Micronized cefditoren pivoxil composition
A technology of cefditoren and composition, applied in the field of preparing micronized granules of cefditoren pivoxil, capable of solving the problems of low solubility of cefditoren pivoxil and inability to administer drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Micronization method of cefditoren pivoxil
[0052] Use air jet mill to micronize cefditoren pivoxil, and the compressed air pressure is 6PSIG to obtain 0.5 2.37μm and d 0.9 6μm powder.
Embodiment 2
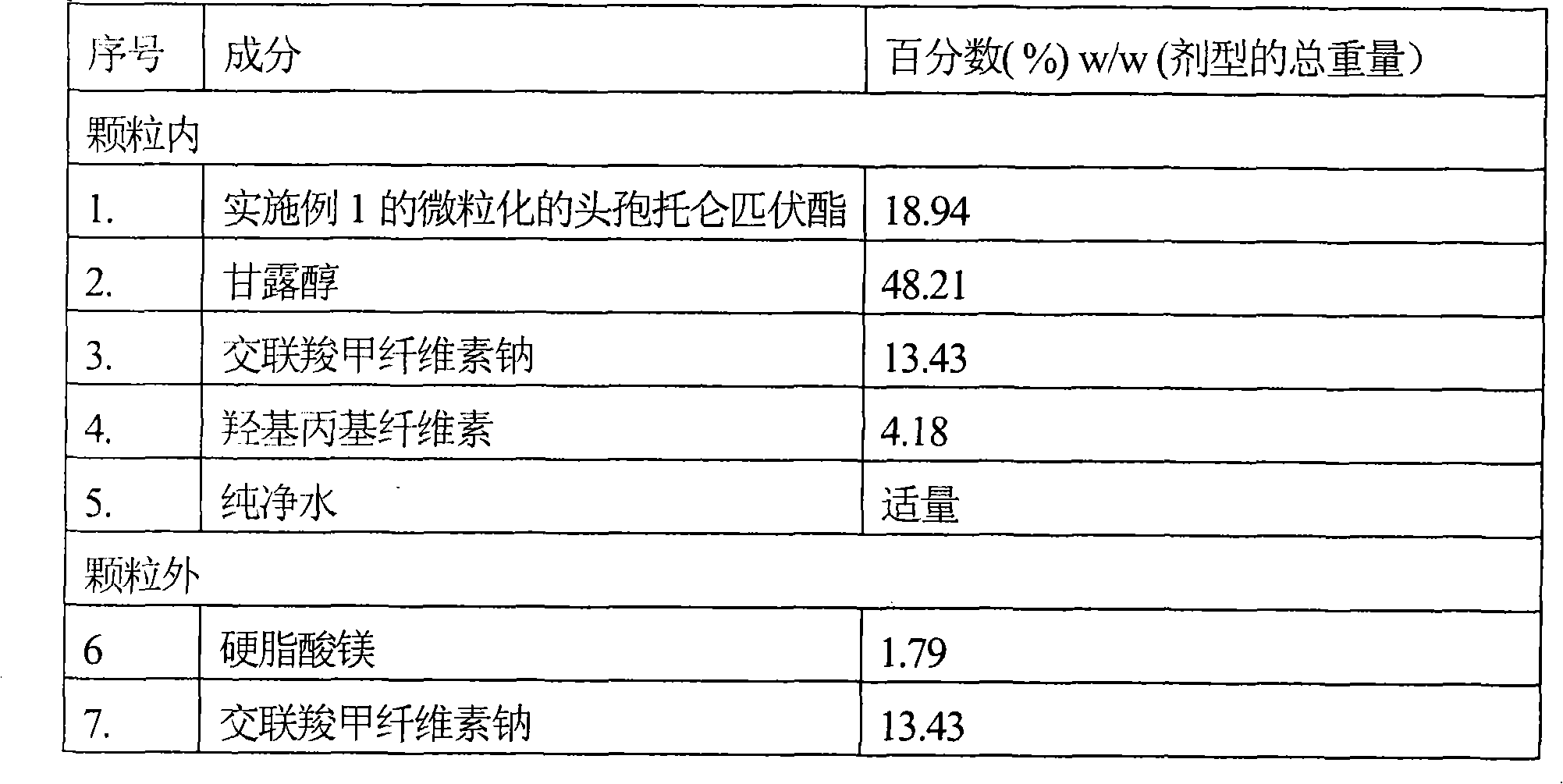
[0054] Tablets containing micronized cefditoren pivoxil
[0055]
[0056] method:
[0057] (i) Sieve micronized cefditoren pivoxil, mannitol and intragranular croscarmellose sodium and mix together.
