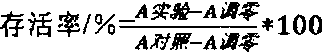Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
63results about How to "Use less solvent" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
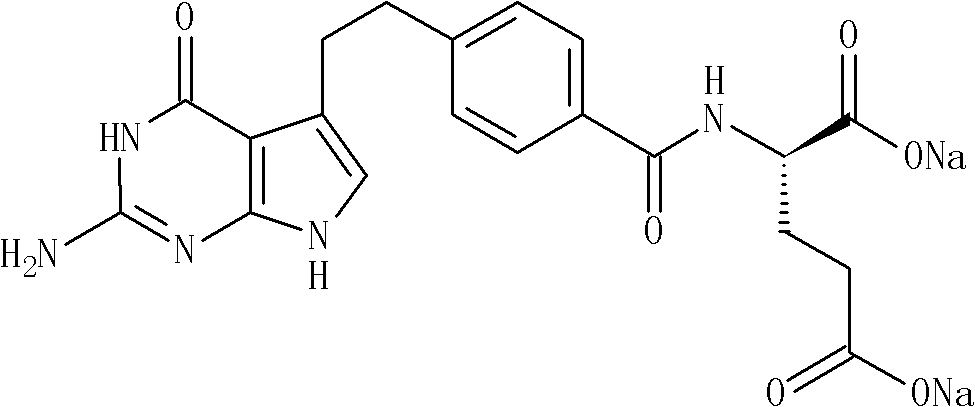
Industrialized production method of high-purity pemetrexed disodium
ActiveCN102086204AReduce Occupational InjuriesEasy to operateOrganic chemistryAcetonitrileSilica gel
The invention provides an industrialized production method of high-purity pemetrexed disodium, comprising the following steps of: (1) adding crude pemetrexed disodium into a reactor, adding water and stirring to dissolve at a temperature of 10-30 DEG C; (2) adding tetrahydrofuran or acetonitrile serving as a dissolvent into the reaction solution of the step (1), dissolving out a part of solids, adding kieselguhr or silica gel and stirring for 5-30 minutes; and (3) filtering the reaction solution of the step (2), adding dissolvent same as the dissolvent added in the step (2) into filtrate, crystallizing for 0.5-10 hours at a temperature of 10-30 DEG C, isolating solids, and drying for 0.5-10 hours at a temperature of 20-40 DEG C to obtain the high-purity pemetrexed disodium. By means of the production method, the shortcomings that in the prior art column chromatography, purification and heating are needed, the product purity is low, the operation is cumbersome and the industrialized production is difficult to realize are overcome; the production method is simple and convenient for operation, is easy to realize the industrialized production and has the advantages of few consumption of dissolvent, energy saving, environmental protection and low labor intensity; and the products have the advantages of white color, high purity, less than 0.05% of impurities in a single product and good stability.
Owner:NANJING HAIRUN PHARM CO LTD
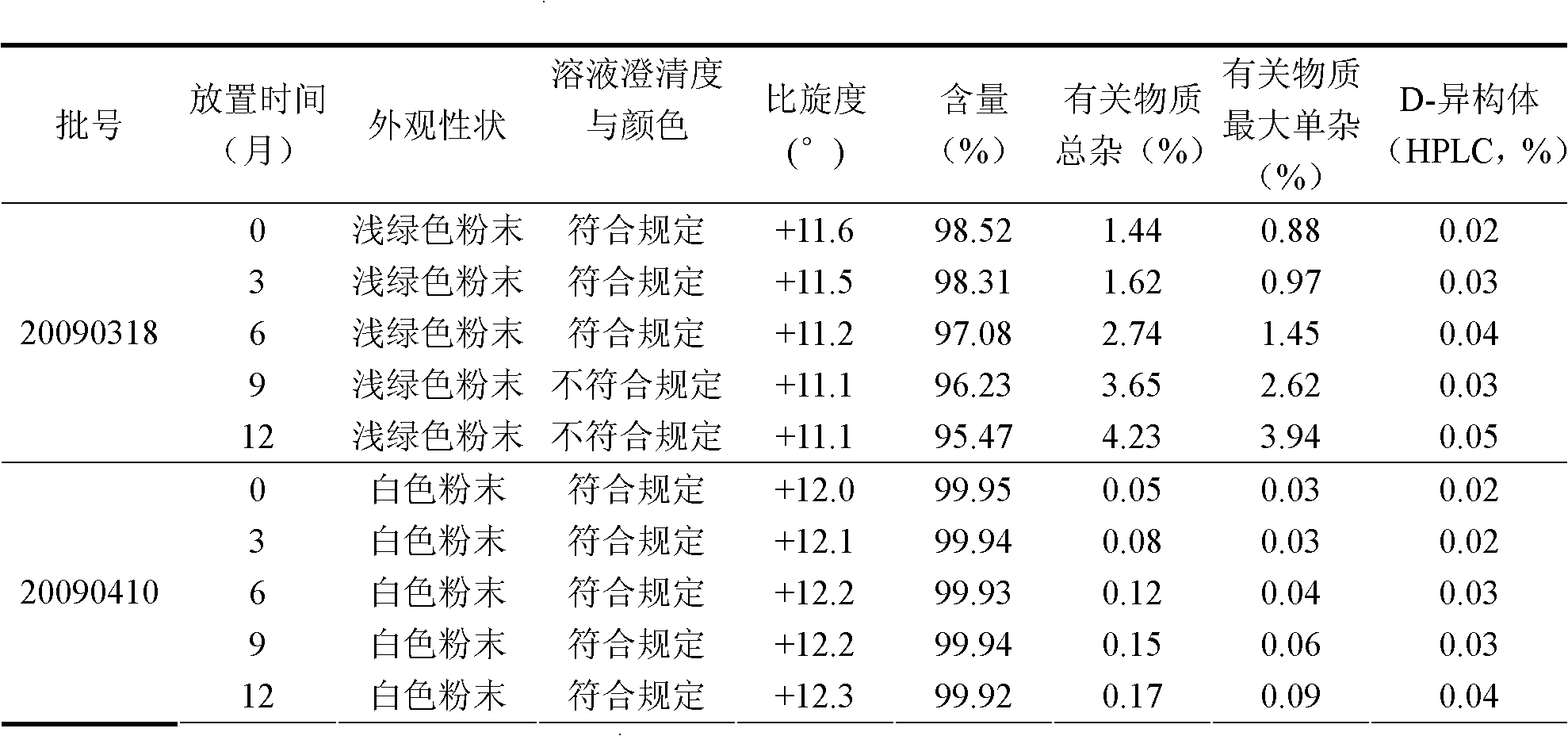
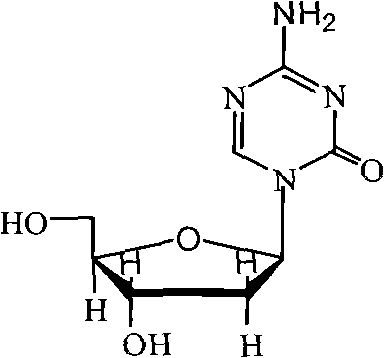
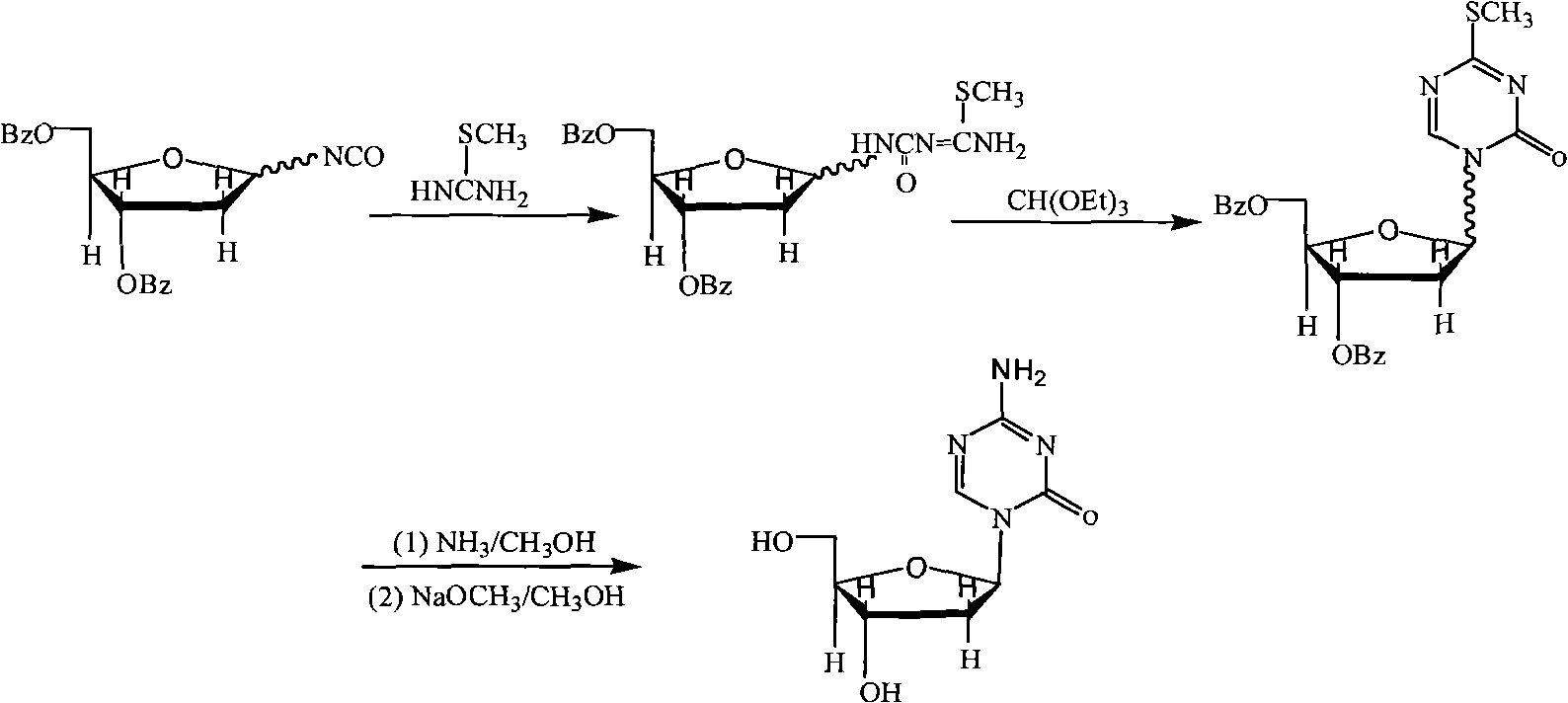
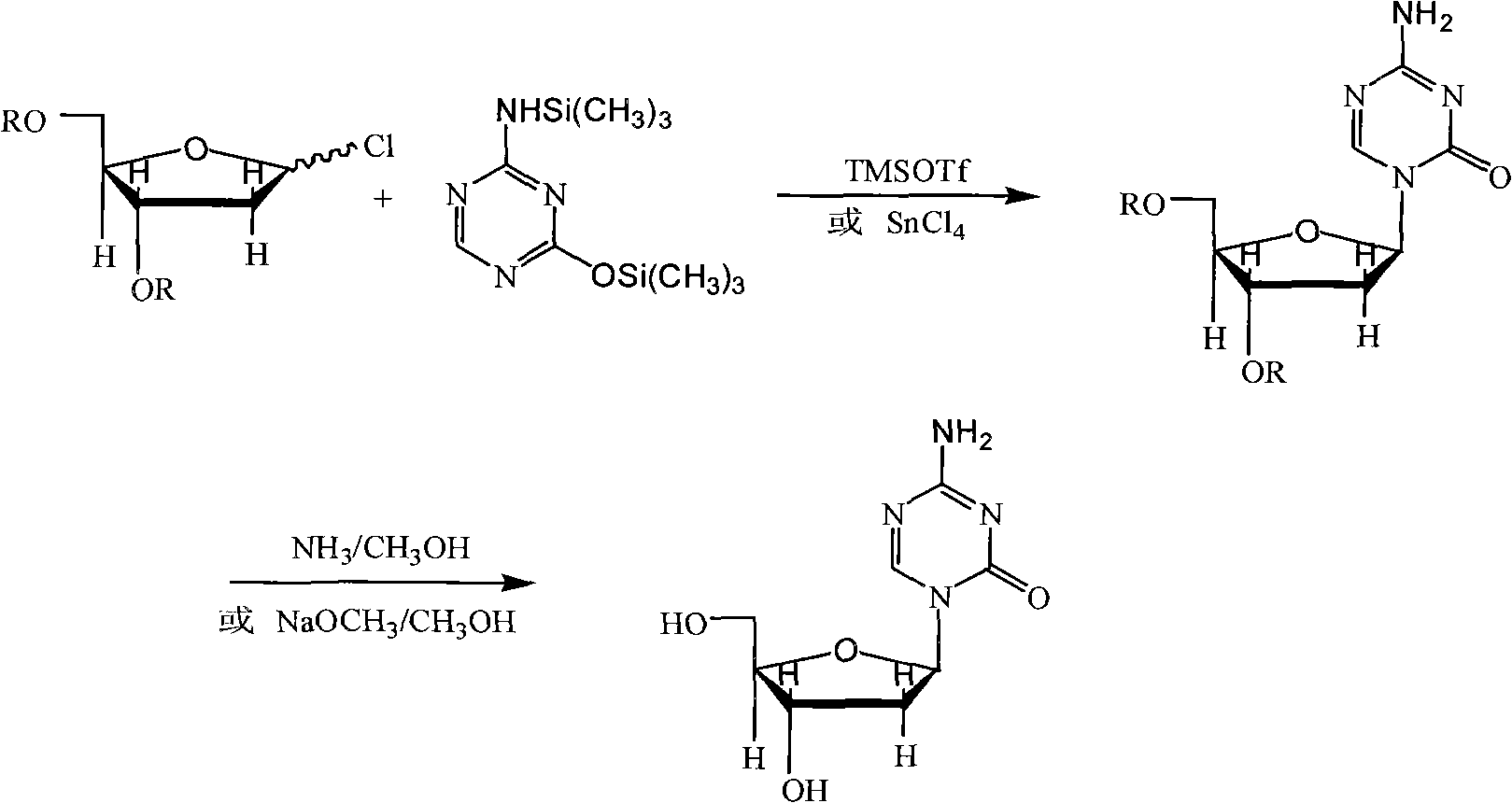
Industrialized production method for high-purity decitabine
InactiveCN101948493AReduce the impactMeet quality requirementsSugar derivativesSugar derivatives preparationSodium methoxideSolvent
The invention provides an industrialized production method for high-purity decitabine. The method comprises the following steps of: 1, performing silanization reaction of 5-azacytosine, bis(trimethylsilyl)amine and trimethyl chlorosilane, which serve as raw materials, to prepare 2,4-bis(trimethylsilyl)-5-azacytosine; 2, performing reaction of the product obtained by the step 1 and 1-chloro-3,5-bis-(4-chlorobenzoyl)-2-deoxy-D-ribofuranose, which serve as raw materials, to prepare a crude product of 1-(3,5-bis-(4-chlorobenzoyl)-2-deoxy-beta-D-ribofuranose)-5-azacytosine; 3, dissolving the product obtained by the step 2 in a C5 to C7 hydrocarbon, stirring, filtering and drying to obtain a refined product; and 4, producing the high-purity decitabine by using the product