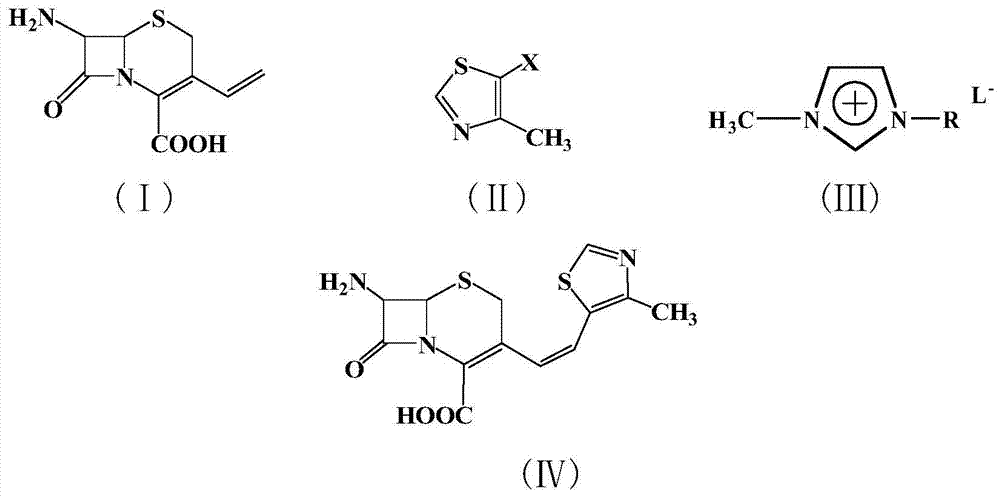
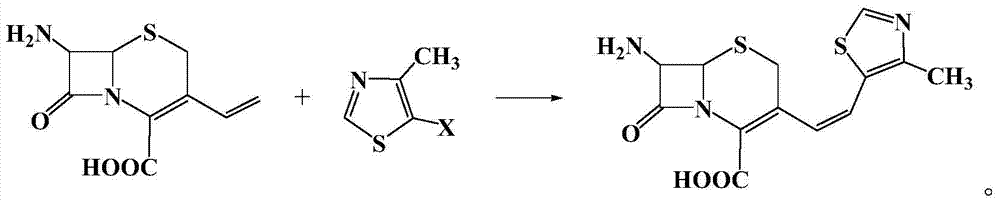
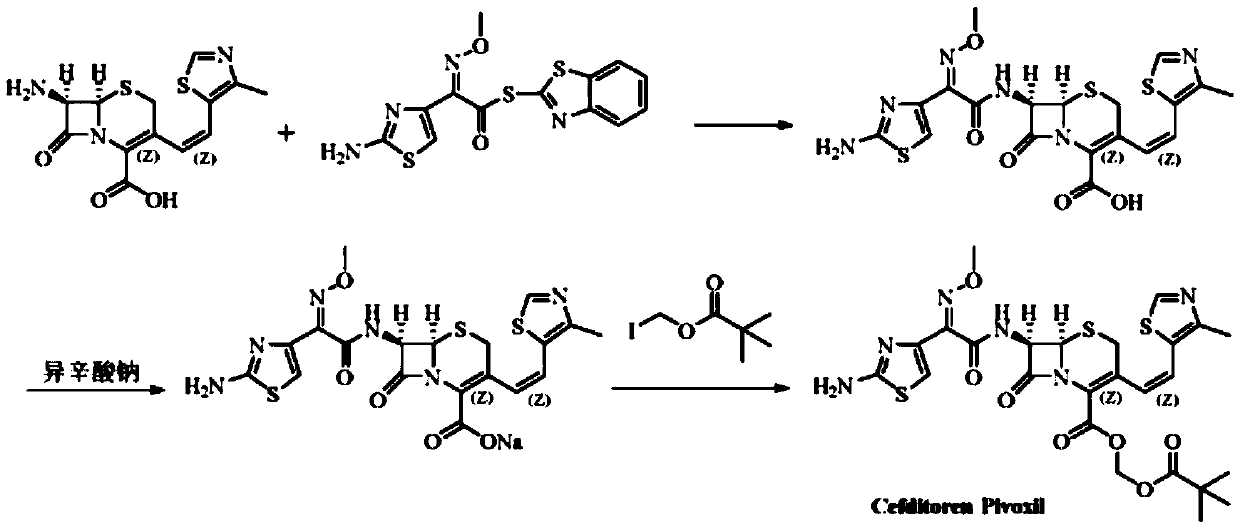
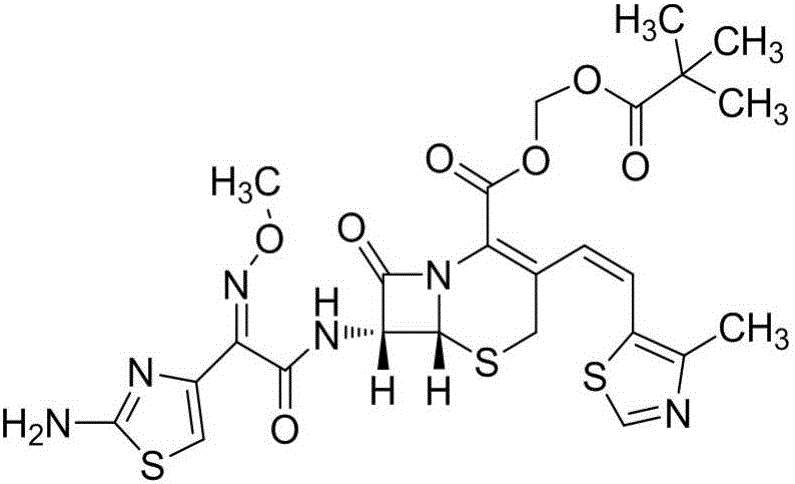
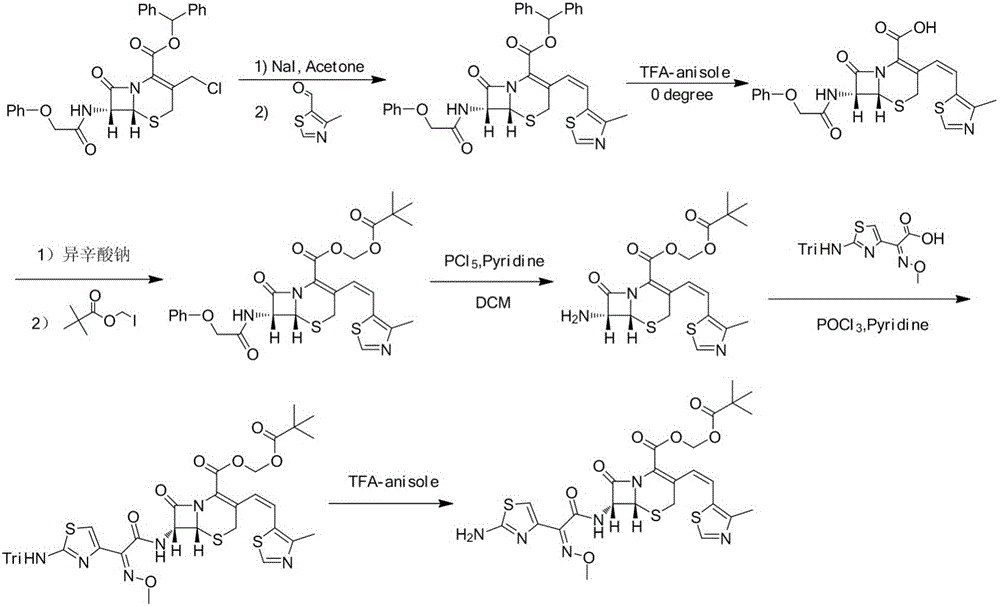
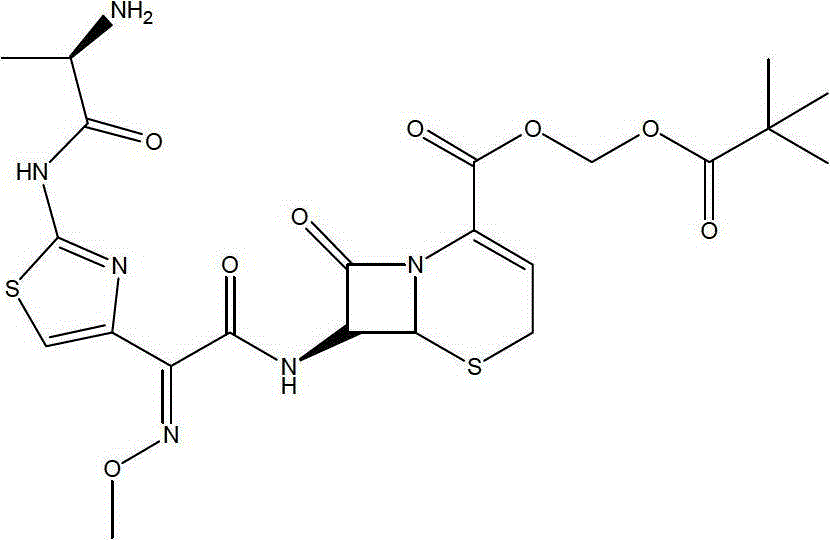
The invention relates to a preparation method of cephalosporin, particularly to a preparation method of cefditoren pivoxil. 7-amino-3-[(Z)-2-(4-methyl-5-thiazolyl) vinyl]-3-cephem-4-carboxylic acid and 2-(2-amino-4-thiazolyl)-2-methoxyiminoacetic are taken as initial raw materials, and the cefditoren pivoxil is obtained through condensation and esterification reactions. The method is simple in process, high in yield, free of highly corrosive solvents and suitable for industrial production.
