Amorphous cefditoren pivoxil composition and process for producing the same
A composition and amorphous technology, applied in the field of pharmaceutical compositions for oral administration, can solve the problems of unsatisfactory production efficiency, process management and long time, and achieve high therapeutic effectiveness, good dissolution and stability excellent effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
manufacture example
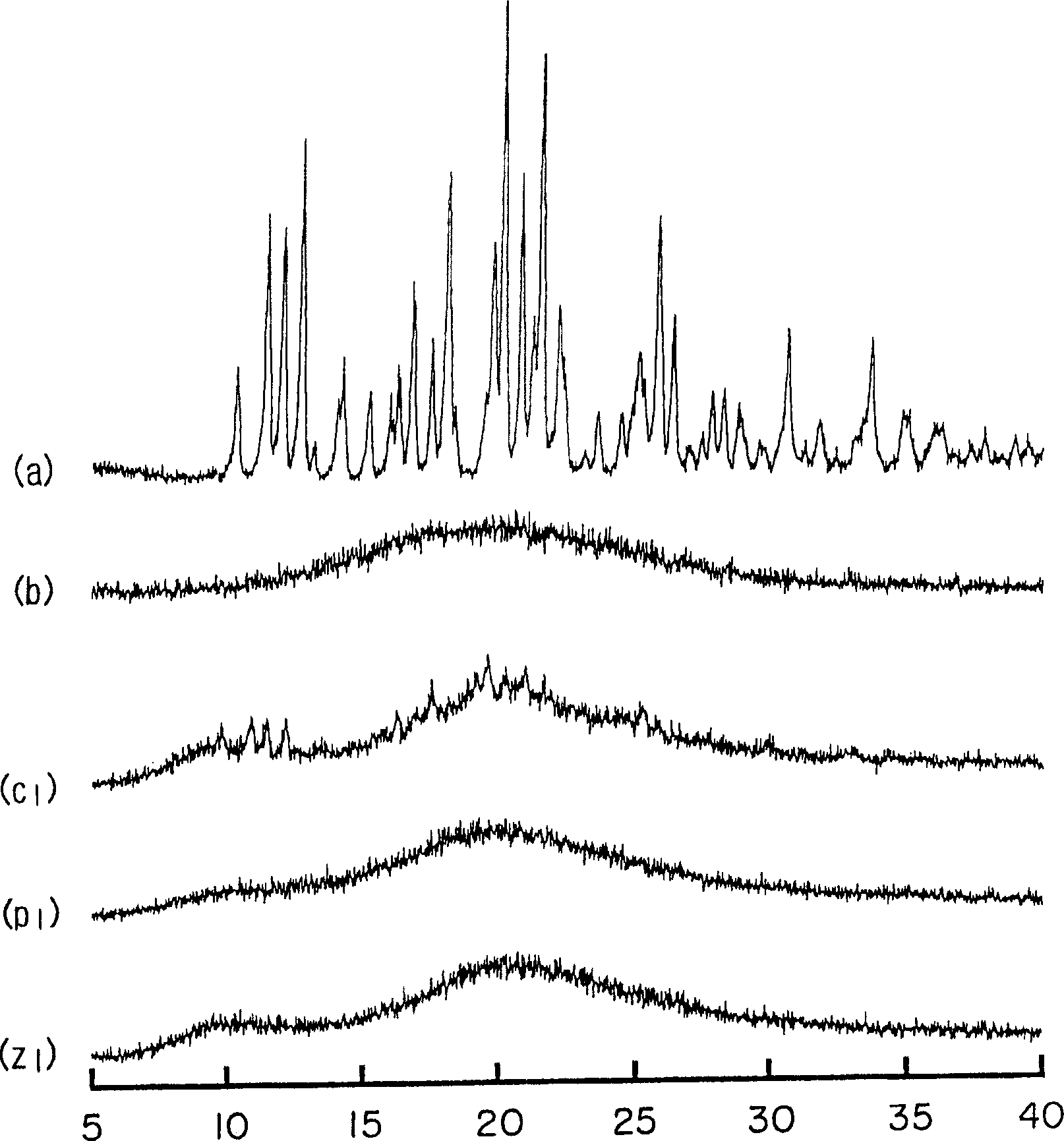
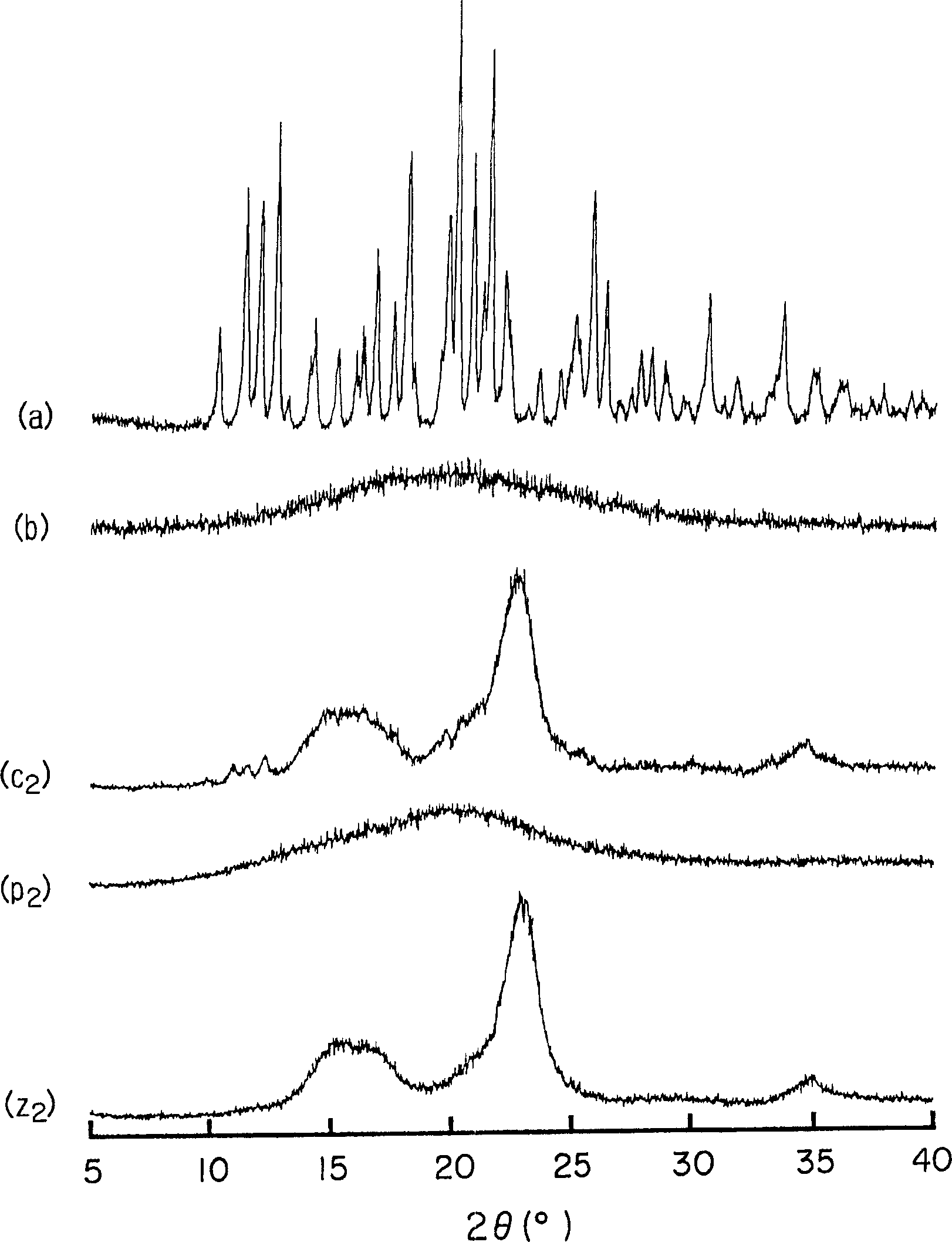