obtained by the step 3, methyl alcohol and sodium methoxide as raw materials. The method overcomes the disadvantages of need of column purification, low purity, difficult industrial production in the prior art, and has the advantages of simple and convenient operation, small solvent consumption, small influence on the environment, low labor intensity, short period, high product purity, single impurity and less than 0.1 percent total impurity content.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Method for preparing magnetic metal and alloy one dimension nanometer material
The present invention is the preparation process of one-dimensional nanometer magnetic metal and alloy material, and relates to functional information material and biomedical material. The one-dimensional nanometer magnetic metal and alloy array material is prepared through utilizing porous polycarbonate or aluminan as template, regulating the metal ion concentration in electric deposited fluid, controlling the pH value and voltage of the depositing solution, assembling metal atoms into the processed template holes to form tubular or columnar structure, dissolving the template and centrifugally washing. Controlling the concentration of metal ions in the solution and other deposition factors can obtain two-component or multi-component alloy with different atom ratio. The prepared material has hollow structure and excellent soft magnetic performance and may be used in information storing and directional medicine releasing.
Owner:BEIJING INSTITUTE OF TECHNOLOGYGY
ZIF-8@SiO2 core-shell spheres as well as preparation method and application thereof
PendingCN110346487ACoating thickness controllableRegular shapeComponent separationMicrosphereMetal-organic framework
The invention discloses ZIF-8@SiO2 core-shell spheres. The ZIF-8@SiO2 core-shell spheres are composite microspheres having a core-shell structure in which SiO2 microspheres are taken as cores while ZIF-8 is taken as shells. The invention further provides a corresponding preparation method. The preparation method comprises the following steps: modifying SiO2 microspheres serving as cores with carboxyl to obtain SiO2-COOH; and then growing a zeolitic metal organic framework ZIF-8 shell layer on the surface layer by layer with a layer-by-layer alternative growth method. As proved by experiments,the composite microspheres have the characteristics of controllable coating thickness, regular appearance and high stability. The composite microspheres can be taken as a solid extraction material forpreparing an online solid extraction column, can be combined with high performance liquid chromatography for online enrichment detection of quinolone medicaments, has high repeatability and batch reproducibility, has high enriching performance for the quinolone medicaments, has the advantages of easiness, rapidness and efficiency in operation and small solvent dosage, and has a wide practical application prospect.
