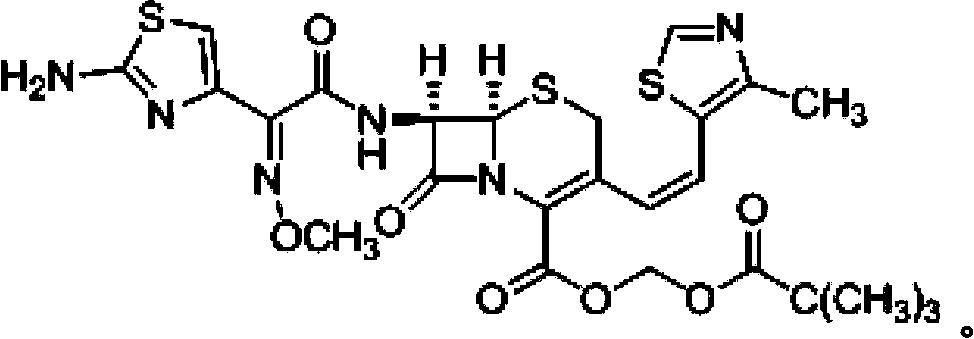
Cefditoren pivoxil composition tablets and preparation method thereof
A technology of cefditoren pivoxil and composition, which is applied in the field of cefditoren pivoxil composition tablet and its preparation field, can solve the problem of slow disintegration speed of cefditoren pivoxil tablet, unsuitable for industrial production and poor stability and other problems, to achieve good disintegration effect, good disintegration effect, good compressibility and good compression formability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Using cefditoren pivoxil as raw material, the effects of the dosage of various excipients on drug hardness, appearance, disintegration, friability and dissolution were investigated. The dosage of excipients in each prescription is shown in Table 1.
[0034] Sieve the raw materials, sieve other auxiliary materials separately, and set aside; cefditoren pivoxil, mannitol, microcrystalline cellulose, hydroxypropyl cellulose, pregelatinized starch, croscarmellose sodium, carboxymethyl starch Sodium and magnesium stearate were mixed according to the prescription amount, and the results of measuring the angle of repose and outflow velocity are shown in Table 2. After tableting, the hardness, appearance, weight difference, disintegration, friability, and dissolution rate were measured, and the results are shown in Table 3. Among them, the method for measuring the outflow speed is: take a certain amount of sample (10g), put it into a funnel, measure the time required for all th...
Embodiment 2
[0044] Using cefditoren pivoxil as raw material, the effects of the dosage of various excipients on drug hardness, appearance, disintegration, friability and dissolution were investigated. The amount of excipients in each prescription is shown in Table 4.
[0045] Sieve the cefditoren pivoxil and other excipients respectively, grind the cefditoren pivoxil and corresponding auxiliary materials according to the prescription ratio through a pulverizer, sieve, and set aside.
[0046] Mix cefditoren pivoxil and the corresponding prescription auxiliary materials according to the relevant proportion, pass through 2 times of sieve, adopt the dry granulation method to make the above-mentioned mixture into granules, add the magnesium stearate of the prescription amount, mix well, measure the angle of repose, The outflow velocity results are shown in Table 5. The hardness, appearance, weight difference, disintegration, friability, and dissolution rate were measured after tableting, and ...
Embodiment 3
[0054] Embodiment 3: cefditoren pivoxil composition tablet of the present invention
[0055]
[0056] Preparation method: 126g of cefditoren pivoxil and 30g of mannitol were mixed, pulverized and sieved to obtain the first mixture for later use; another remaining 55g of mannitol was pulverized, and then mixed with 55 parts by weight of microcrystalline cellulose, 15 parts by weight of Carboxymethyl starch sodium, 12g croscarmellose sodium are sieved respectively, and it is for subsequent use; the mannitol, microcrystalline cellulose, carboxymethyl starch sodium and croscarmellose sodium obtained by sieving the first mixture are Mix evenly to obtain the second mixture, sieve it twice, then use dry granulation method to make the second mixture into granules, add 1g of magnesium stearate, mix evenly, and press into tablets to obtain.
PUM
| Property | Measurement | Unit |
|---|---|---|
| angle of repose | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com