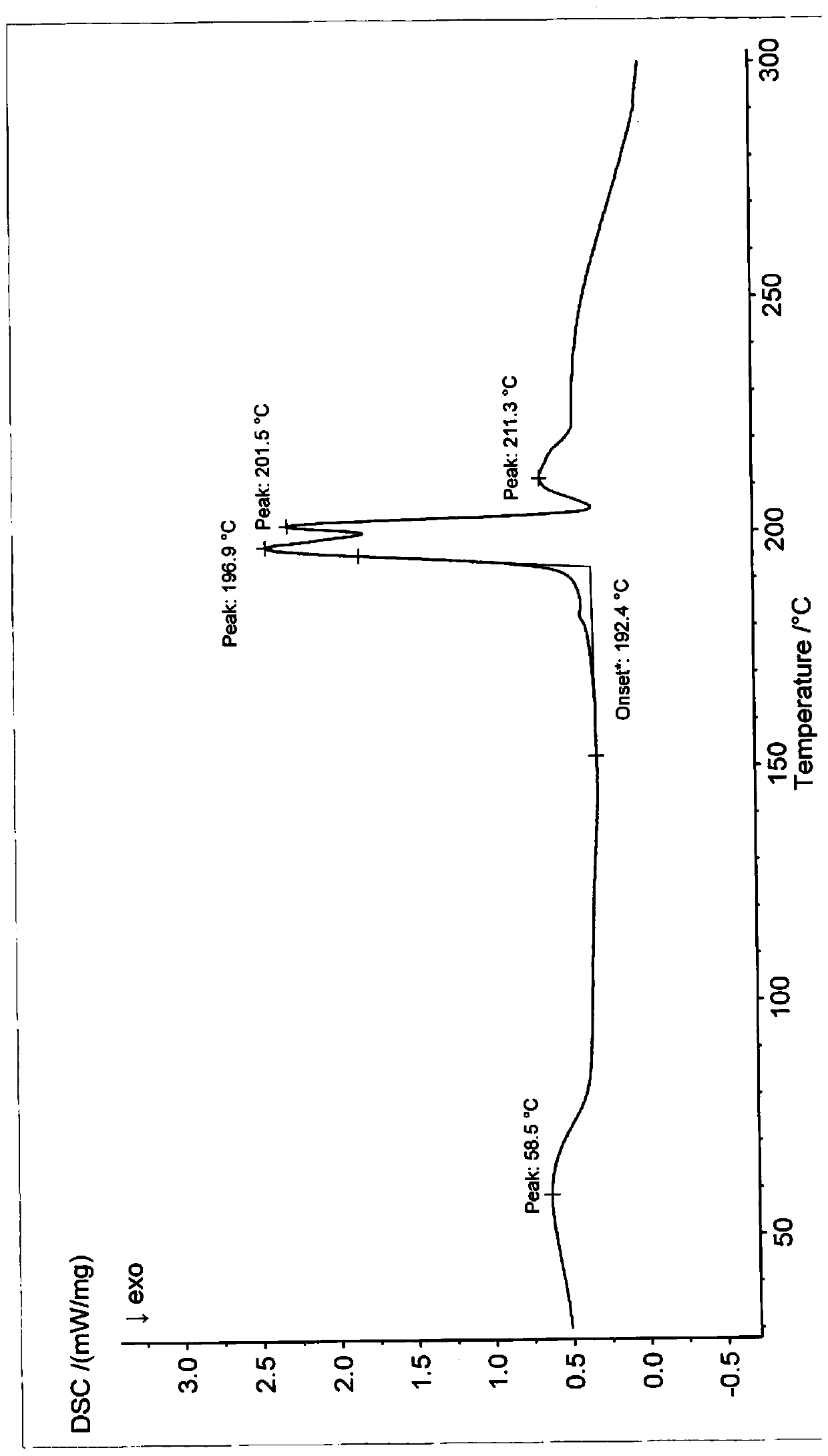
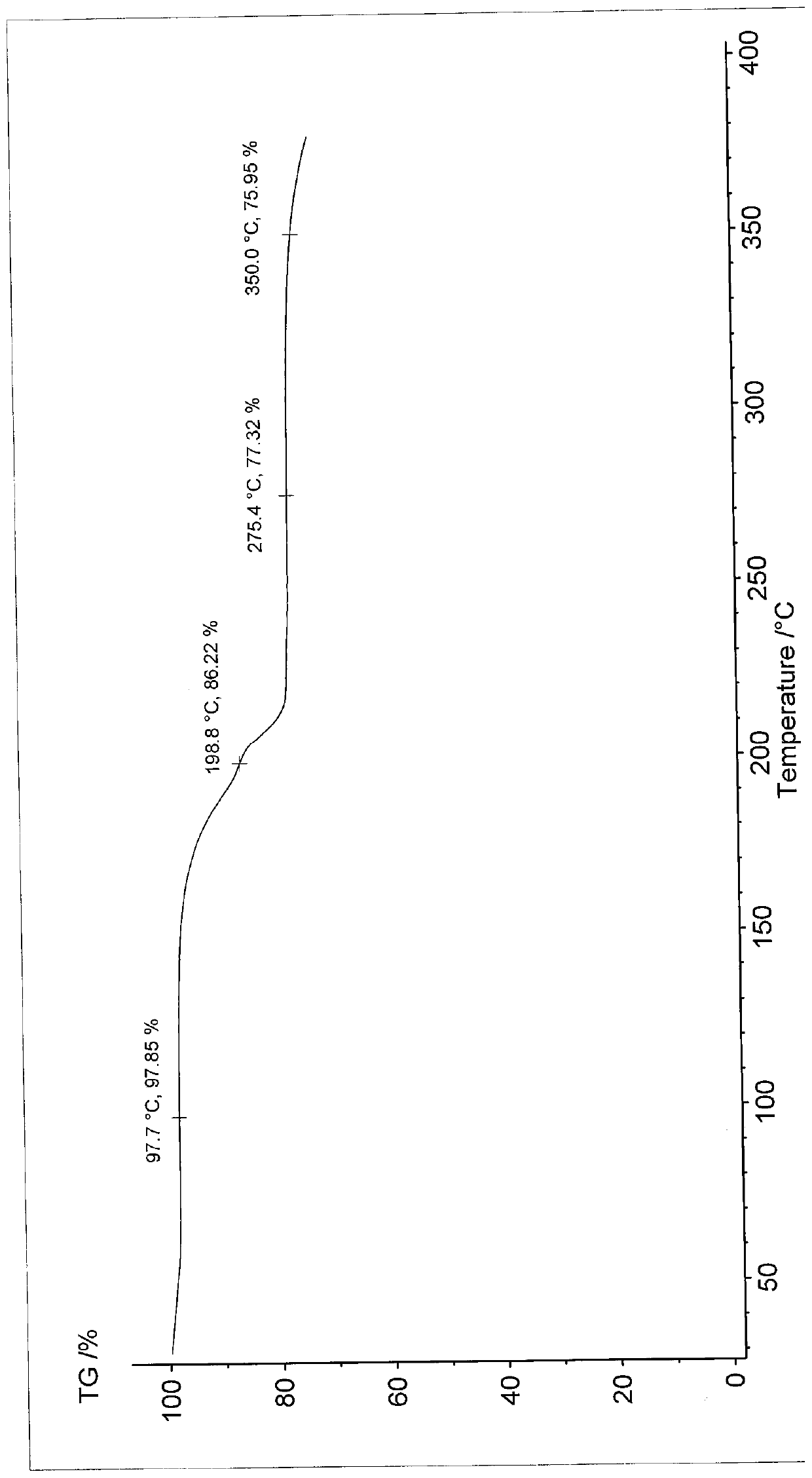