Owner:CHENGDU YILUKANG MEDICAL TECH & SERVICE
Method for synthesizing cefditoren pivoxil
The invention relates to a method for synthesizing cefditoren pivoxil. The method comprises that D-7ACA reacts with an oxidizing reagent to produce a compound 1, the compound 1 is protected through silanization to produce a compound 2, 4-methylthiazole-5-methanol and NaI undergo an iodination reaction in the presence of a small amount of sulfuric acid for catalysis, triphenylphosphine is added into the reaction system and undergoes a reaction to produce a compound 3, the compound 3 is added into the compound 2 liquid and undergoes a reaction, the reaction product is concentrated, methanol anda small amount of concentrated hydrochloric acid are added into the concentrated product, the concentrated product is deprotected and crystallized to form cefditoren mother nucleuses, 7-ATCA and an AEactive ester undergo a reaction under alkaline conditions, the reaction product is crystallized to form a cefditoren sodium wet product, the cefditoren sodium wet product is added into iodomethyl pivalate and undergoes a reaction in the presence of a phase transfer catalyst and the product is crystallized to form a cefditoren pivoxil crude product. In preparation of the compound 1, cefditoren sodium and cefditoren pivoxil, single solvents are used and are easy to recover. The method has the advantages of simple operation, high product conversion rate, few impurities and low production cost and is suitable for industrial production of cefditoren pivoxil.
Owner:QILU ANTIBIOTICS PHARMA
Preparation method of Cefditoren Pivoxil
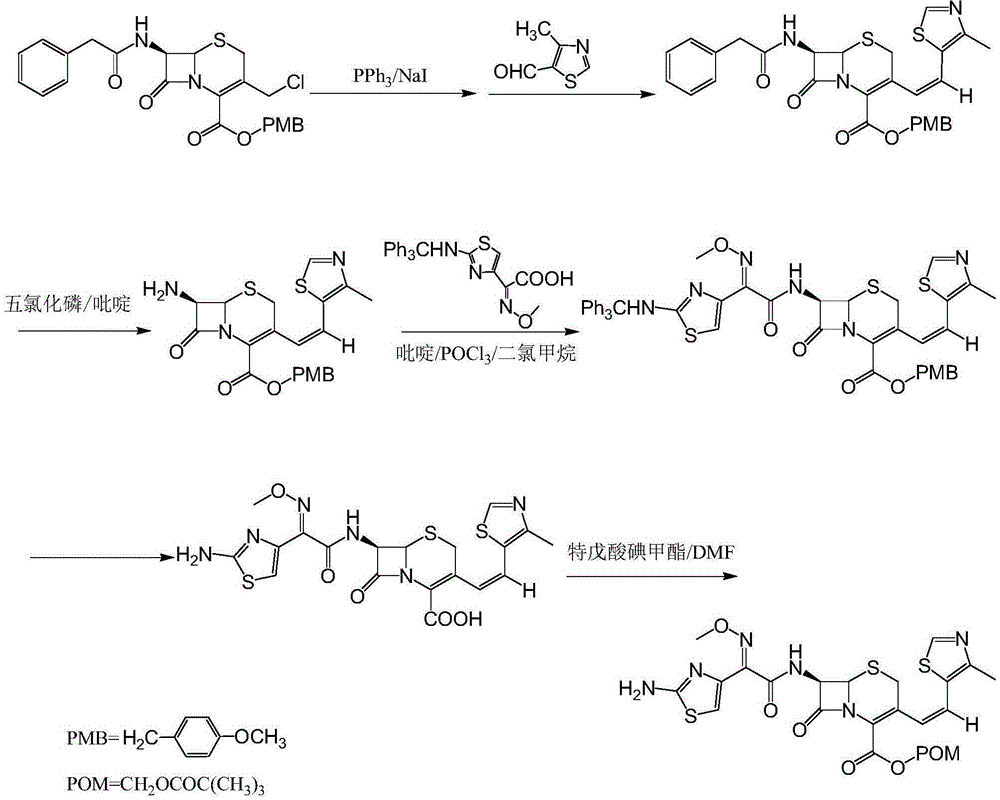
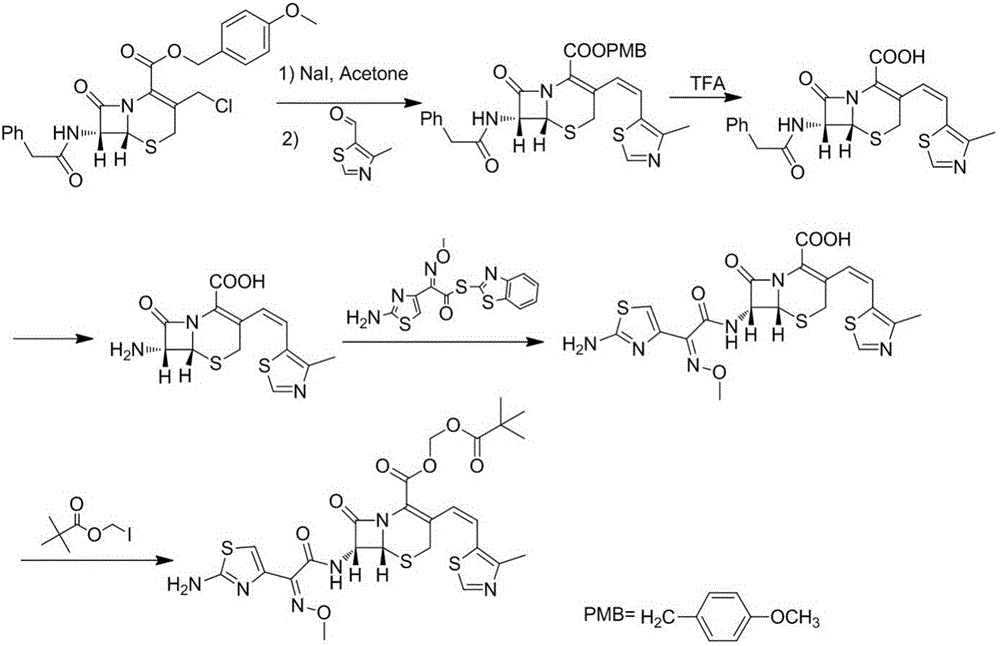
The invention relates to a preparation method of Cefditoren Pivoxil. The preparation method comprises steps as follows: 7-ACA (3-acetyloxymethyl-5-thio-7-amino-8-oxy-1-nitrogen heterobicyclic octyl-2-ene-2 carboxylic acid) is taken as a starting raw material and is subjected to iodination and Wittig reaction after silanization protection, and a Cefditoren parent nucleus 7-ATCA (7-amino-3-[(Z)-2-(4-methyl-5-thiazole) vinyl]-3-cephem-4-carboxylic acid) is generated; after amino protection of aminothiazole ethyl gallate, a compound 2 is produced from 7-ATCA under catalysis of AlMe3; the compound2 is subjected to an esterification reaction with iodomethyl pivalate under actions of a phase transfer catalyst and an acid adsorbent, the amino protection is removed, and a target product CefditorenPivoxil is obtained. According to the preparation method, reaction conditions are mild, product purity and yield are high, the process is stable, amplification is easy, and the method is applicable to industrial production.
Owner:SHANDONG YUXIN PHARMA CO LTD
Method for preparing cefditoren pivoxil
The invention discloses a method for preparing cefditoren pivoxil in the technical field of medicine, which comprises: dissolving a cefditoren pivoxi crude product in a water-soluble solvent; preparing a cefditoren pivoxil organic phase; preparing a cefditoren pivoxil aqueous phase; and adjusting the pH value of the solution, performing acid extraction treatment and menstruum crystallization treatment, and obtaining the cefditoren pivoxil with the purity of 98.1 to 98.8 percent. The method ensures that the cefditoren pivoxil can obtain high-purity products according with pharmacopoeia through purification under stable condition by simple operation.
