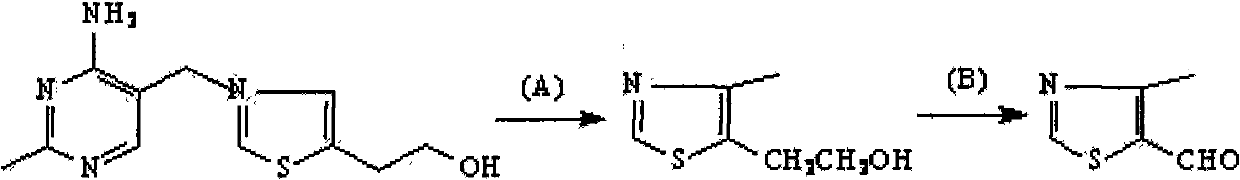Preparation method of 4-methylthiazolaldehyde-5
A technology of methylthiazole aldehyde and methylthiazole, applied in the direction of organic chemistry, can solve the problems of unsatisfactory hydrogenolysis reaction, less synthesis methods, positional isomer impurities, etc.
Inactive Publication Date: 2010-10-27
CHENGDU KAOENSI SCI & TECH
View PDF0 Cites 6 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
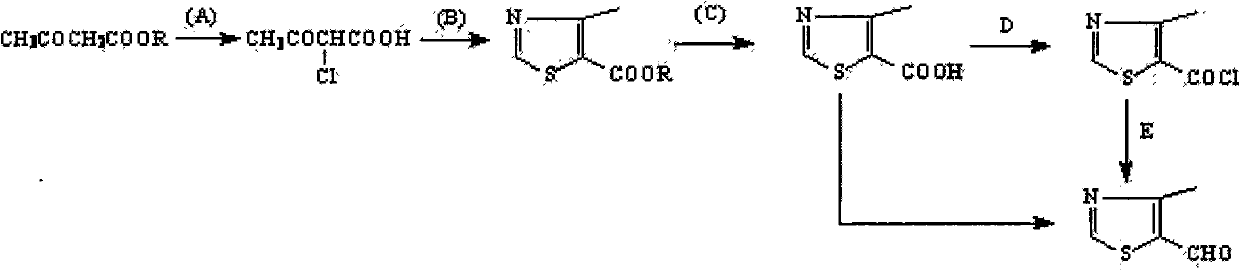
4-Methylthiazole aldehyde-5 is an important intermediate for the synthesis of cefditoren pivoxil, and there are many studies on its synthesis process, but there are very few synthetic methods for industrial production
take NH 2 As a cyclizing agent, CSH is more convenient from the perspective of cyclization, but there are positional isomer impurities, which affect the yield
If the thiazole acid is made into an acid chloride and then hydrogenated (Rosenmund Reduction) to form an aldehyde compound, but the effect of the hydrogenolysis reaction is not ideal
Make the price of the product higher than the existing cost
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
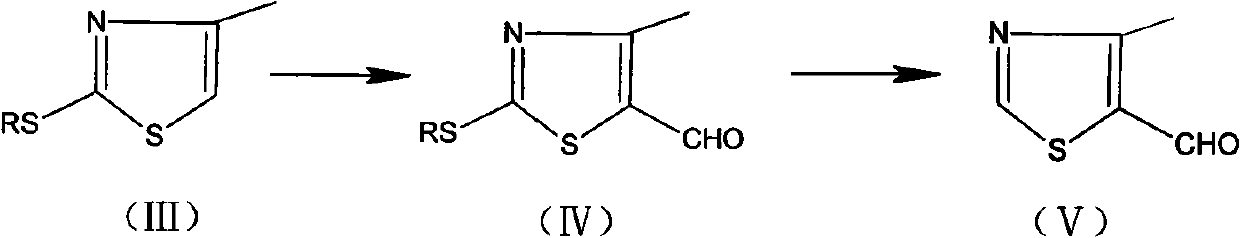
The invention relates to a preparation method of 4-methylthiazolaldehyde-5. The method comprises the following steps of: carrying out a Vilsmeier reaction to obtain 2-thiol-4-methylthiazolaldehyde-5 (IV) by using 2-thiol-4-methylthiazol (III) as a raw material; and then in the presence of a temperature not exceeding 50 DEG C, a gage pressure of 0.06-0.2 MPa and a hydrogenation catalyst which is 0.5-7.5 times of the weight of the raw material, removing thiol groups through hydrogenation reaction to obtain the 4-methylthiazolaldehyde-5 (V) as a target product. The method has the advantages of high reaction yields in all steps, good product purity, easy obtaining of chemical raw materials, no harsh environmental protection requirement and suitability for industrial scale production. The reaction flow of the method is disclosed in the specification, wherein R in the formula is a methyl group.
Description
technical field The invention relates to a new method for preparing side chain compound 4-methylthiazolein-5 used for producing cefditoren pivoxil. Background technique Cefditoren axetil is a third-generation oral cephem drug. It is a prodrug, which is hydrolyzed into an active body after oral administration, and has a broad antibacterial spectrum and strong antibacterial activity from staphylococci to Gram-negative bacteria, and is characterized by various strains producing β-lactamase Also very stable. The drug was researched and developed by Japan's Meiji Pharmaceutical Co., Ltd., and phase I clinical research began in 1987. It was approved on April 1, 1994 and is now widely used clinically. 4-Methylthiazole aldehyde-5 is an important intermediate in the synthesis of cefditoren pivoxil, and there are many studies on its synthesis technology, but there are very few synthesis methods for industrial production. The preparation method of this compound can comprise at pres...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): C07D277/24
Inventor 郑虎翁玲玲
Owner CHENGDU KAOENSI SCI & TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com