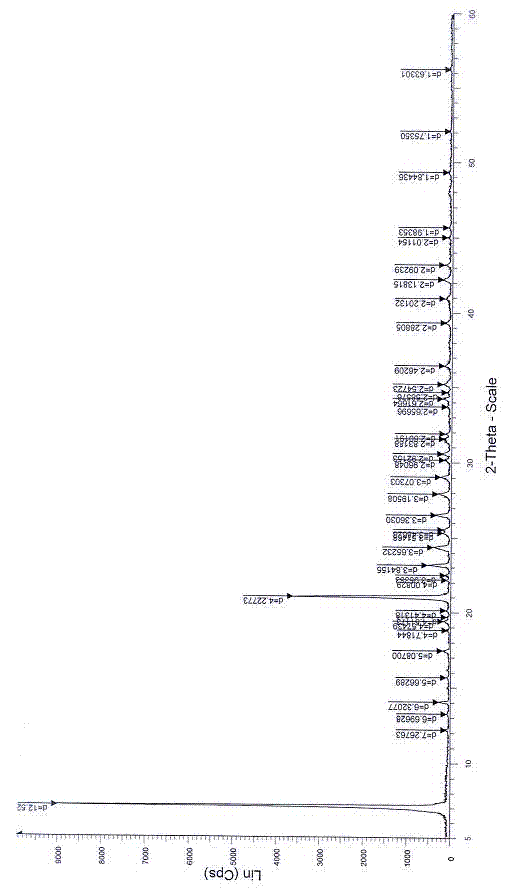
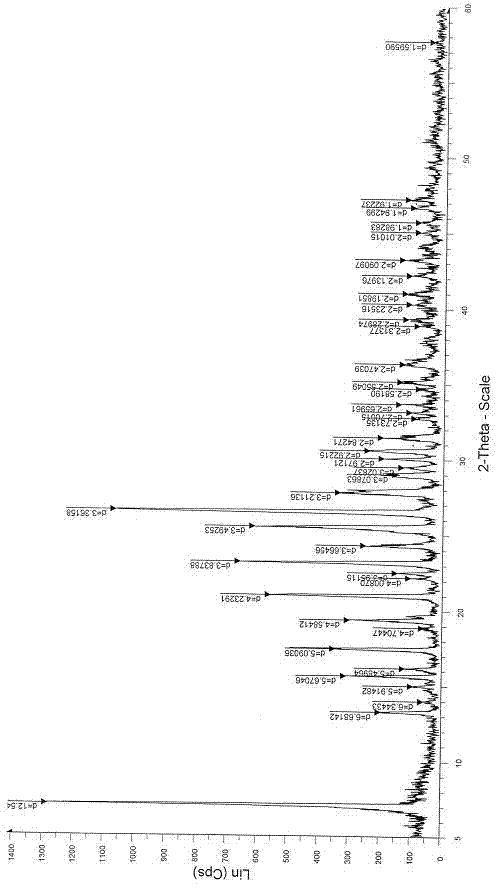
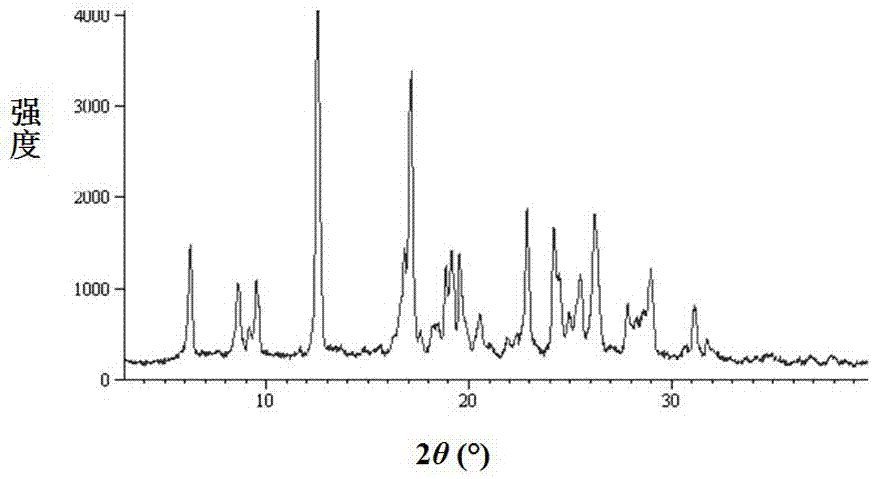