Owner:SHANGHAI JIAO TONG UNIV
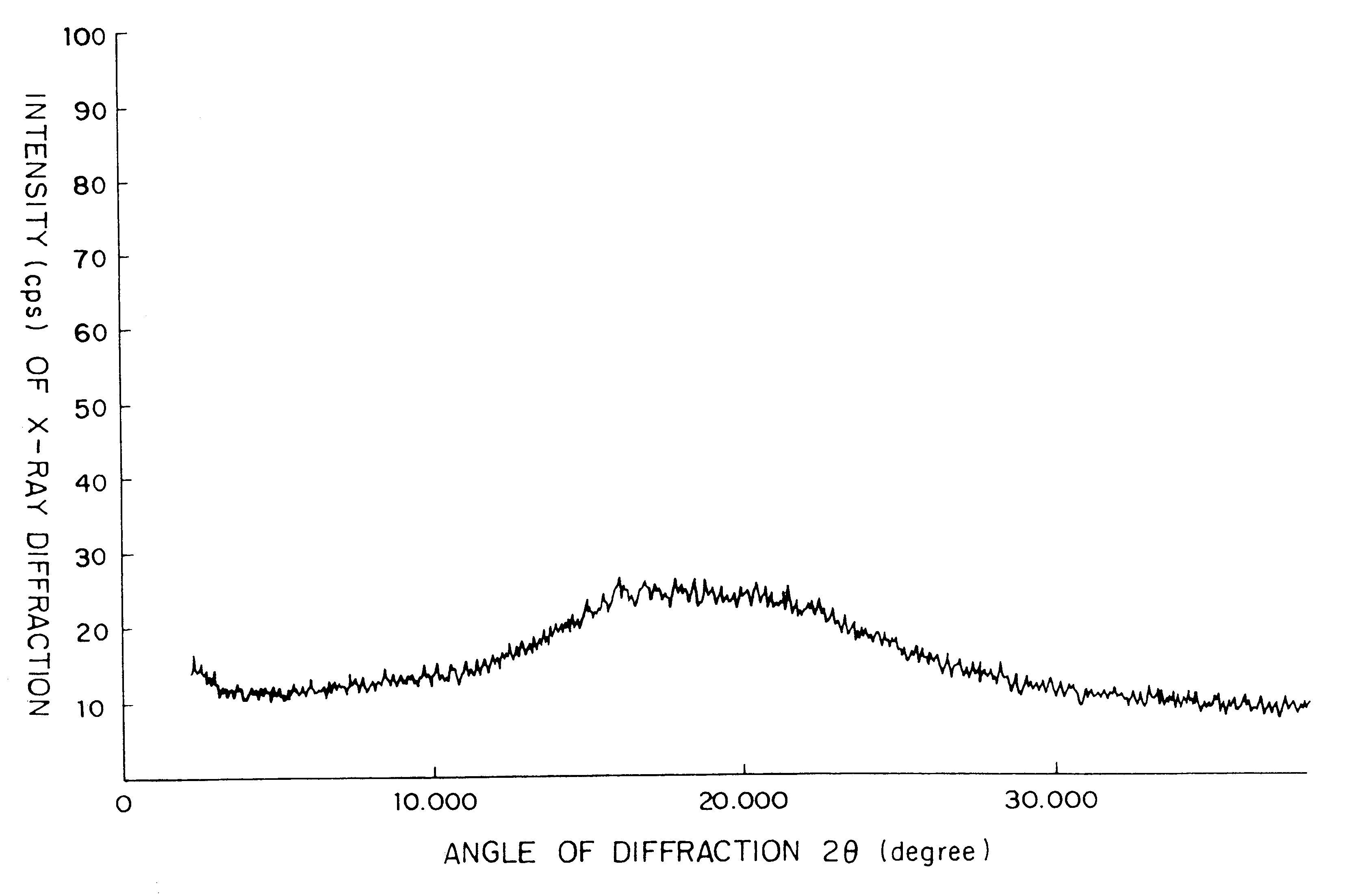
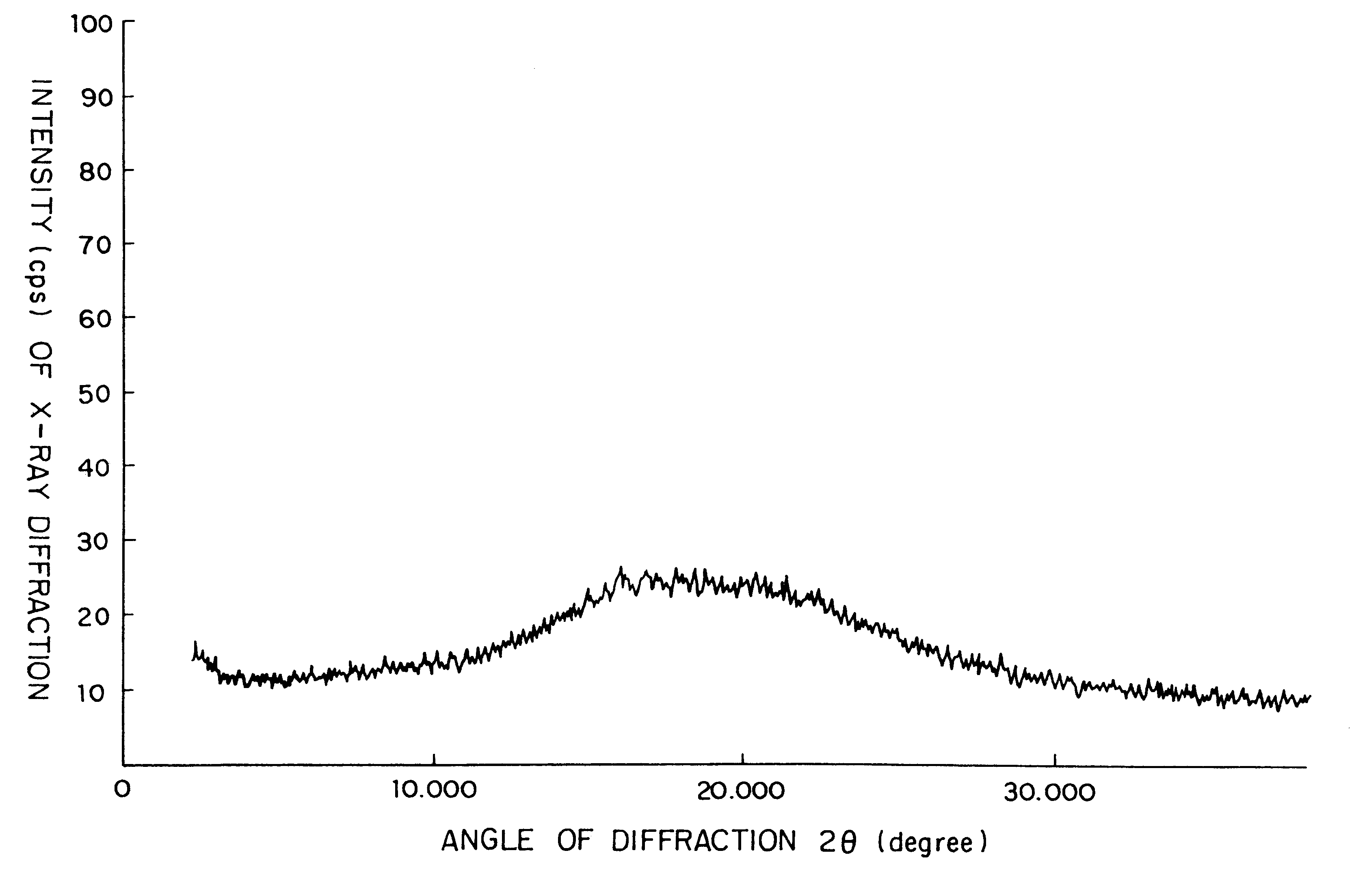
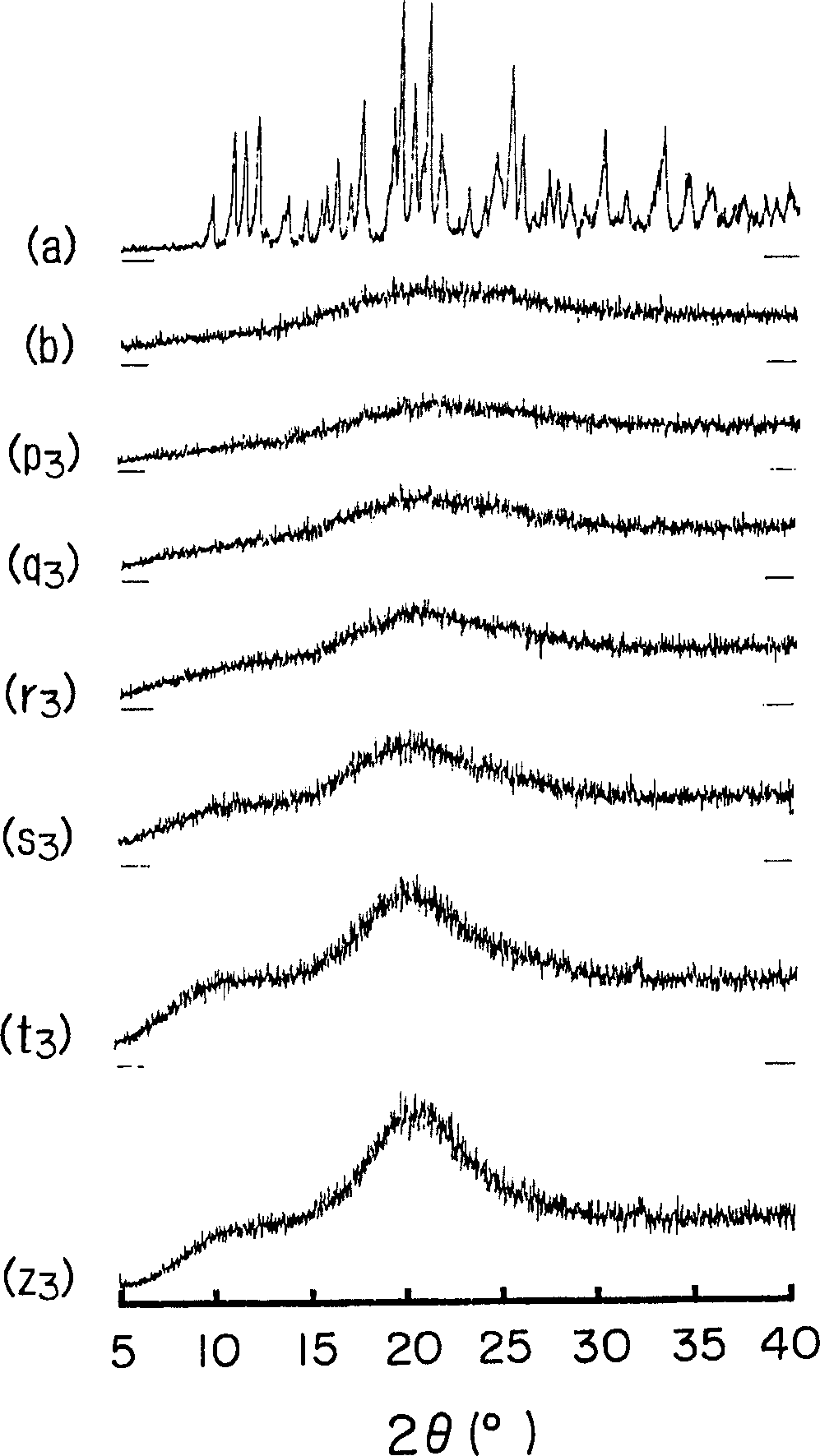
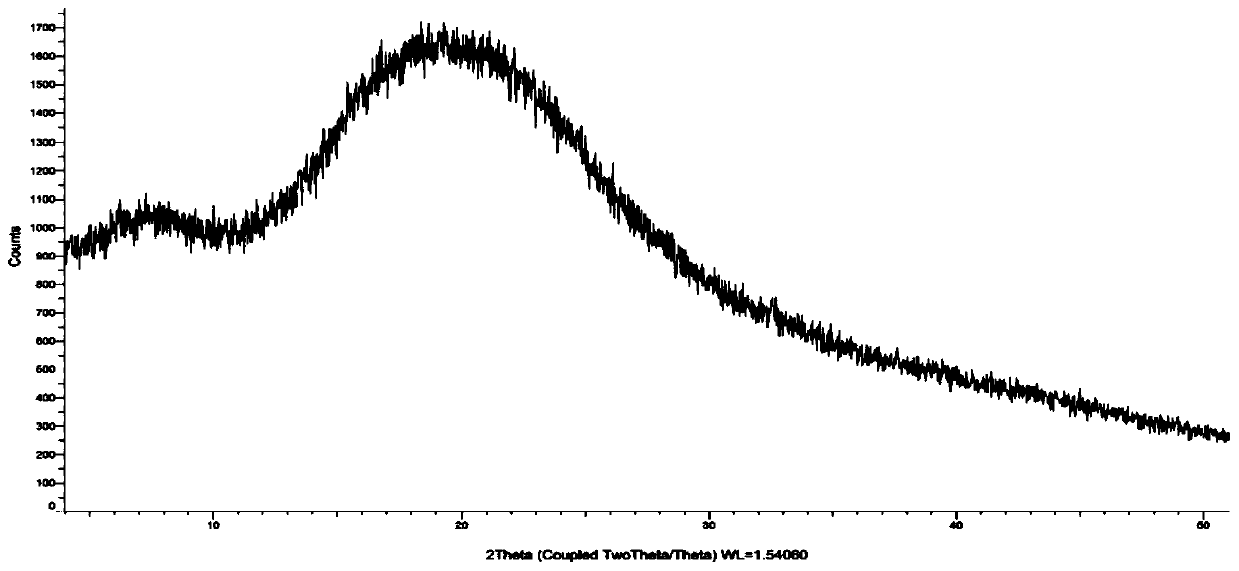
Amorphous cefditoren pivoxil composition and process for producing the same
A composition of an amorphous cefditoren pivoxil excellent in stability and releasability; and a process for producing the amorphous composition. The composition comprises cefditoren pivoxil and a pharmaceutically acceptable organic polymer, and is characterized by being obtained by pulverizing crystalline cefditoren pivoxil in the presence of a pharmaceutically acceptable organic polymer and making the cefditoren pivoxil amorphous.
Owner:MEIJI SEIKA KAISHA LTD
Micronized cefditoren pivoxil composition
The invention relates to a medicament composition and method of preparing the same. The medicament composition includes micronized particles of cefditoren pivoxil, wherein the particles have the d0.5 from 1[mu]m to 40 [mu]m. The invention also relates to method for preparing micronized cefditoren pivoxil particles.
