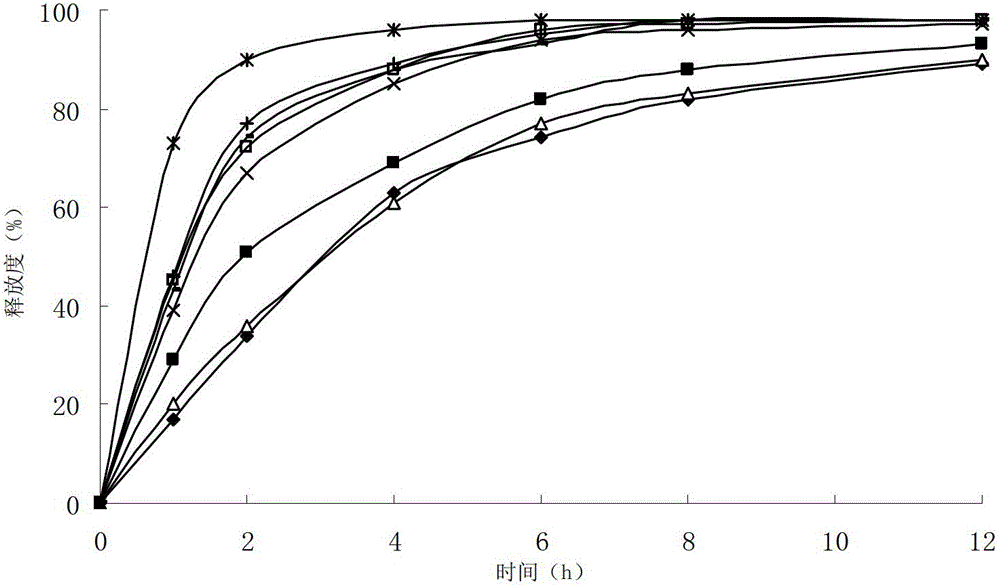
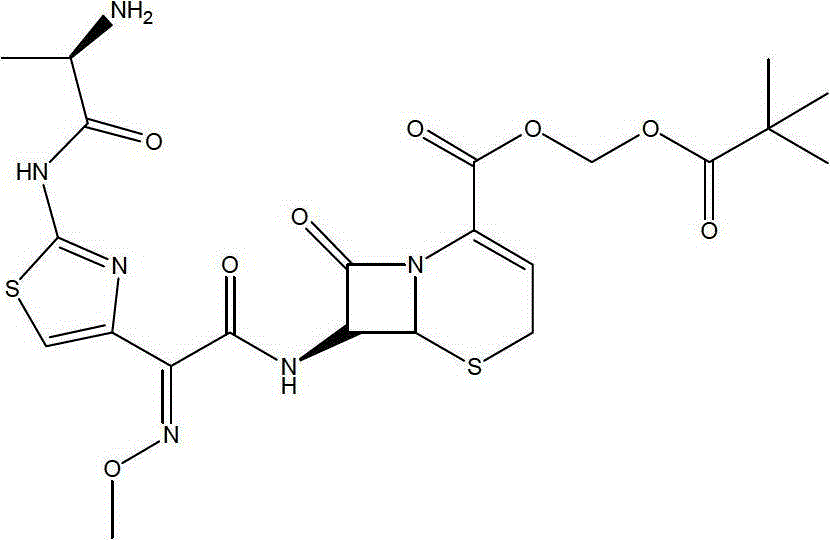
Cefditoren pivoxil liposome solid preparation
A technology of cefditoren pivoxil and cefditoren, which is applied in the field of medicine and achieves the effects of good sustained release effect, high bioavailability and high stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] Embodiment 1 cefditoren pivoxil liposome tablet
[0080] The ingredients used are as follows:
[0081]
[0082] Adopt following production process to prepare cefditoren pivoxil liposome tablet:
[0083] (1) Accurately weigh 100g of cefditoren pivoxil, 100g of soybean phosphatidylserine, 50g of cholesterol, and 50g of polyoxyethylene alkylamine, and dissolve them in 1000ml of a mixed solvent of ethanol and chloroform with a volume ratio of 2:1, Stir to dissolve;
[0084] (2) Put the above solution in an eggplant-shaped bottle, remove ethanol and chloroform under reduced pressure in a 50°C water bath, and form a uniform transparent film on the wall of the bottle;
[0085](3) Add 1000ml of phosphate buffer solution with a pH value of 6.6 to the eggplant-shaped bottle, and continue to rotate in a water bath at 50°C under normal pressure to swell and hydrate the film;
[0086] (4) Filter the above solution with a 0.45 μm microporous membrane, place the filtrate in a ...
Embodiment 2
[0090] Embodiment 2 cefditoren pivoxil liposome tablet
[0091] The ingredients used are as follows:
[0092]
[0093] Adopt following production process to prepare cefditoren pivoxil liposomal capsules:
[0094] (1) Accurately weigh 100g of cefditoren pivoxil, 50g of soybean phosphatidylserine, 50g of cholesterol, and 80g of polyoxyethylene alkylamine, and dissolve them in 1000ml of a mixed solvent of ethanol and chloroform with a volume ratio of 2:1, Stir to dissolve;
[0095] (2) Put the above solution in an eggplant-shaped bottle, remove ethanol and chloroform under reduced pressure in a 50°C water bath, and form a uniform transparent film on the wall of the bottle;
[0096] (3) Add 1000ml of phosphate buffer solution with a pH value of 6.6 to the eggplant-shaped bottle, and continue to rotate in a water bath at 50°C under normal pressure to swell and hydrate the film;
[0097] (4) Filter the above solution with a 0.45 μm microporous membrane, place the filtrate in...
Embodiment 3
[0101] Embodiment 3 cefditoren pivoxil liposome tablet
[0102] The ingredients used are as follows:
[0103]
[0104] Adopt the following production process to prepare cefditoren pivoxil liposome dry suspension:
[0105] (1) Accurately weigh 100g of cefditoren pivoxil, 150g of soybean phosphatidylserine, 100g of cholesterol, and 100g of polyoxyethylene alkylamine, and dissolve them in 1000ml of a mixed solvent of ethanol and chloroform at a volume ratio of 2:1, Stir to dissolve;
[0106] (2) Put the above solution in an eggplant-shaped bottle, remove ethanol and chloroform under reduced pressure in a 50°C water bath, and form a uniform transparent film on the wall of the bottle;
[0107] (3) Add 1000ml of phosphate buffer solution with a pH value of 6.6 to the eggplant-shaped bottle, and continue to rotate in a water bath at 50°C under normal pressure to swell and hydrate the film;
[0108] (4) Filter the above solution with a 0.45 μm microporous membrane, place the f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com