[0058] (ii) Hydroxypropyl cellulose was dissolved in purified water.
[0059] (iii) Granulating the mixture of step (i) using the binder solution of step (ii).
[0060] (iv) Drying the granules of step (iii) in a fluid bed drier.
[0061] (v) Sifting the dried granules of step (iv).
[0062] (vi) Magnesium stearate and extragranular croscarmellose sodium are sieved and blended with the granules of step (v).
[0063] (vi) Finally, the mixture of step (vi) is compressed into tablets.
Embodiment 3
[0065] With ingredients similar to Example 2, non-micronized cefditoren pivoxil (with d 0.5 greater than 100 μm) to prepare tablets comprising unmicronized cefditoren pivoxil.
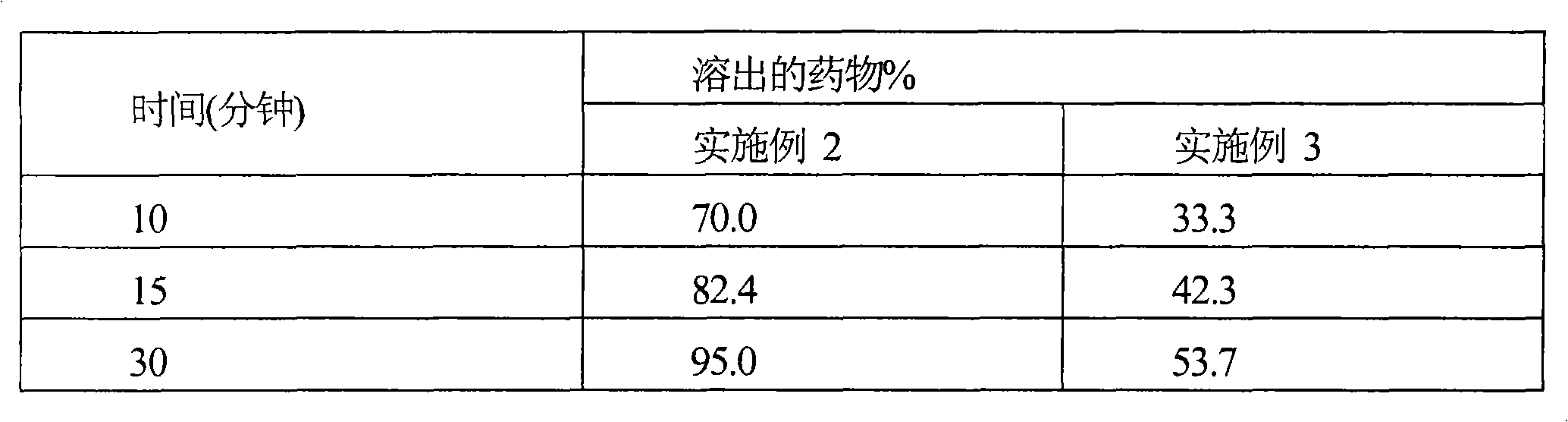
[0066] Comparison of In Vitro Dissolution Profiles
[0067] Dissolution studies were performed on tablets of cefditoren pivoxil prepared according to the compositions of Examples 2 & 3 in 900 ml of 0.1N HCl at 37°C using USP Apparatus II with a paddle speed of 50 rpm. Table 1 provides a comparison of the dissolution profile of tablets comprising micronized (Example 2) and non-micronized cefditoren pivoxil (Example 3).
[0068] Table 1
[0069]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com