Owner:RANBAXY LAB LTD
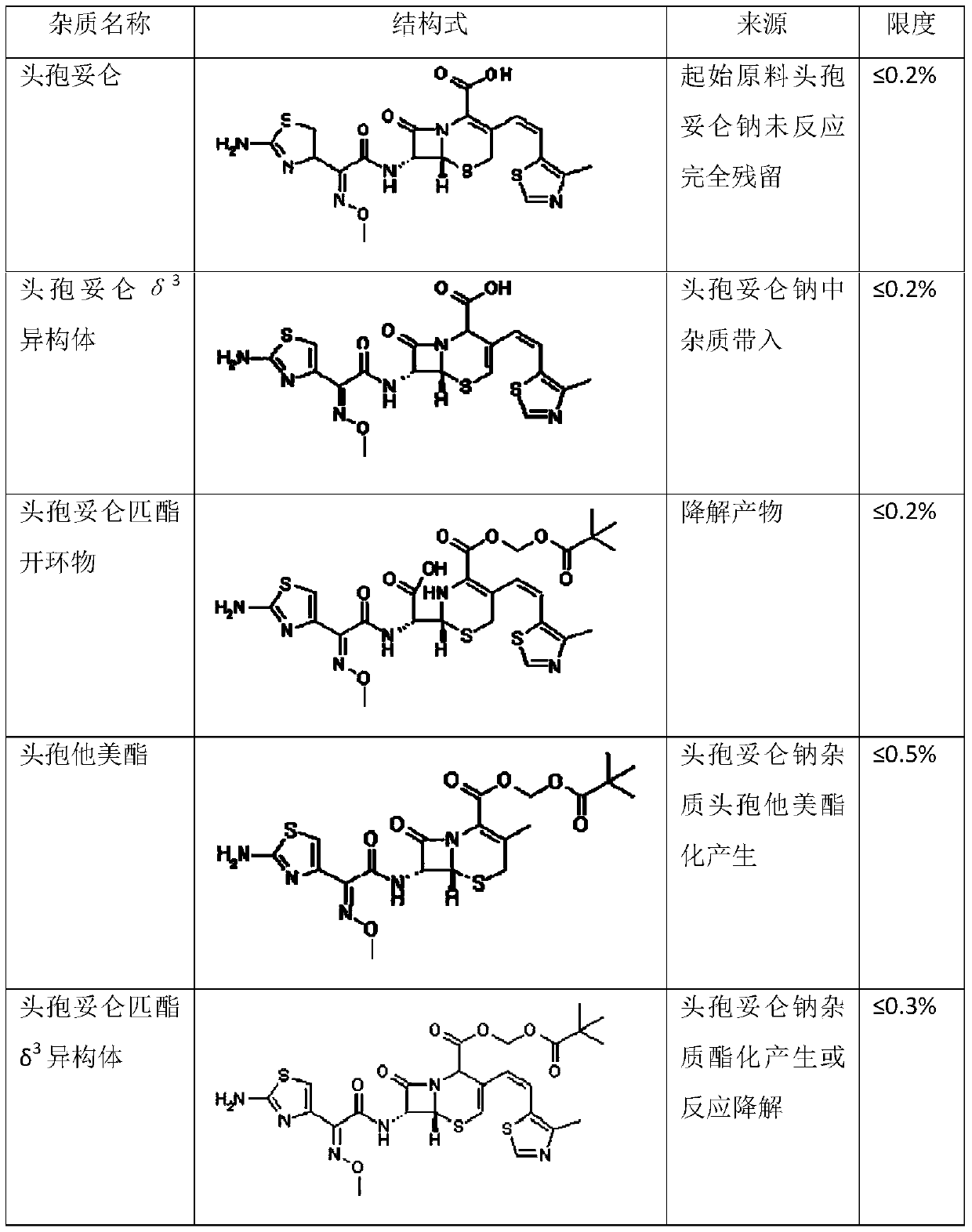
Preparation methods of Cefditoren acid delta 3 isomer and cefditoren pivoxil delta 3 isomer
The invention discloses preparation methods of Cefditoren acid delta 3 isomer and cefditoren pivoxil delta 3 isomer. The cefditoren acid delta 3 isomer is prepared from the following steps that pure water and tetrahydrofuran are added in a reaction vessel, the temperature is lowered to 0-5 DEG C, a 7-ATCA delta 3 isomer and AE active ester (MAEM) are added, after adding is completed, then organicalkali is dropwise added to control pH of 8.0-8.5, the organic alkali is needed to be additionally added to keep the pH of 8.0-8.5, dichloromethane and water are added after reaction is completed, stirring and extraction are conducted, still standing and layering are conducted, a lower organic layer is discarded, the pH of an upper water layer is 3.0-3.5, stirring and filtering are conducted, andwashing and drying are conducted to obtain a product. The content of the cefditoren acid delta 3 isomer and the cefditoren pivoxil delta 3 isomer which are prepared through the preparation method canreach above 93%.
Owner:CHONGQING MEDICAL & PHARMA COLLEGE
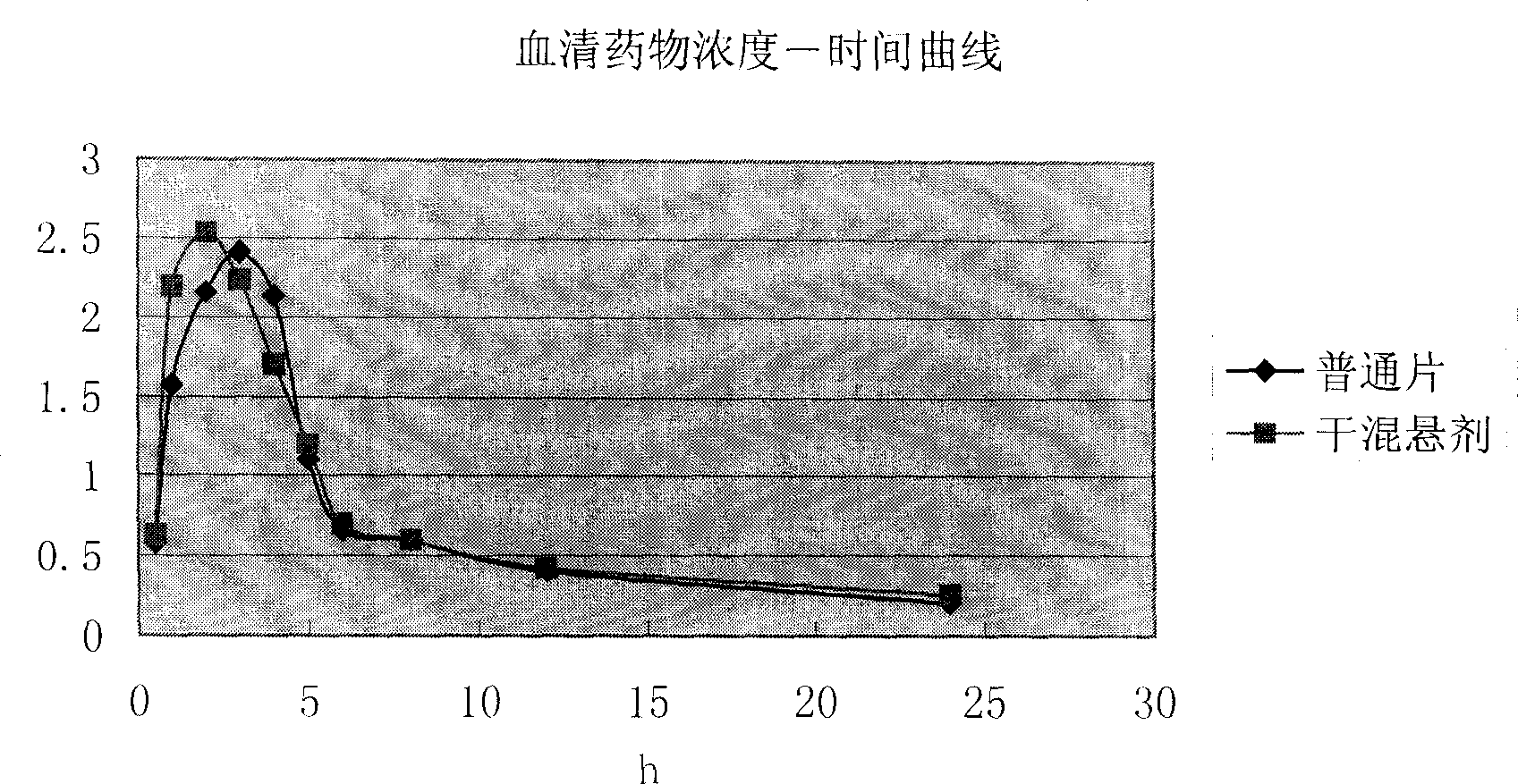
Antibacterial medicinal composition of enhanced oral absorptivity
An objective of the present invention is to provide a cefditoren pivoxil pharmaceutical preparation which can safely be administered to a patient and not only improves wettability of cefditoren pivoxil, but also further improves absorbability through the intestinal tracts by maintaining amorphous particles having a high oral absorbability in a liquid for a long period of time. The present invention is a pharmaceutical composition comprising amorphous cefditoren pivoxil and a sugar ester fatty acid, which is obtainable by mixing or wet-granulating particles containing amorphous cefditoren pivoxil with the sugar ester fatty acid while amorphous cefditoren pivoxil maintains its particle state.
Owner:MEIJI SEIKA KAISHA LTD
Cefditoren pivoxil composition tablets and preparation method thereof
ActiveCN102846570BFast disintegrationHigh dissolution rateAntibacterial agentsOrganic active ingredientsCarboxymethyl celluloseMannitol
The invention belongs to the field of medicine preparation, and discloses cefditoren pivoxil composition tablets and a preparation method thereof. The cefditoren pivoxil composition tablets adopt cefditoren pivoxil as a raw material, and are prepared with certain weight parts of auxiliary materials of mannitol, microcrystalline cellulose, sodium carboxymethyl starch, cross-linked sodium carboxymethyl cellulose, and magnesium stearate. The cefditoren pivoxil composition tablets provided by the invention have high disintegration rate, high dissolution rate, good stability, accurate dosage, convenient application, easy-to-obtain raw materials, and low cost. The tablets are easy to carry. The preparation method of the cefditoren pivoxil composition tablets provided by the invention is simple to operate and easy to control. The preparation method is labor-saving and time-saving, such that the method is suitable for large-scale industrialized productions.
