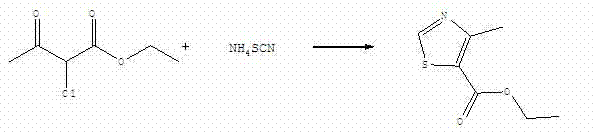Method for synthesizing ethyl 4-methylthiazole-5-formate employing single step
A technology of methylthiazole and ethyl formate, applied in the field of synthesis of cefditoren axetil intermediates, can solve the problems of many operation steps, large environmental pollution, large amount of three wastes, etc. less effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Add 164.5g (1mol) of ethyl 2-chloroacetoacetate and 87.5g (1.15mol) of ammonium thiocyanate to the reaction kettle, start stirring, raise the temperature to 160°C, and keep it warm for 5 hours at 160-165°C. After the reaction is completed Add 150 g of water, stir, filter, and air-dry naturally to obtain 158 g of light yellow ethyl 4-methylthiazole-5-carboxylate, with a yield of 92.28%.
Embodiment 2
[0024] Add 164.5g (1mol) of ethyl 2-chloroacetoacetate and 99g (1.3mol) of ammonium thiocyanate to the reaction kettle, start stirring, raise the temperature to 170°C, and keep it warm for 3 hours at 170-175°C. After the reaction is completed, add 130 g of water was stirred, filtered, and air-dried to obtain 144 g of pale yellow ethyl 4-methylthiazole-5-carboxylate, with a yield of 84.10%.
Embodiment 3
[0026] Add 164.5g (1mol) of ethyl 2-chloroacetoacetate and 80g (1.05mol) of ammonium thiocyanate to the reaction kettle, start stirring, raise the temperature to 130°C, and keep it warm for 6 hours at 130-135°C. After the reaction is completed, add 160 g of water was stirred, filtered, and air-dried to obtain 150 g of pale yellow ethyl 4-methylthiazole-5-carboxylate, with a yield of 87.61%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com