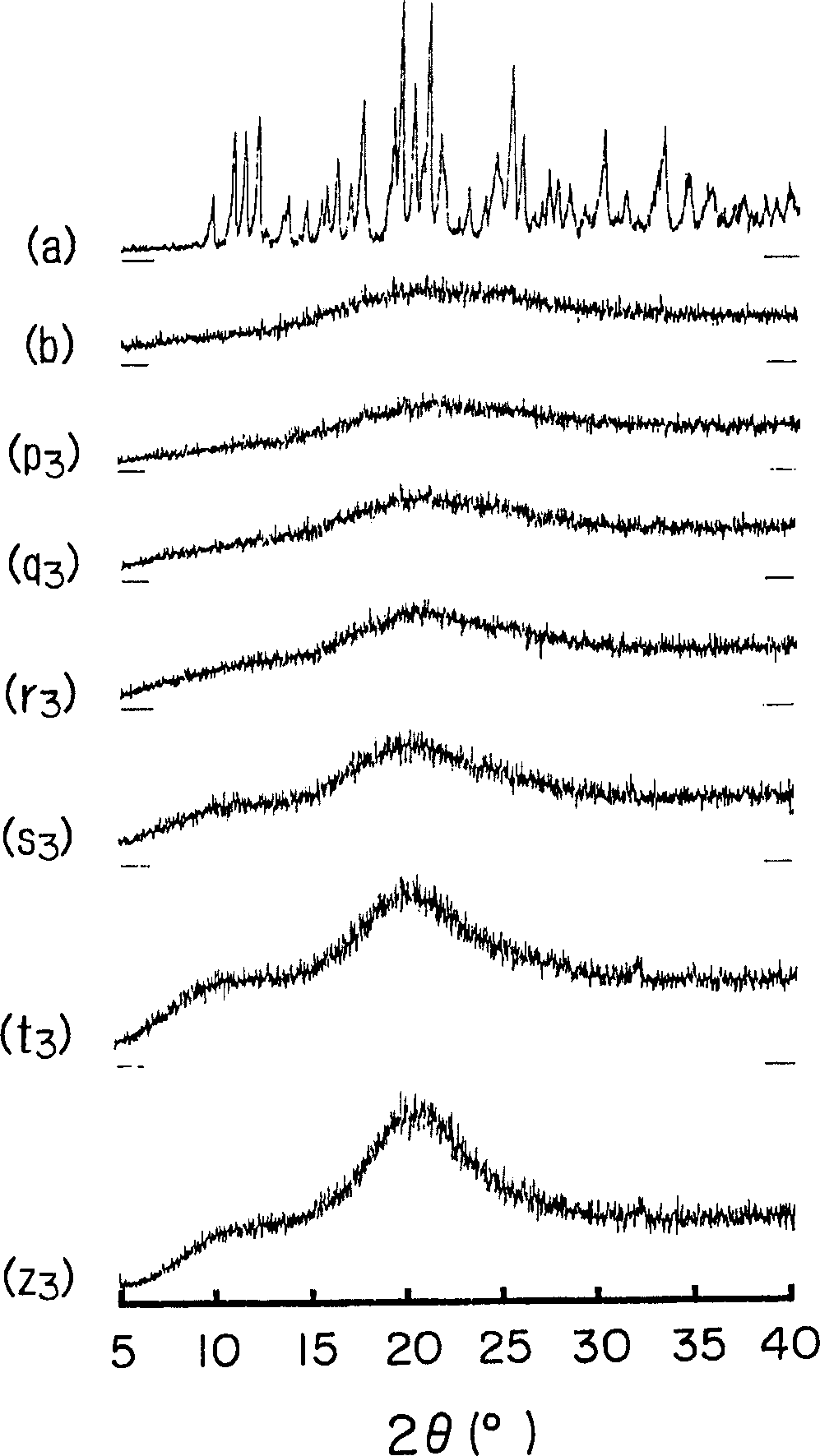
[0063] Amorphous cefditoren and pivoxil compositions 1 to 7 of the present invention were prepared as follows.
[0064] Amorphous Composition 1
[0065] With 0.3g crystalline cefditoren pivoxil (manufactured by Meiji Seika Co., Ltd.), 2.7g sodium caseinate (manufactured by New Zealand Dairy Board) (the weight ratio of cefditoren pivoxil / casein sodium is 10 / 90) was put into a pulverizing container made of alumina, and pulverized for 30 minutes using a vibrating rod mill (manufactured by CMT, model TI-200) to obtain an amorphous composition 1.
[0066] Amorphous Composition 2
[0067] Mix 0.3 g of crystalline cefditoren pivoxil (manufactured by Meiji Seika Co., Ltd.), 2.7 g of crystalline cellulose (manufactured by Asahi Kasei Co., Ltd.) (the weight ratio of cefditoren pivoxil / crystalline cellulose is 10 / 90) was placed in a pulverizing container made of alumina, and pulverized for 30 minutes using a vibrating rod mill (manufactured by CMT: TI-200) to obtain an amorphous c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com