Owner:SHANDONG LUOXIN PHARMA GRP CO LTD
Cefditoren pivoxil liposome solid preparation
Owner:HAINAN MEIDA PHARMA
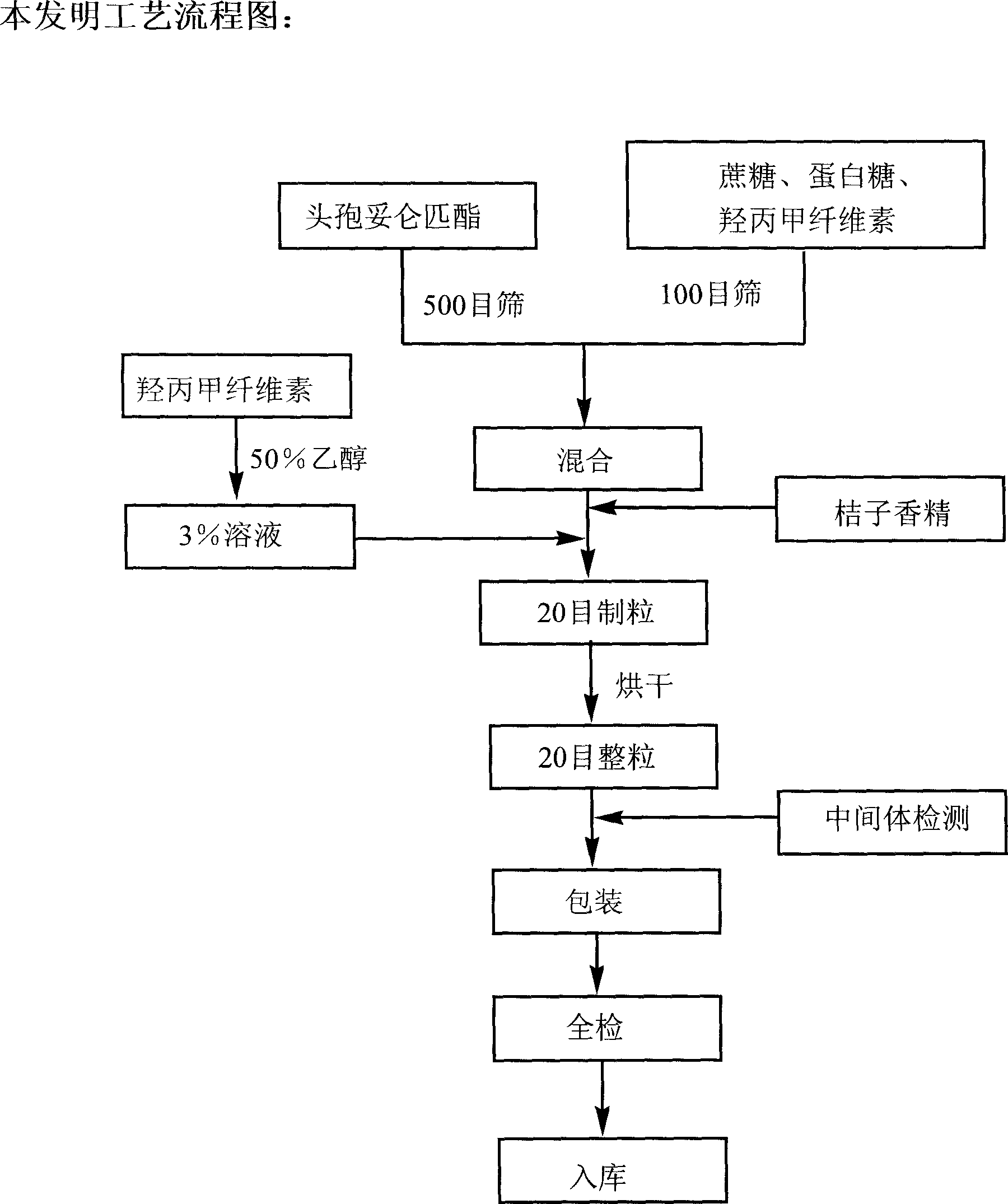
Cefditoren pivoxil dry suspensoid and its preparing process
InactiveCN1843361AGreat tasteQuality improvementAntibacterial agentsPowder deliveryHypromelloseSugar
The invention relates to a Cefditoren pivoxil for suspension and its preparing process, wherein the preparation is prepared from Cefditoren pivoxil, cane sugar, methyl hydroxypropylcellulose, egg white sweets and orange essence through sieving, weighing, mixing homogeneously, charging adhesive, palletizing, forced air drying and granulating.
Owner:楼剑波 +1
Preparation method of amorphous cefditoren pivoxil composition
InactiveCN110251467APromote growthControl growthAntibacterial agentsPowder deliveryChemical synthesisOrganic solvent
The invention relates to the field of medicinal chemistry synthesis and provides a preparation method of an amorphous cefditoren pivoxil composition. The preparation method is characterized by including: dissolving crystalline cefditoren pivoxil into a mixed organic solvent heated to certain temperature, suspending a water-soluble polymer material of a certain proportion into the mixed organic solvent, and drying through a spraying dryer to obtain the amorphous cefditoren pivoxil composition (with the water-soluble polymer material of a certain proportion wrapped inside).
Owner:BEIJING JIMEITANG MEDICINE RES CO LTD
Preparation method of cefditoren pivoxil dimer
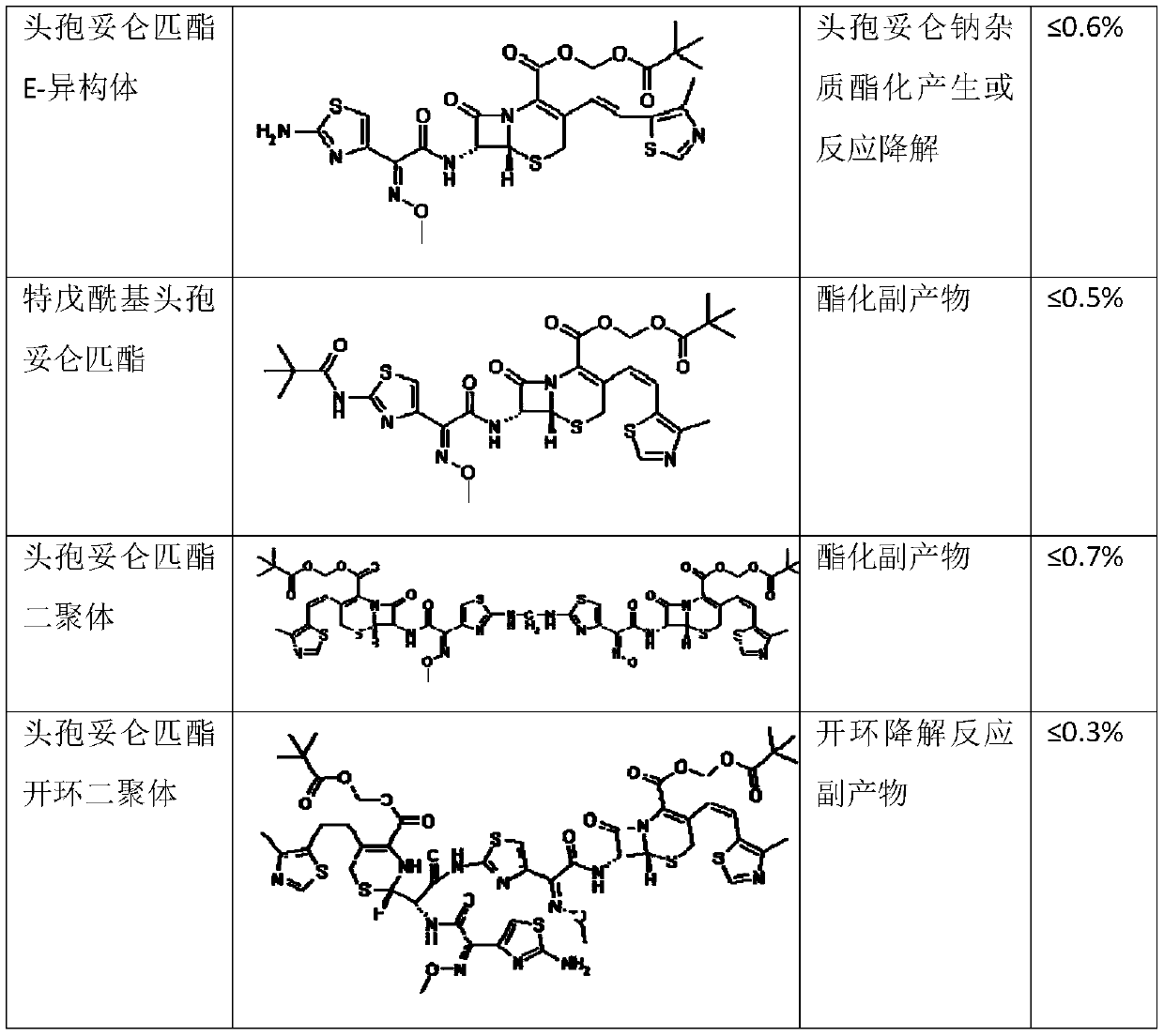
ActiveCN110183468AClinical Safe Use GuaranteeThe preparation method is simple and easyOrganic chemistryOrganic solventCefditoren
The invention discloses a preparation method of a cefditoren pivoxil dimer. The preparation method is characterized in that an organic solvent and cefditoren sodium are added to a reaction container,and are stirred and dissolved, the temperature is reduced to subzero 20-subzero 50 DEG C, iodomethyl pivalate is added in batches, and iodomethyl pivalate is added again after reacting for a certain time after each addition of iodomethyl pivalate; inorganic or organic base is required to be added to control pH at 7.5-8.5 during the reaction, and subsequent separation and purification are performedafter the reaction to obtain the product. The preparation method has simple steps, simple raw materials and low cost, and is suitable for large-scale preparation. The content of the cefditoren pivoxil dimer prepared with the preparation method provided can reach 92.0% or higher, a theoretical basis is provided for safe use of drugs, effective data support is provided for the quality standard of cefditoren pivoxil, and effective guarantee is provided for safe clinical use of drugs.