Owner:GUANGXI UNIV FOR NATITIES
Water-soluble nano material supercritical carbon dioxide anti-solvent preparing device
InactiveCN101264431ACrystallize fastIncrease the areaCrystallization separationGranulation by liquid drop formationFiltration membraneHigh pressure
The invention relates to a preparation device for water-soluble nanometer material supercritical carbon dioxide anti-solvent, comprising a high-pressure pump for carbon dioxide, a high-pressure pump for solution, a nozzle, a crystallization reactor, a whole filtration membrane crystallizer, a separation reactor, and a cyclone separator, which is characterized in that: the nozzle and the whole filtration membrane crystallizer are arranged in the crystallization reactor, the aperture of the nozzle outlet is 1 to 1000um, the aperture of the whole filtration membrane crystallizer is 50 to 500nm, the cyclone separator is connected with the whole filtration membrane crystallizer arranged in the crystallization reactor, thus forming a closed recovery system for products. The preparation device has the advantages of large whole filtration membrane crystallizer area, fast crystallization, small particle size, uniform distribution, high crystallization rate of more than 80 percent, automatic collection of products without opening the cover of the crystallization reactor, ability to conversely blow the whole filtration membrane crystallizer utilizing carbon dioxide, and closely select products through the cyclone separator, thorough collection of products, rapid recovery of filter membrane flux, no pollution to environment, not harm to human body, less solvent consumption, high separation efficiency, fast speed, short cycle, low energy consumption, and easy operation.
Owner:NORTHEAST FORESTRY UNIVERSITY +1
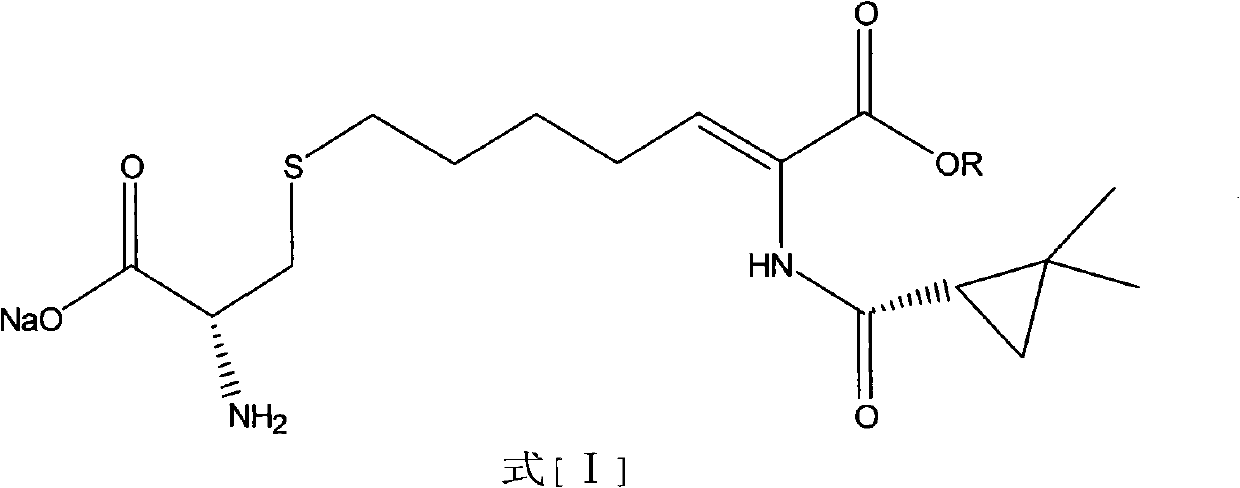
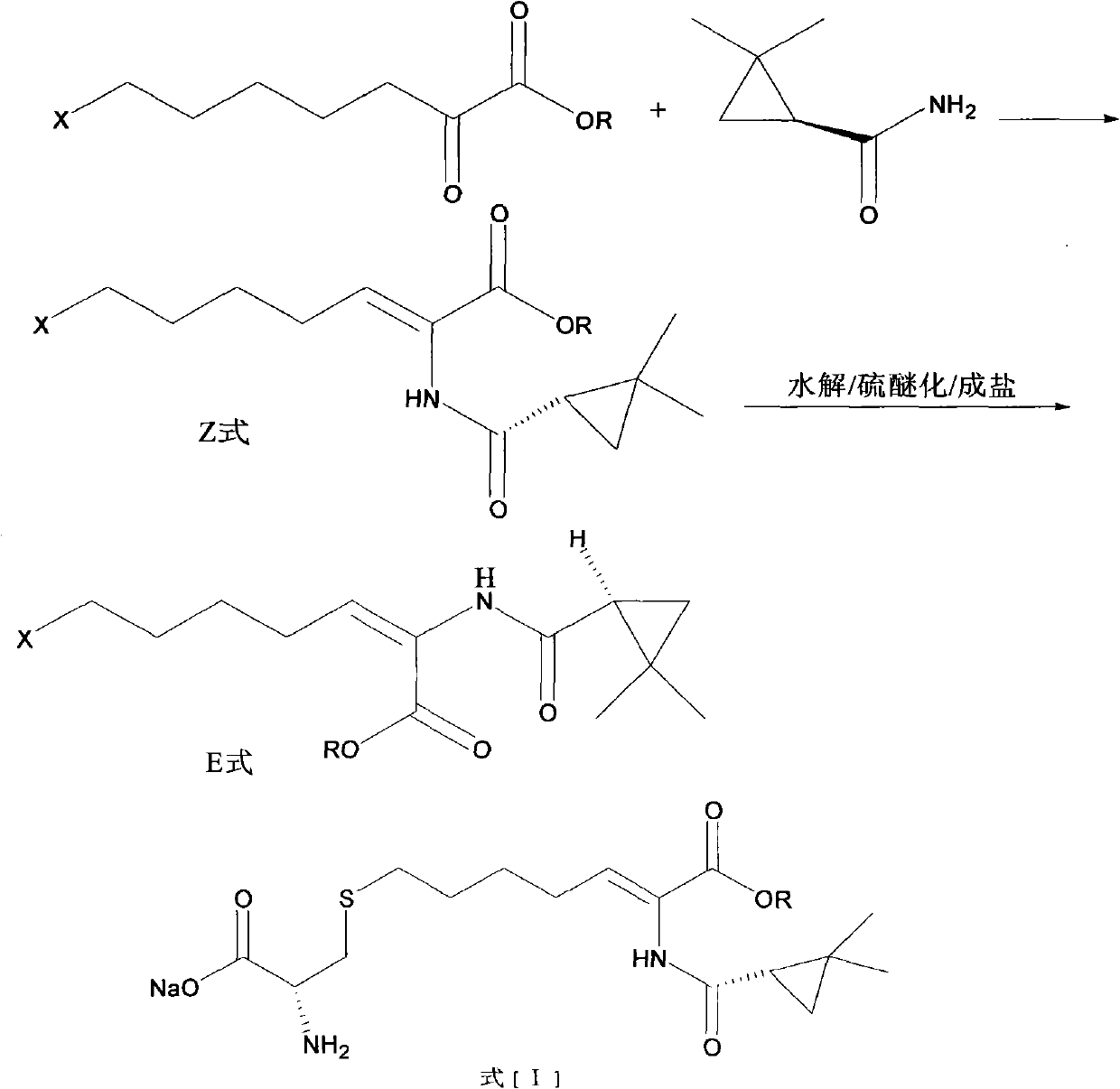
Preparation method of cilastatin sodium
ActiveCN102702051AReduce generationHigh yieldOrganic compound preparationSulfide preparationIsomerizationCilastatin sodium
The invention belongs to the field of pharmaceutical synthesis, and provides a preparation method of cilastatin sodium, and the method comprises the following steps: performing condensation, alkaline hydrolysis, and thioetherification of raw materials of 7-oxyhalogen alkyl heptylate and (s)-2,2-dimethylcyclopropane methanamide, adjusting the pH value of the thioetherified solution, washing by a nonpolar solvent, performing isomerization, purification by a neutral macroporous adsorption resin, and salt formation so as to obtain the cilastatin sodium solid. The invention reduces the generation of impurities, improves the isomerization efficiency, simplifies the preparation process, and effectively improves the yield and purity of cilastatin sodium.
Owner:SHANDONG NEWTIME PHARMA
Method for extracting O-methyl nandinine from Nandina domestica
The invention provides a method for extracting O-methyl nandinine from Nandina domestica, which mainly solves the problems of complex operation, low product content, low recovery rate and inadaptability to mass production operation in the prior art. The method comprises the following steps: 1] primary extraction; 2] secondary extraction; 3] cationic resin adsorption purification; and 4] anionic resin adsorption decolorization. The method can be utilized to obtain the O-methyl nandinine product with the purity of greater than 98% only by several steps, and is convenient to operate. The method has the advantages of high product content, high recovery rate and small solvent hazard, and is suitable for mass production operation.