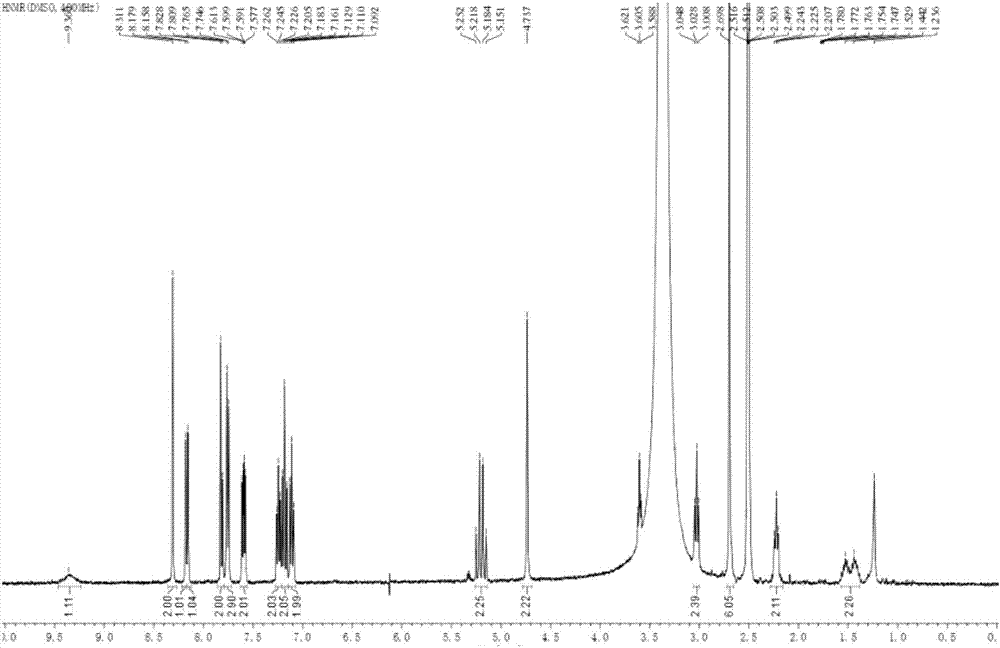
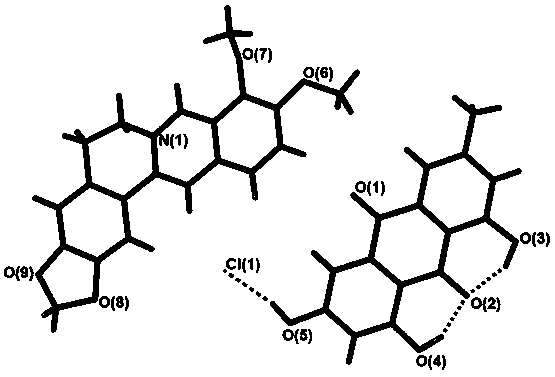