Owner:CHONGQING MEDICAL & PHARMA COLLEGE
A kind of preparation method of cefditoren pivoxil intermediate
ActiveCN103695522BSuitable for synthesisSimple processOrganic chemistryFermentationWittig reactionSynthesis methods
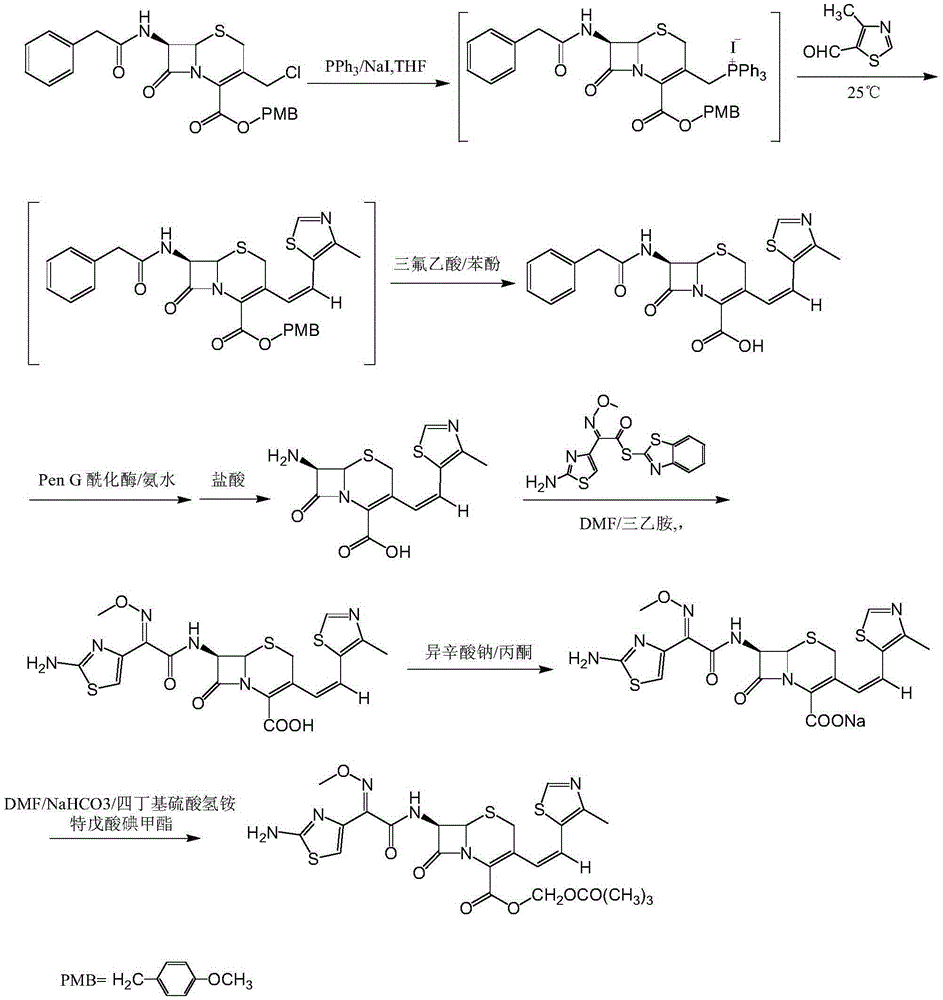
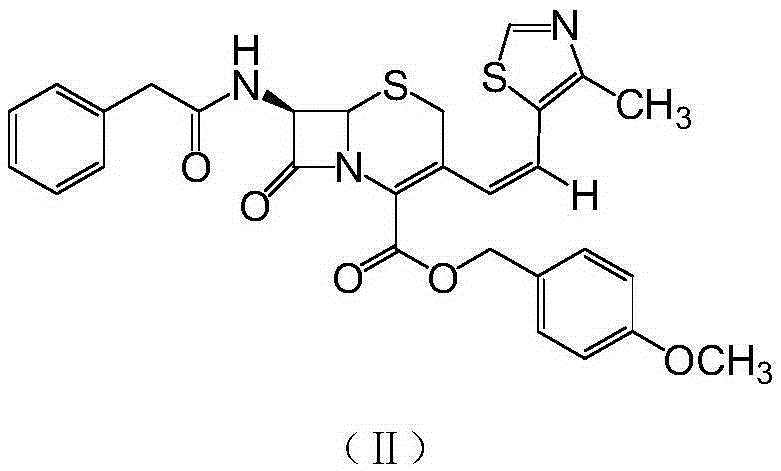
The invention discloses a synthesis method of a drug intermediate, and particularly relates to a preparation method of a cefditoren pivoxil cephalosporins intermediate. The preparation method comprises the step of reacting through phosphorus Ylide-Wittig reaction and hydrolysis reaction by using 7-phenylacetamide-3-chloromethylcephalosporanic acid p-methoxybenzyl ester to obtain cefditoren pivoxil cephalosporins. The preparation method is simple in process, safe, environment-friendly, high in yield, and suitable for industrialized production.
Owner:CHENGDU YILUKANG MEDICAL TECH & SERVICE
Noncrystalline antibacterial composition containing cefditoren pivoxil
According to the present invention, there is provided a solid dispersion composition which can maintain amorphous cefditoren pivoxil in a suspension for a long period of time. The present invention is a solid dispersion composition comprising at least 0.1 mg of a sugar ester fatty acid on the basis of an amount equivalent to 100 mg efficacy of cefditoren pivoxil.
Owner:MEIJI SEIKA KAISHA LTD
A kind of synthetic method of cefditoren pivoxil mother nucleus
Owner:山东昌邑四方医药化工有限公司
Method for preparing cefditoren pivoxil open-cycle dimer
The invention discloses a method for preparing a cefditoren pivoxil open-cycle dimer. The cefditoren pivoxil open-cycle dimer is characterized by being prepared by the following steps of reactions: firstly, protecting amino on cefditoren pivoxil by using Cbz (carbobenzoxyl), carrying out cycle opening so as to obtain a Cbz protected cefditoren pivoxil open-cycle substance, further enabling the Cbz protected cefditoren pivoxil open-cycle substance to react with the cefditoren pivoxil so as to obtain a Cbz protected cefditoren pivoxil open-cycle dimer, and finally removing Cbz protection, so as to obtain a product. The method disclosed by the invention is simple in step, low in cost and applicable to on-scale preparation. The content of the cefditoren pivoxil open-cycle dimer prepared by using the preparation method disclosed by the invention is up to 95.0% or greater, the yield can be up to 58%, theoretic bases are provided for medicine use security, effective data support is provided for quality standards of the cefditoren pivoxil, and effective insurance is provided for clinical use security of medicines.