Owner:SHAANXI JIAHE PHYTOCHEM
Method for synthesizing pretilachlor
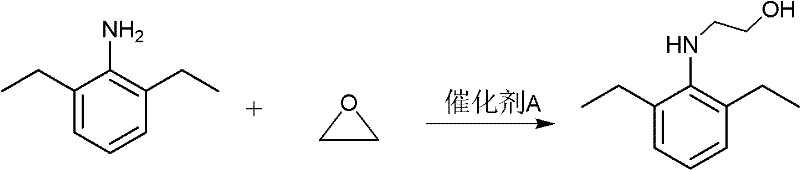
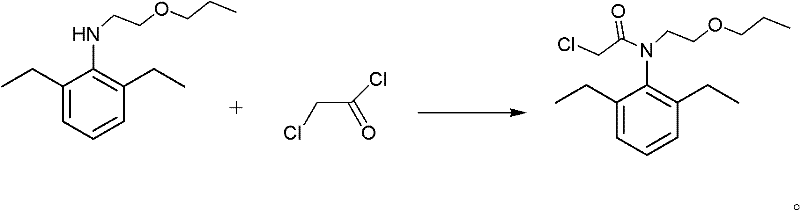
InactiveCN102229542ALow toxicityUse less solventOrganic compound preparationAmino-hyroxy compound preparationSolventEthyl phosphate
The invention discloses a method for synthesizing pretilachlor. The method for synthesizing the pretilachlor sequentially comprises the following steps of: synthesizing N-(1-methyl-2-hydroxy ethyl)-2,6-diethylaminobenzene; synthesizing 2-ethyl-6-methyl-N-(1'-methoxyl-propyl-2-group)phenylamine; and synthesizing the pretilachlor and the like. The method is simple and practical and high in efficiency; yield of products in each step is over 92 percent; in the synthetic process, the adopted reagent has low toxicity, few solvents are used and few side reactions are generated, so corrosivity to equipment is low and environmental friendliness is achieved; and the raw materials are low in price, so the production cost is reduced, economical efficiency is high, and a good application prospect is achieved.
Owner:NANTONG WEILIKE CHEM
Chromatography device with negative pressure
InactiveCN1446611AUse less solventImprove separation efficiencyIon-exchange process apparatusIon-exchanger regenerationEngineeringSolvent
A negative-pressure chromatographic equipment is composed of cylindrical main body, the quick-unlocked locking top conic closure head with negative pressure port connected to negative pressure pump via valve and vapour-liquid separator, conic top fastener for said conic top closure head, the quickly-unlocked locking bottom conic closure head with liquid inlet connected to liquid outlet of three-way value via liquid inlet tube, and conic bottom fastener for said conic bottom closure head. Its advantages are high separation efficiency and speed, less consumption of solvent and energy, and no noise and pollution.
Owner:NORTHEAST FORESTRY UNIVERSITY +1
Polyisoprene preparation method
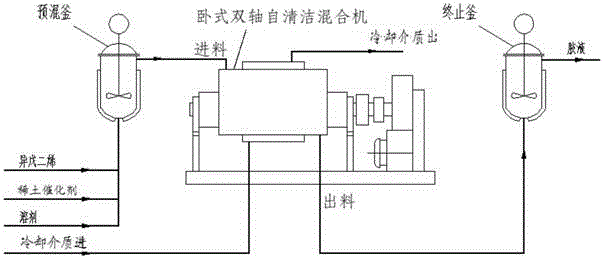
ActiveCN105085755AIncrease concentrationImprove conversion rateRotary stirring mixersChemical/physical/physico-chemical stationary reactorsRare earthEngineering
The present invention discloses a polyisoprene preparation method. According to the method, the method is performed in a single horizontal double-shaft self-cleaning mixer, the horizontal double-shaft self-cleaning mixer comprises a housing with a jacket and at least two stirring shaft in the housing and with stirring paddle blades, and the stirring shafts and the stirring paddle blades are hollow structures. The method comprises: introducing a cooling medium into the jacket and the hollow structures, conveying a reaction material containing a rare earth catalyst, a solvent and an isoprene monomer into the housing, and carrying out a solution polymerization reaction to obtain the polyisoprene. With the method of the present invention, the concentration of the isoprene monomer performing the polymerization is improved, the conversion rate of the isoprene monomer is improved, and the quality of the obtained polyisoprene product is good.
Owner:CHINA PETROLEUM & CHEM CORP +1
Method for synthesizing 5,7-dihydroxy flavone
The invention discloses a method for synthesizing 5,7-dihydroxy flavone. The method comprises the following steps of: acetylating 1,3,5-trimethoxybenzene to generate 2,4,6-trimethoxybenzene ethyl ketone; condensing the 2,4,6-trimethoxybenzene ethyl ketone and methyl benzoate to generate 1-(2'-4'-6')-trimethoxy-3-phenyl-1,3-propanedione; and performing cyclization and demethylation on the 1-(2'-4'-6')-trimethoxy-3-phenyl-1,3-propanedione to prepare the 5,7-dihydroxy flavone so as to obtain chrysin. In the method, the 1,3,5-trimethoxybenzene is directly taken as a starting raw material, a target product is obtained by performing friedel-crafts acylation reaction, ester exchange, cyclization and demethylation, and the cyclization and the demethylation are performed in one step instead of two steps. The method has the advantages of low cost, low environmental pollution, low equipment requirement and mass production.
Owner:SHAANXI JIAHE PHYTOCHEM
Method for extracting and purifying tobacco sucrose tetraester
InactiveCN102838641AHigh purityDeepen understandingEsterified saccharide compoundsSugar derivativesNicotiana tabacumSucrose
The invention discloses a method for extracting and purifying tobacco sucrose tetraester. The method comprises the following steps of: (1) screening by a 20-60-mesh sieve after smashing tobacco leaves, adding dichloromethane, enabling a mass ratio of the tobacco leaves to the dichloromethane to be 1:10-20, extracting for 20-40 minutes through ultrasonic waves, conducting liquid-liquid extraction on extraction liquid by using a methanol-water solution, enabling a mass ratio of the carbinol to water to be 4-6:1, enabling a mass ratio of the tobacco leaves to the methanol-water solution to be 1:2-3, and obtaining a sucrose tetraester compound; and (2) separating the compound by using silicagel column chromatography and semi-prepared high performance liquid chromatography to obtain the sucrose tetraester. Purity of the sucrose tetraester obtained by using the method provided by the invention is higher than 98%, and an extraction ratio after purification is 0.1-0.3%.
Owner:ZHENGZHOU UNIVERSITY OF LIGHT INDUSTRY
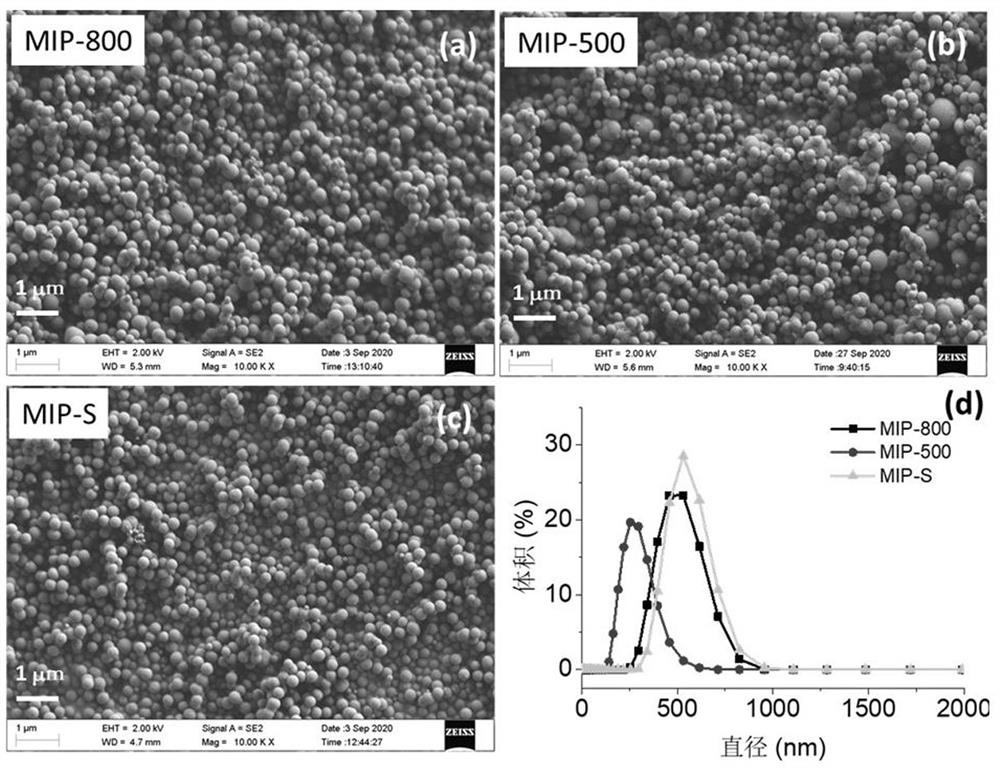
Molecularly-imprinted nanoparticle for glycoprotein and synthesis method thereof
ActiveCN112675823AUniform shape and sizeEasy to controlOther chemical processesColor/spectral properties measurementsFunctional monomerCombinatorial chemistry
The invention discloses a molecularly-imprinted nanoparticle for glycoprotein and a synthesis method thereof, belongs to the field of analytical chemistry, and relates to synthesis of molecularly-imprinted polymers. The synthesis method comprises the following steps: firstly, preparing a solution phase A from template glycoprotein molecules, preparing a solution phase B from a functional monomer and a cross-linking agent, and preparing solution phase C from an initiator; secondly, respectively fixing the solution phase A, the solution phase B and the solution phase C on three micro-fluidic injection pumps with adjustable flow rates, connecting and mixing the solution phase A and the solution phase B through a Y-shaped three-way valve, connecting and mixing the mixed solution and the solution phase C through another Y-shaped three-way valve, and enabling the obtained mixed solution and the solution phase C to flow into a reactor for a polymerization reaction; and finally, collecting a polymerized nanoparticle at the tail end of the reactor, and removing the glycoprotein template molecules to obtain the molecularly-imprinted nanoparticle for glycoprotein. The molecularly-imprinted nanoparticle for glycoprotein is prepared in a microfluidic mode, operation is easy, continuous production can be achieved, and the obtained molecularly-imprinted nanoparticle can be used for specific recognition of target glycoprotein molecules.