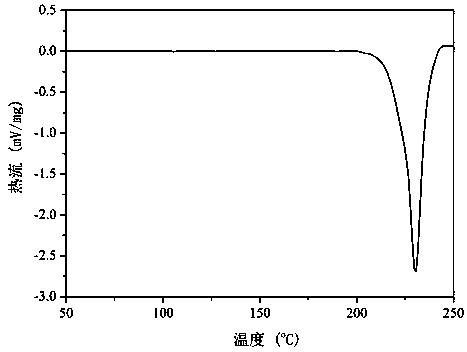
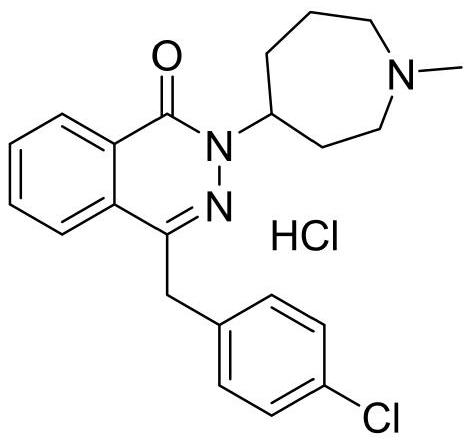
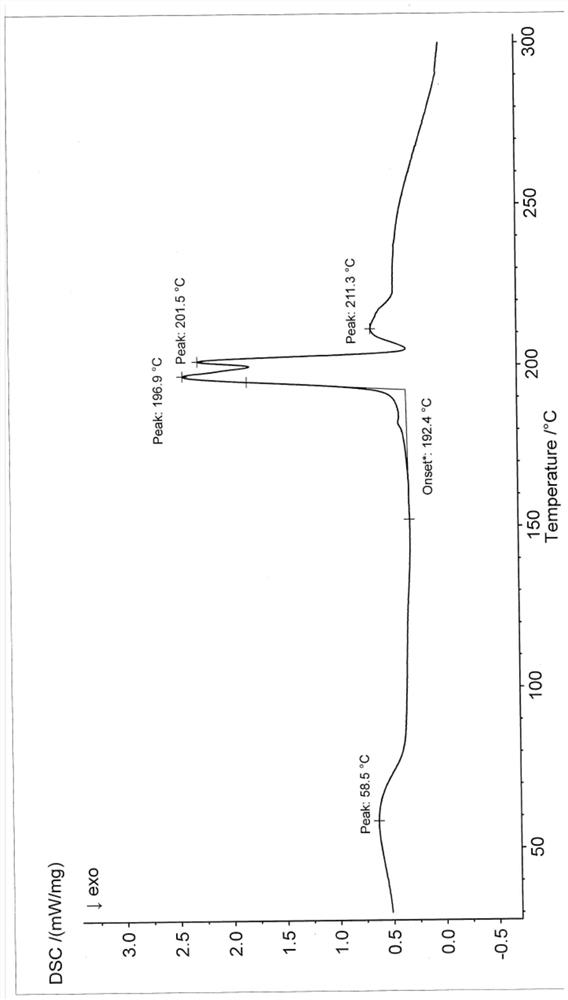