Owner:CHONGQING MEDICAL & PHARMA COLLEGE
Preparation method of thiazole compounds
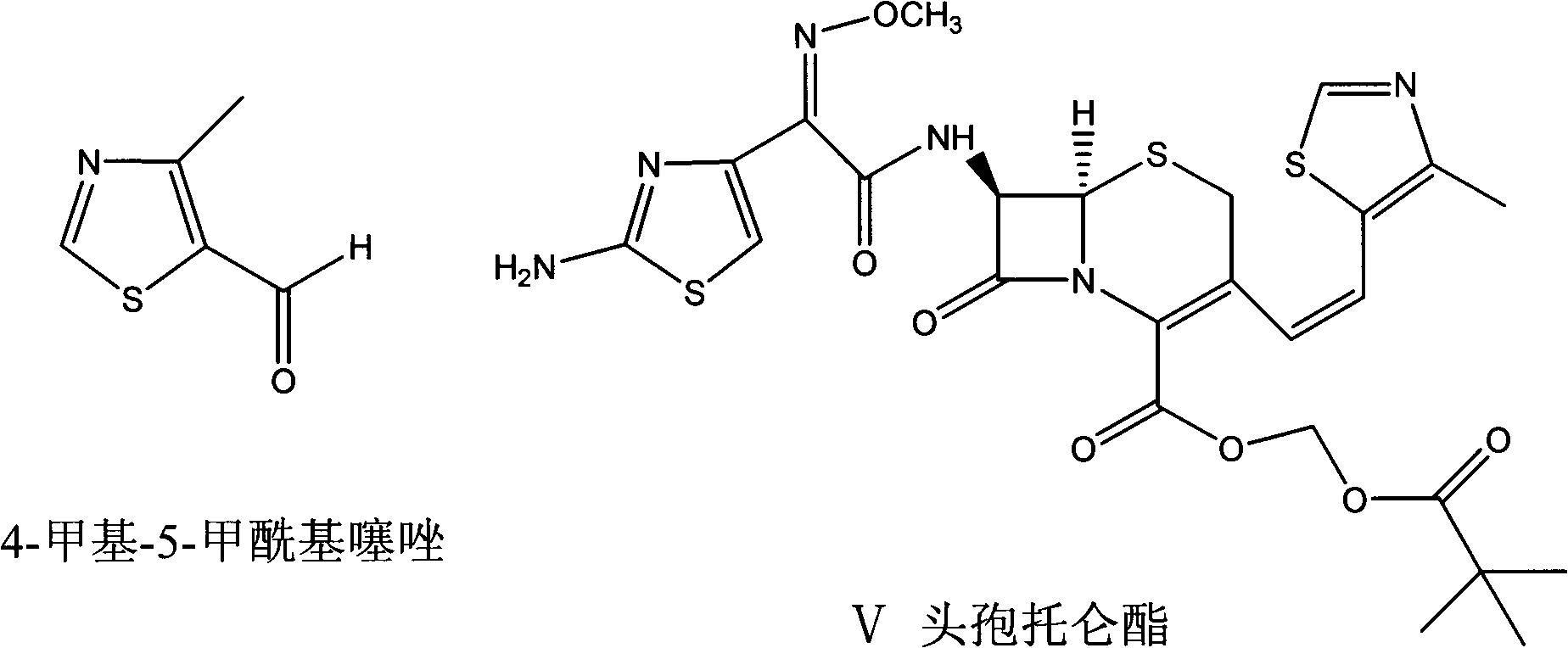
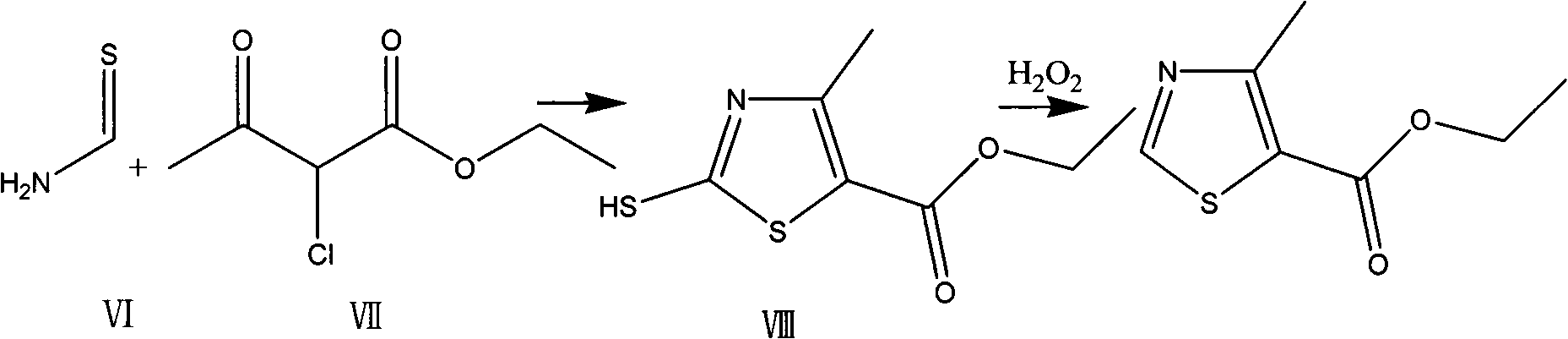
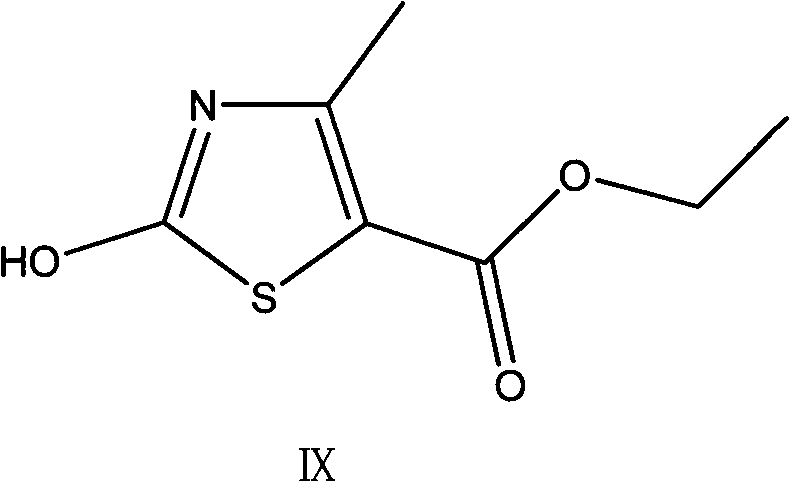
The invention provides a preparation method of a thiazole compound as shown in a formula II, comprising the following steps of: by taking alkyl nitrite R3NO2 as a diazotization reagent, performing a diazotization deamination reaction on a compound of a formula I in an anhydrous polar solvent at a reaction temperature of 30-80 DEG C to generate the compound as shown in the formula II. The invention further provides a preparation method of a thiazole compound shown in a formula IV, comprising the following steps of: converting an ester group in the compound of the formula II to an aldehyde group so as to prepare the compound shown as the formula IV. The preparation method provided by the invention is moderate in reaction conditions, simple and convenient to operate, few in by-products, small in reaction molar volume, simple in after-treatment, saved in solvent dosage, suitable for industrialized production, environment-friendly, and capable of preparing the high-yield and high-purity key intermediate, namely, 4-methyl-5-formoxyl thiazole, of cefditoren pivoxil.
Owner:SHANGHAI INST OF PHARMA IND +1
Cefditoren pivoxil purifying method
InactiveCN106243128AEasy to operateEasy to operate in industrialized productionOrganic chemistryOrganic solventOrganic layer
The invention relates to a cefditoren pivoxil purifying method. The method concretely comprises the following steps: dissolving crude cefditoren pivoxil with low purity in an organic solvent, adding an acid to form a salt, washing the salt, carrying out liquid separation, adding an organic solvent, adjusting the pH value of the obtained solution to 4.0-8.0 by using an alkali, adding the above obtained organic layer to a non-polar solvent in a dropwise manner to obtain an amorphous solid, and crystallizing the amorphous solid by using an organic solvent to obtain purified cefditoren pivoxil with a crystal form. The purity of the final product is 99.0-99.7%, the content of E-isomer is smaller than 0.1%. The method has the advantages of simplicity in operation, mild reaction conditions, obvious improvement of the purity of the product, and facilitation of industrial production.
Owner:JINAN KANGHE MEDICAL TECH
Cefditoren pivoxil liposome solid preparation
The invention discloses a cefditoren pivoxil liposome solid preparation and a method for preparing the same. The active ingredient namely cefditoren pivoxil and a specific combination of soybean phosphatidylserine, cholesterol, and polyoxyethylene alkylamine are prepared into liposome, so the stability, dissolution property and bioavailability of the preparation are greatly improved, and moreover, the preparation has a stable and lasting effect, small side effects and obvious efficacy.
Owner:HAINAN MEIDA PHARMA
Refinement method of cefditoren pivoxil
The present invention belongs to the technical field of medicine, and particularly relates to a refinement method of cefditoren pivoxil, wherein a cefditoren pivoxil crude product is sequentially subjected to dissolution, extraction, concentration crystallization and slurry beating to finally obtain the cefditoren pivoxil pure product. According to the present invention, the current situations of poor purification effect of the cefditoren pivoxil crude product and high dimer content in the existing refinement process can be overcome, and the refinement method has advantages of simple operation and high refinement efficiency, and is suitable for large-scale industrial production.
Owner:REYOUNG PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com