Owner:NANJING NORMAL UNIVERSITY
A kind of minodronic acid crystal form ii and preparation method thereof
ActiveCN102268042AEasy to prepareUse less solventOrganic active ingredientsGroup 5/15 element organic compoundsSolubilityPowder diffraction
The invention relates to the field of synthesis of medicines, in particular to a minodronate crystalform II and a preparation method thereof. The minodronate crystalform II is characterized in that an X-ray powder diffraction pattern has characteristic peaks when a reflection angle 2theta is close to 10.32, 11.16, 13.14, 15.02, 15.72, 17.44, 20.28, 20.72, 21.46, 22.30, 23.48, 25.64, 26.48, 28.80, 30.14, 32.30, 34.58, 35.50 and 36.34. The minodronate crystalform II has high solubility and dissolution rate.
Owner:广东宏远集团药业有限公司
Preparation method of amorphous dasatinib
The invention relates to a preparation method of amorphous dasatinib, wherein the preparation method includes the steps: dissolving raw materials of various crystal types of dasatinib in an anhydrous organic solvent at the temperature of 60-100 DEG C, adding a cosolvent for helping dissolution, and thus obtaining a fully-dissolved dasatinib solution; carrying out spray drying on the dasatinib solution, carrying out cyclone isolation, and rapidly precipitating to obtain a dasatinib solid powder; drying the dasatinib solid powder under reduced pressure, removing the solvent, and thus obtaining the dasatinib amorphous product. Through testing, the prepared amorphous dasatinib has the moisture content generally less than 2%, and is nearly anhydrous dasatinib. The method is suitable for converting various crystal types of dasatinib into the dasatinib amorphous product having the residual solvent consistent with Chinese pharmacopoeia, and the preparation yield reaches up to more than 99%. Moreover, the method has the advantages of simple operation, less operation steps, less using solvent, small process pollution, low cost and the like, and is suitable for large-scale industrialized production.
Owner:深圳市新浩瑞医药科技有限公司
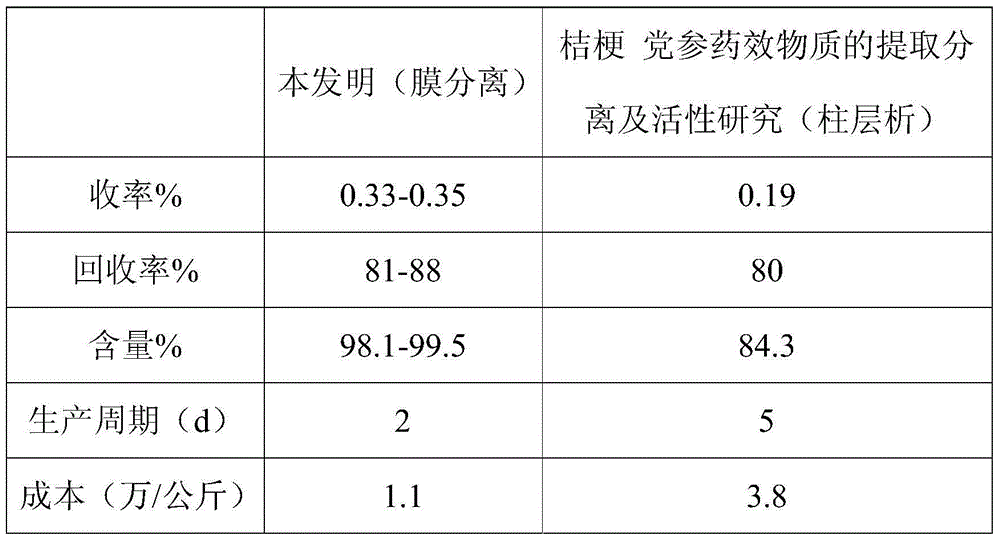
Method for extracting platycodin D from platycodon grandiflorum
The invention provides a method for extracting platycodin D from platycodon grandiflorum and aims to mainly solve the problems that the prior art is complex to operate, low in product content, low in recycle rate and unsuitable for large-scale production operation. The method for extracting platycodin D from the platycodon grandiflorum comprises the following steps: (1) extraction; (2) primary ultrafiltration; (3) secondary nanofiltration; and (4) recrystallization. The method for extracting platycodin D from the platycodon grandiflorum can be used for preparing a platycodin D product of which the purity is higher than 98% by just a plurality of steps, and is convenient to operate, high in product content, high in recycle rate, small in harms caused by solvents and suitable for large-scale production operation.
Owner:SHAANXI JIAHE PHYTOCHEM
Method for extracting polyphenolic compounds from seabuckthorn fruit
InactiveCN102908370AShort extraction timeUse less solventCosmetic preparationsToilet preparationsSolventDried fruits
The invention provides a method for extracting polyphenolic compounds from seabuckthorn fruit. According to the invention, seabuckthorn dried fruit powder or dry seabuckthorn fruit pomace are well mixed with diatomite, and the mixture is subjected to extraction in an extraction tank; filtering is carried out after extraction is finished, such that an extraction liquid is obtained; the liquid is concentrated and lyophilized, such that polyphenolic compounds in seabuckthorn fruit are obtained. The method provided by the invention has the advantages of short extraction time, low solvent dosage, and high extraction efficiency.
Owner:苏州宝泽堂医药科技有限公司
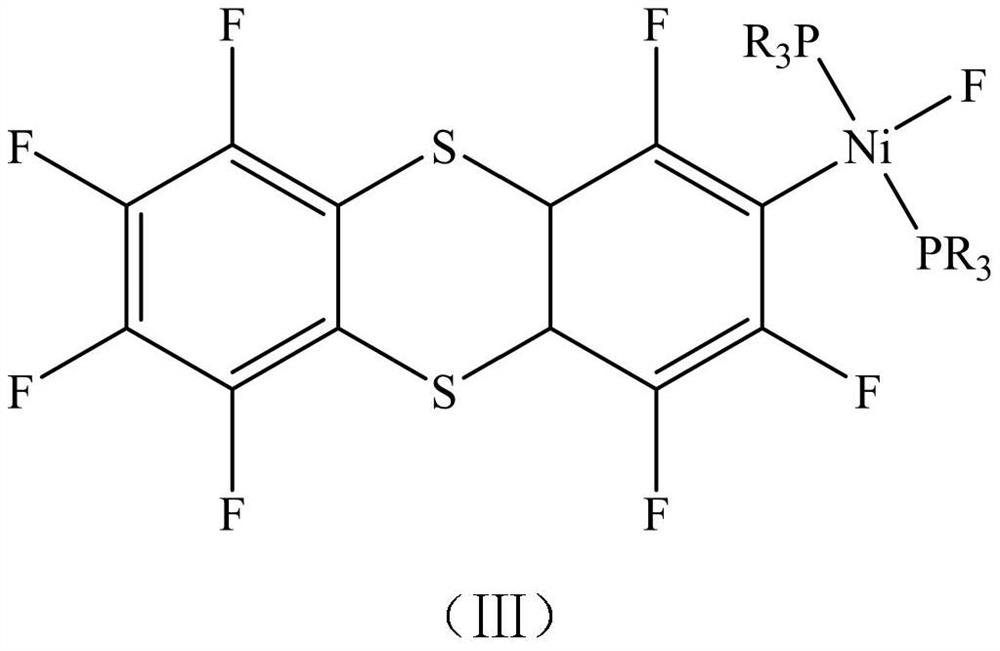
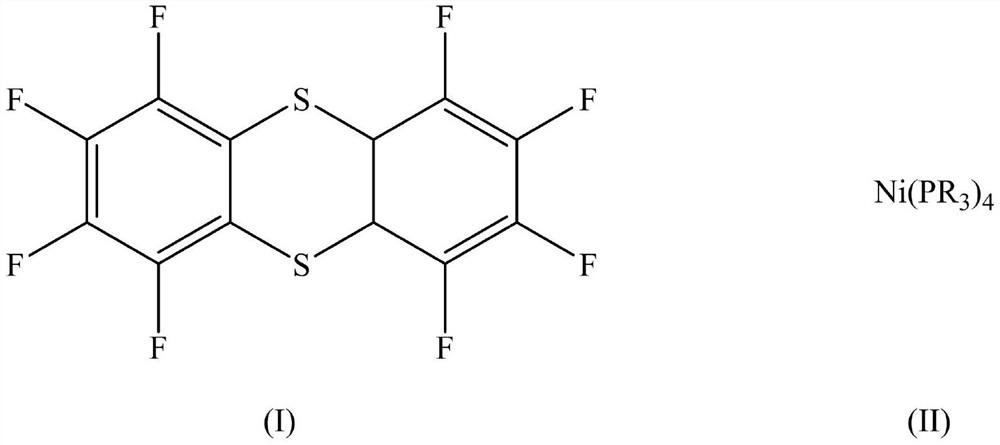
Nickel metal complex as well as preparation method and application thereof
InactiveCN112480178AHigh yieldHigh degree of polymerizationNickel organic compoundsPolymer scienceIsopropyl
The invention discloses a nickel metal complex as well as a preparation method and an application thereof. The structural formula of the nickel metal complex is shown as a formula (III), wherein R isethyl, isopropyl or cyclohexyl in the formula (III). The nickel metal complex prepared by the preparation method disclosed by the invention is applied to the process of preparing the K-type perfluoropolyether oil as a catalyst and can effectively prepare the K-type perfluoropolyether oil with high polymerization degree, improves the product yield and reduces the amount of used solvents and the like, thereby effectively promoting the reduction of the production cost of the K-type perfluoropolyether.
