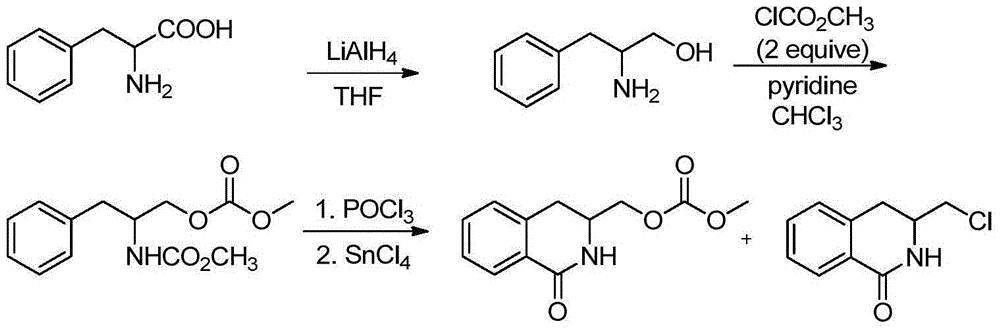
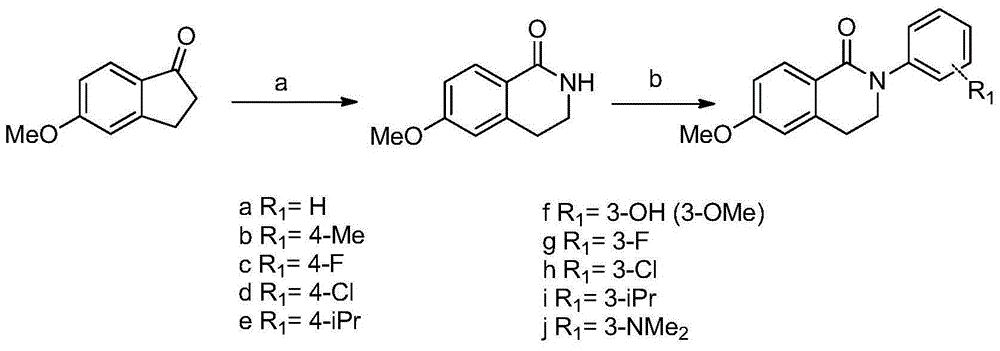
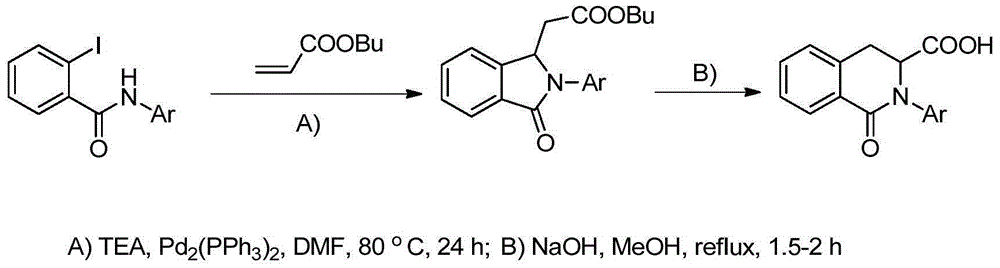
Method for synthesizing isoquinoline ketone compounds
A technology for isoquinolinones and compounds, which is applied in the field of synthesizing isoquinolinones and their derivatives, can solve the problems of complex operation, low total yield, harsh reaction conditions, etc. Effects of functional group tolerance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Taking the preparation of 2-phenyl-3,4-dihydroisoquinolin-1-one with the following structural formula as an example, the raw materials used and the preparation method are:
[0032]
[0033] Dinuclear salicylic acid copper complex 1.9mg (0.0025mmol), TBAC (tetrabutylammonium chloride) 1.4mg (0.005mmol), 2-phenyl-1,2,3,4-tetrahydroisoquinoline 52mg (0.25mmol), 3-benzyl-5-(2-hydroxyethyl)-4-methylthiazoline chloride (provided by Shanghai Aladdin Biochemical Technology Co., Ltd.) 13.4mg (0.01mmol), 1.0mL acetonitrile Put it into the Scheck tube, and the reaction solution was ventilated with oxygen for 3 to 5 times under liquid nitrogen freezing, and stirred at 30°C for 12 hours. After the reaction was completed, 10 mL of distilled water was added to the mixture, and then extracted three times with 15 mL of dichloromethane. The organic phase was collected and washed with anhydrous Na 2 SO 4 Drying, suction filtration, removal of dichloromethane with a rotary evaporator,...
Embodiment 2
[0035] In embodiment 1, the 3-benzyl-5-(2-hydroxyethyl)-4-methylthiazoline chloride used is replaced with equimolar thiamine hydrochloride (vitamin B1), other steps are the same as in embodiment 1 Likewise, 2-phenyl-3,4-dihydroisoquinolin-1-one was obtained in 90% yield.
Embodiment 3
[0037] Taking the preparation of 2-p-tolyl-3,4-dihydroisoquinolin-1-one with the following structural formula as an example, the raw materials used and the preparation method are:
[0038]
[0039] In Example 1, 2-phenyl-1,2,3,4-tetrahydroisoquinoline was used with equimolar 2-p-methylphenyl-1,2,3,4-tetrahydroisoquinoline Phenyl is replaced, and other steps are identical with embodiment 1, obtains white solid 2-p-tolyl-3,4-dihydroisoquinolin-1-one, and its productive rate is 92%, and spectral data is: 1 HNMR (400MHz, CDCl 3 )δ: 8.16(d, J=7.6Hz, 1H), 7.47(td, J=1.6Hz, J=7.2Hz, 1H), 7.37(t, J=7.2Hz, 1H), 7.28-7.21(m, 5H), 3.97(t, J=6.4Hz, 2H), 3.13(t, J=6.4Hz, 2H), 2.37(s, 3H); 13 CNMR (100MHz, CDCl 3 )δ: 164.2, 140.5, 138.2, 136.0, 131.9, 129.8, 129.5, 128.7, 127.1, 126.9, 49.5, 28.6, 21.0; IR (KBr), ν (cm -1 ): 3411, 3127, 1658, 1402, 1320, 826, 741, 619; HRMS (ESI) m / z: C 16 h 15 NNaO[M+Na] + The theoretical value is 260.1051, and the measured value is 260.1045.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com