Owner:浙江诺亚氟化工有限公司
A method for extracting high-purity ceramide from rice bran
ActiveCN106008253BHigh purityHigh yieldCarboxylic acid amide separation/purificationThermal insulationFiltration
The invention discloses a method for extracting high-purity ceramide from rice bran. The method comprises the following steps: (1) pretreatment: cleaning the rice bran raw material, grinding and sieving; and then performing enzymolysis and filtration to obtain enzymolysis rice bran; (2) microwave countercurrent extraction: adding an organic solvent into the enzymolysis rice bran, and performing microwave countercurrent extraction and thermal-insulation filtration to obtain rice bran extract; (3) concentration: concentrating the rice bran extract and recycling the organic solvent to obtain a rice bran concentrate; (4) organic solvent extraction and separation: stirring and extracting the rice bran concentrate with the organic solvent, and performing vacuum concentration to obtain a tarry lipid mixture; (5) performing silica gel chromatography adsorption separation, eluting the organic solvent and collecting the ceramide target fraction; and (6) concentrating and drying to obtain a ceramide product. The method disclosed by the invention has the advantages of simple technology and low energy consumption and cost and is suitable for industrial continuous production; and the purity of the obtained ceramide product is greater than or equal to 99%, and the yield is greater than or equal to 0.075%.
Owner:HUNAN HUACHENG BIOTECH
A kind of method for preparing polyisoprene
ActiveCN105085755BIncrease concentrationImprove conversion rateRotary stirring mixersChemical/physical/physico-chemical stationary reactorsPolymer scienceRare earth
The present invention discloses a process for preparing polyisoprene carried out in a single horizontal twin-shaft self-cleaning mixer comprising a jacketed housing and a At least two stirring shafts with stirring blades in the housing, the stirring shafts and the stirring blades are hollow structures; the method includes: passing a cooling medium into the jacket and the hollow structure, and The reaction material containing the rare earth catalyst, the solvent and the isoprene monomer is fed into the shell for solution polymerization to obtain polyisoprene. The method of the invention improves the concentration of the isoprene monomer to be polymerized and the conversion rate of the isoprene monomer, and the quality of the obtained polyisoprene product is better.
Owner:CHINA PETROLEUM & CHEM CORP +1
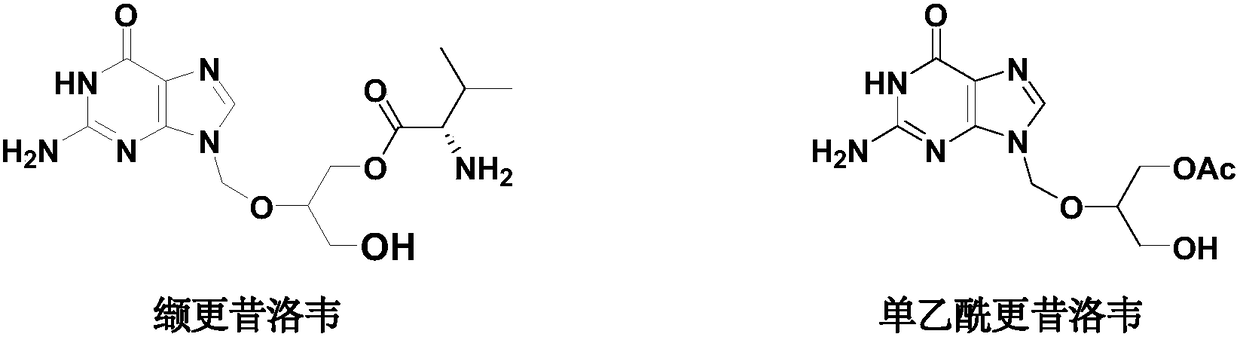
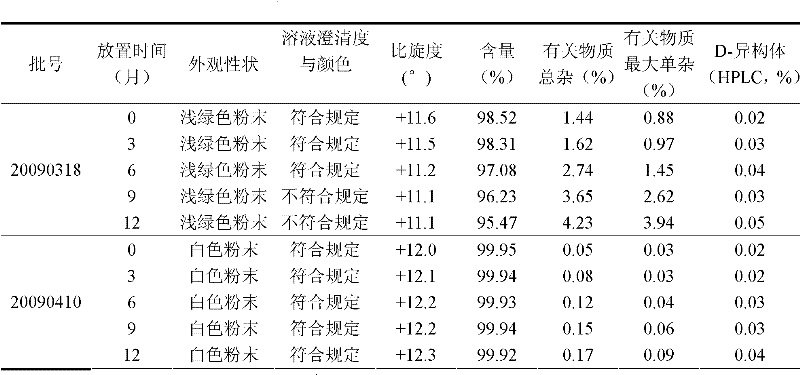
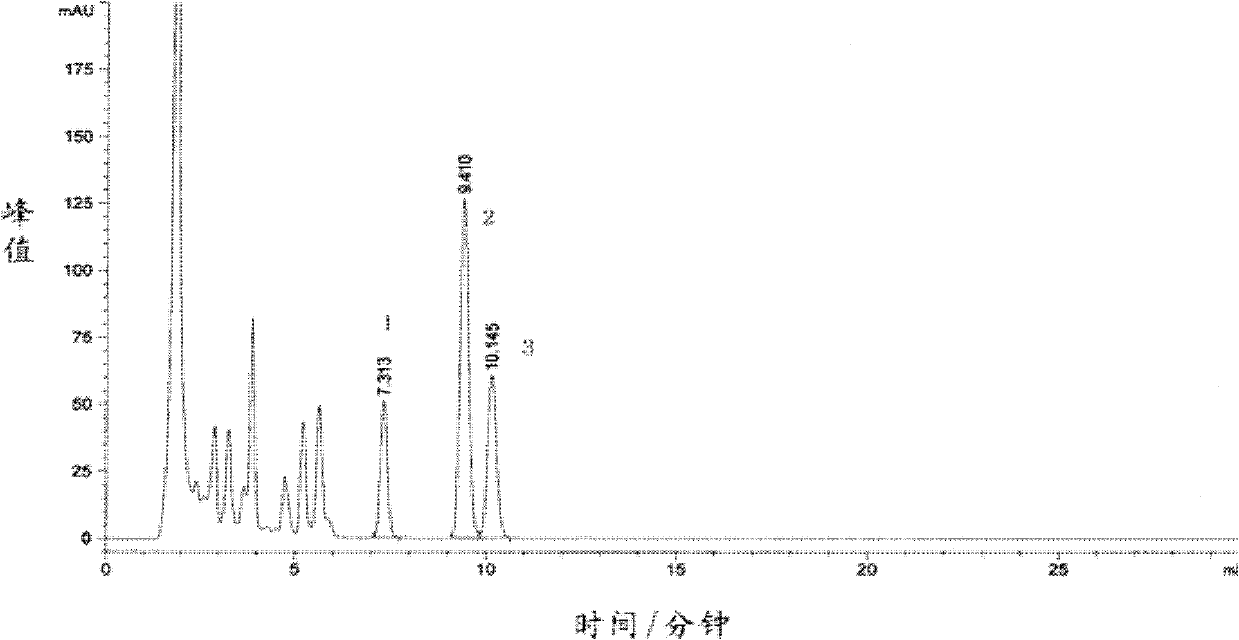
Preparation method of high-purity monoacetyl ganciclovir
The invention discloses a preparation method of high-purity monoacetyl ganciclovir. The method comprises the steps of taking ganciclovir as a raw material; protecting an alcoholic hydroxyl group through sulfite; then adding an acetylation agent to perform selective acetylation, thus obtaining the monoacetyl ganciclovir. The method is high in raw material conversion rate, and high in acetylation selectivity; the content of the obtained monoacetyl ganciclovir reaches 99% or higher, thus the synthesizing quality of following valganciclovir products can be improved; the production efficiency is high; the method is suitable for massive industrial production.
Owner:ANHUI HAIKANG PHARMA
Industrialized production method of high-purity pemetrexed disodium
ActiveCN102086204BReduce Occupational InjuriesEasy to operateOrganic chemistryState of artAcetonitrile
The invention provides an industrialized production method of high-purity pemetrexed disodium, comprising the following steps of: (1) adding crude pemetrexed disodium into a reactor, adding water and stirring to dissolve at a temperature of 10-30 DEG C; (2) adding tetrahydrofuran or acetonitrile serving as a dissolvent into the reaction solution of the step (1), dissolving out a part of solids, adding kieselguhr or silica gel and stirring for 5-30 minutes; and (3) filtering the reaction solution of the step (2), adding dissolvent same as the dissolvent added in the step (2) into filtrate, crystallizing for 0.5-10 hours at a temperature of 10-30 DEG C, isolating solids, and drying for 0.5-10 hours at a temperature of 20-40 DEG C to obtain the high-purity pemetrexed disodium. By means of the production method, the shortcomings that in the prior art column chromatography, purification and heating are needed, the product purity is low, the operation is cumbersome and the industrialized production is difficult to realize are overcome; the production method is simple and convenient for operation, is easy to realize the industrialized production and has the advantages of few consumptionof dissolvent, energy saving, environmental protection and low labor intensity; and the products have the advantages of white color, high purity, less than 0.05% of impurities in a single product andgood stability.
Owner:NANJING HAIRUN PHARM CO LTD
Label ink capable of continuously resisting yellowing at a temperature of 300 DEG C and used for PI substrates
The invention discloses label ink capable of continuously resisting yellowing at a temperature of 300 DEG C and used for PI substrates, wherein the label ink has excellent adhesion and excellent high-temperature yellowing resistance, solves the problem of low adhesion of the existing PI label ink, particularly solves the problem of poor high-temperature yellowing resistance of the existing label ink, and particularly can increase the carbon tape printing line width from 0.08 mm to 0.03 mm.
Owner:SUZHOU BETELY POLYMER MATERIALS CO LTD
Detection method of genotoxic impurity diisopropyl sulfate in medicine
ActiveCN108693286AEliminate distractionsImprove sensitivity and accuracyComponent separationMicrogramDrug
The invention discloses a detection method of a genotoxic impurity diisopropyl sulfate in a medicine. The detection method comprises dissolving a thymol drug raw material, carrying out extraction, carrying out centrifugal separation at a high speed to obtain a supernatant, and analyzing the supernatant through a triple tandem quadrupole high-performance liquid chromatography-mass spectrometer to detect the content of the genotoxic impurity diisopropyl sulfate in the thymol drug raw material. According to the detection method, a diisopropyl sulfate recovery rate is in a range of 90-110%, a standard deviation (RSD) is 2.33%, a detection limit is 1.74 micrograms / kilogram and a quantitation limit is 5.81 micrograms / kilogram. The method has a low detection limit, a high recovery rate, high sensitivity and good repeatability and can be used for detection of genotoxic impurity diisopropyl sulfate in pharmaceutical raw materials including drugs difficult to gasify.
Owner:中科广化(重庆)新材料研究院有限公司 +2
Preparation method of fine line ribbon printing label ink resistant to yellowing at 300 DEG C continuously and used for PI (polyimide) base material
InactiveCN110079154ASolve low adhesionSolve the problem of poor high temperature yellowing performanceInksPolyesterFine line
The invention discloses a preparation method of label ink resistant to yellowing at 300 DEG C continuously and used for a PI (polyimide) base material. The preparation method comprises following steps: (1), resin, a dispersing agent, a dispersing solvent, a modifier and a stabilizer are stirred uniformly at a low speed, pigment and filler are added in sequence, the components are stirred uniformlyat a high speed, and a dispersed material is obtained, wherein the resin is saturated polyester and the filler is inorganic micro-powder; (2), the dispersed material is ground, and a main agent is obtained; (3), the main agent is mixed with a curing agent and a diluent, and the label ink resistant to yellowing at 300 DEG C continuously and used for the PI base material is obtained. Multiple problems about adhesive force, ribbon printing performance, yellowing resistance at 300 DEG C for 60 min and the like are solved while the cost is greatly reduced, the unexpected technical effect is realized, and the label ink can perfectly replace the same foreign imported products.
Owner:SUZHOU BETELY POLYMER MATERIALS CO LTD
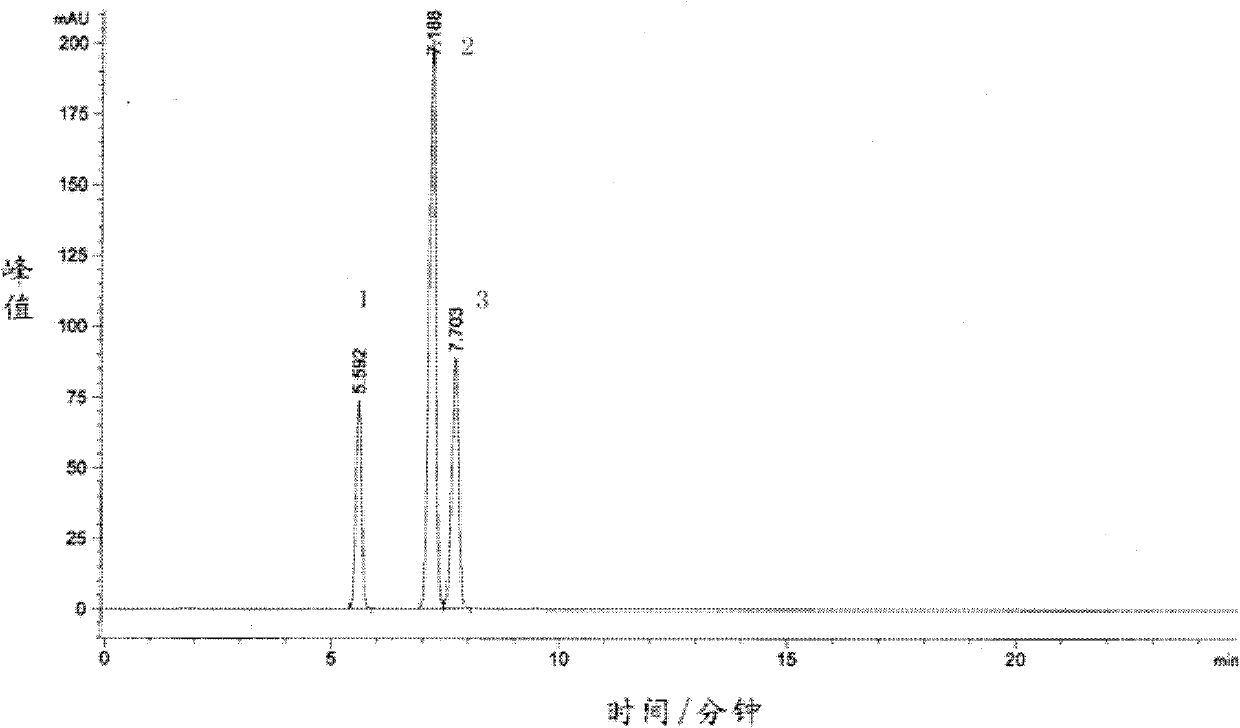
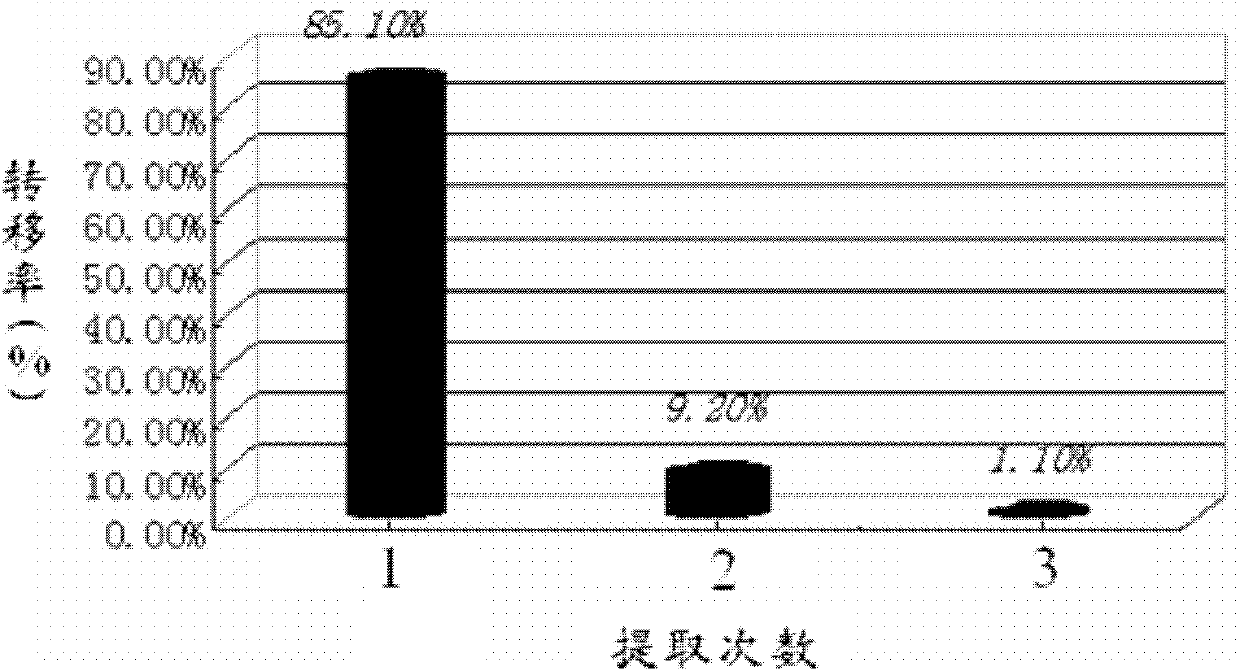
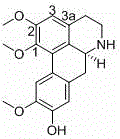
Traditional Chinese medicine venenum bufonis extract and preparation method thereof
ActiveCN102512453BImprove extraction efficiencyExcellent transfer rateAmphibian material medical ingredientsAntineoplastic agentsMedicinal herbsGradient elution
Owner:北京协和制药二厂有限公司
Preparation method for alkaloids by performing extraction on lindera glauca root and uses thereof
InactiveCN105481770AReduce dosageIncrease concentrationOrganic active ingredientsOrganic chemistryHigh concentrationAlcohol
The invention discloses a preparation method for alkaloids by performing extraction on lindera glauca root and uses thereof. The preparation method comprises the following steps: 1) processing raw materials; 2) performing percolating extraction by using an acidic alcohol aqueous solution; 3) preparing a lindera-glauca-root total alkaloids extractive concentrate; 4) preparing an eluent; and 5)-8) preparing and purifying the compounds. By employing the acidic alcohol aqueous solution as the extraction solvent, the extraction solvent can be repeated recovered for utilization, and cost is saved. Percolating extraction is employed, the leachate can reach a relatively high concentration, and extraction efficiency can be improved. The method employs normal-temperature operation, does not need heating, the solvent usage amount is less, the filtration requirement is relatively low, the separation operation process is simplified, the extractive is less in impurities, extraction efficiency is high, and the solvent usage amount is less.
Owner:HUNAN UNIV OF SCI & ENG
A kind of preparation method and application of extracting alkaloid from mountain pepper root
InactiveCN105481770BReduce dosageIncrease concentrationOrganic active ingredientsOrganic chemistryHigh concentrationAlcohol
The invention discloses a preparation method for alkaloids by performing extraction on lindera glauca root and uses thereof. The preparation method comprises the following steps: 1) processing raw materials; 2) performing percolating extraction by using an acidic alcohol aqueous solution; 3) preparing a lindera-glauca-root total alkaloids extractive concentrate; 4) preparing an eluent; and 5)-8) preparing and purifying the compounds. By employing the acidic alcohol aqueous solution as the extraction solvent, the extraction solvent can be repeated recovered for utilization, and cost is saved. Percolating extraction is employed, the leachate can reach a relatively high concentration, and extraction efficiency can be improved. The method employs normal-temperature operation, does not need heating, the solvent usage amount is less, the filtration requirement is relatively low, the separation operation process is simplified, the extractive is less in impurities, extraction efficiency is high, and the solvent usage amount is less.
Owner:HUNAN UNIV OF SCI & ENG
A method for fast separation of phthalates by supercritical chromatography
InactiveCN105548394BFast wayGood reproducibilityComponent separationEnvironmental resistanceAdjuvant
The invention discloses a method for supercritical fluid chromatography-based fast separation of phthalate and belongs to the technical field of separation. The fast, saving and eco-friendly method provided through the invention solves the problem that the existing fluid chromatography or gas chromatography method for separating a phthalate plasticizer consumes long time and a large amount of materials. The method utilizes a supercritical fluid chromatography method to separate a desired substance, utilizes a CO<2+> adjuvant as a mobile phase, realizes separation of more than ten kinds of phthalate plasticizers in several minutes, is fast and eco-friendly, is poisonless and harmless for an experimenter and has good reappearance. Through combination of the method and UV, FID and MS detecting instruments, fast separation and detection of phthalate plasticizers are realized.
Owner:CHONGQING UNIV
Method for detecting related substances of palonosetron hydrochloride injection
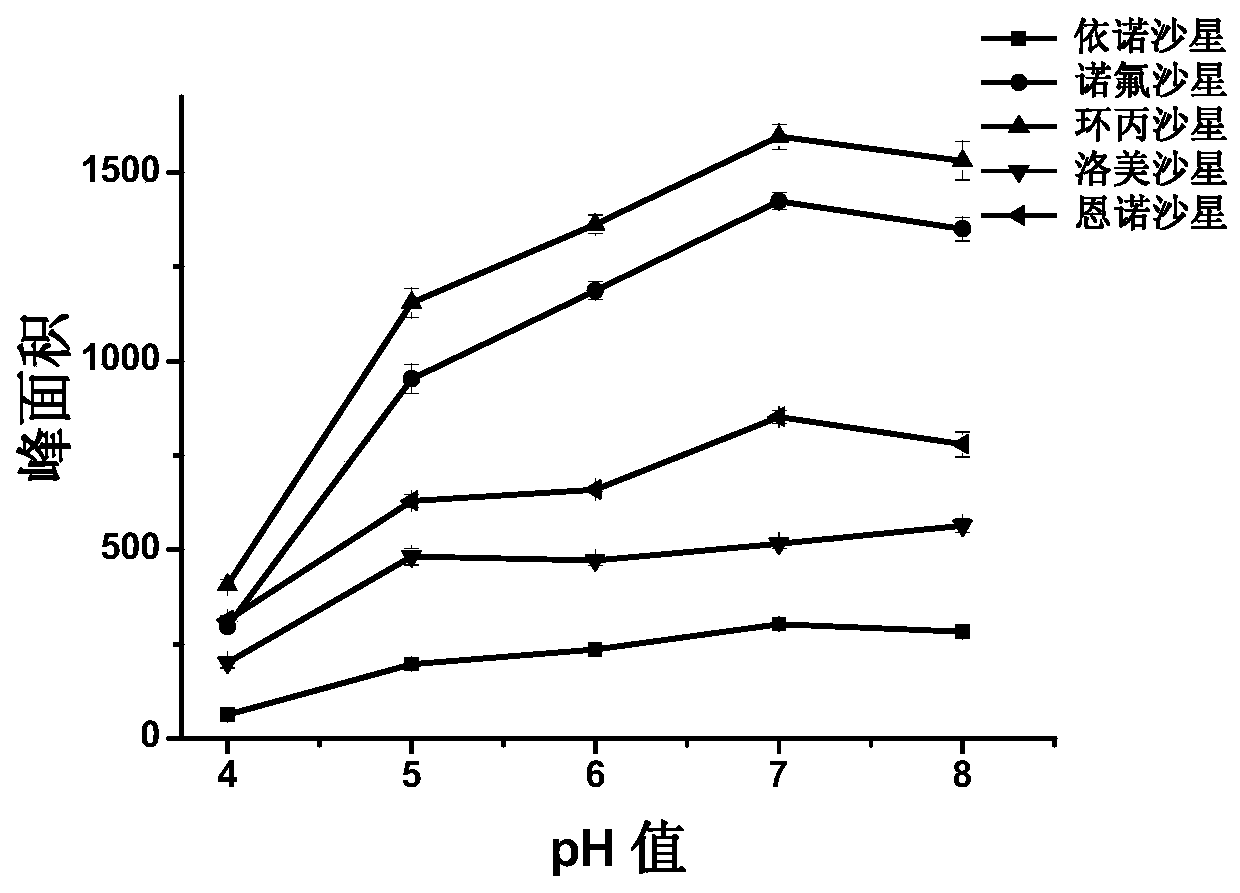
PendingCN110146601ALess solventHuman and environment friendlyComponent separationChromatographic columnChemistry
The invention provides a method for detecting related substances of palonosetron hydrochloride injection, which comprises the steps of enriching the palonosetron hydrochloride injection by adopting asolid phase extraction cartridge, eluting, and performing liquid chromatography analysis under the conditions that: chromatographic column: the filler is bonded vancomycin; detection wavelength: 235 to 245 nm; column temperature: 30-40 DEG C; flow rate: 1.4-1.6 mL / min; mobile phase: ammonium acetate solution and tetrahydrofuran mixed solution. The related substances of the palonosetron hydrochloride enriched by the method can be concentrated by more than 10 times, the influence of auxiliary materials can be removed at the same time, one-time extraction and detection of fat-soluble and water-soluble impurities in a sample can be realized, the related substances can be accurately detected, therefore, the method is high in sensitivity, simple and convenient to operate and accurate in result.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com