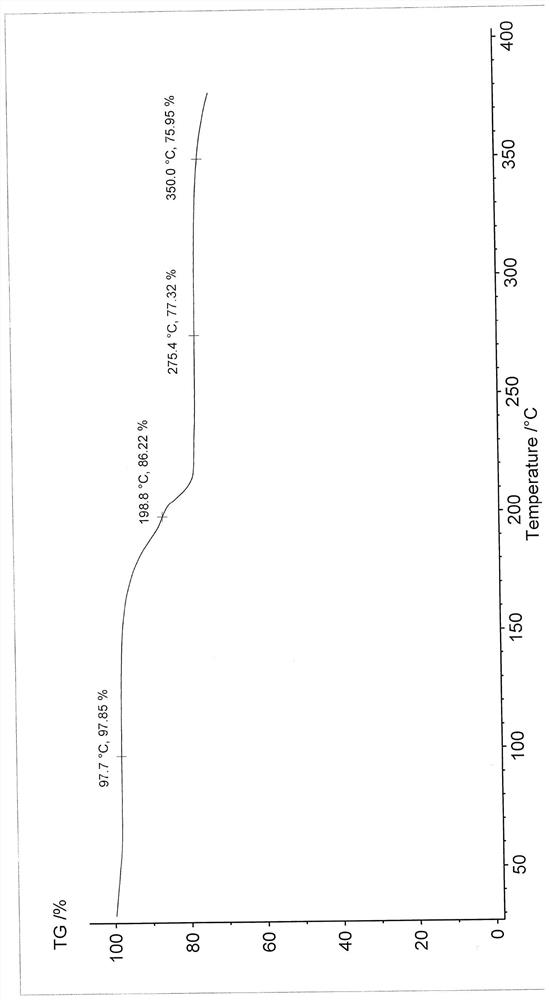
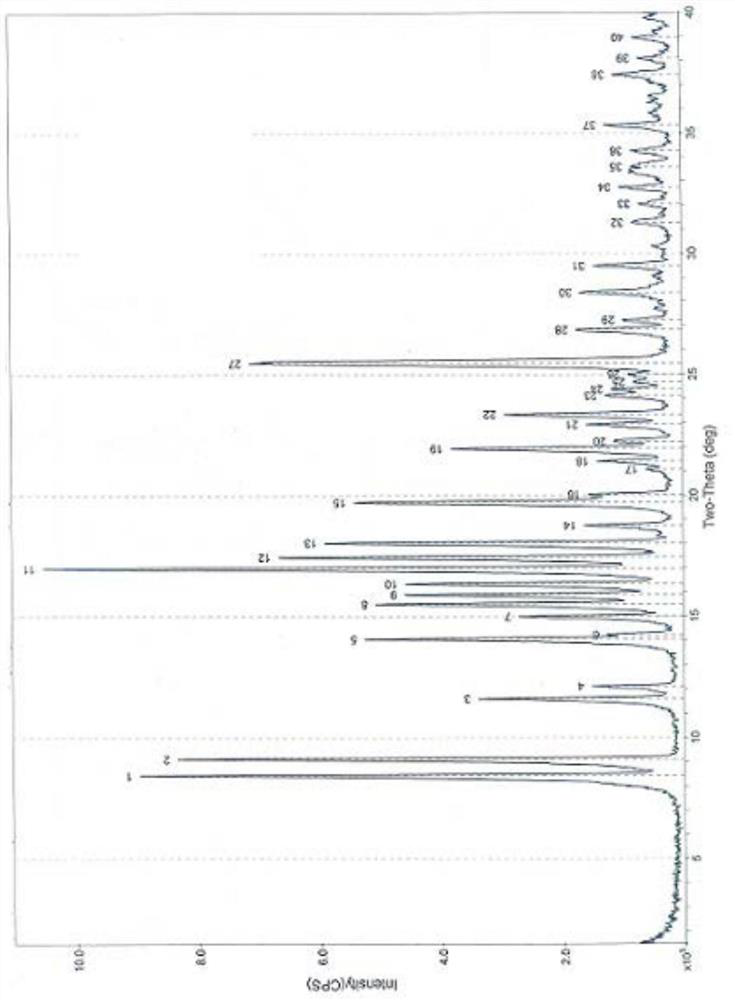
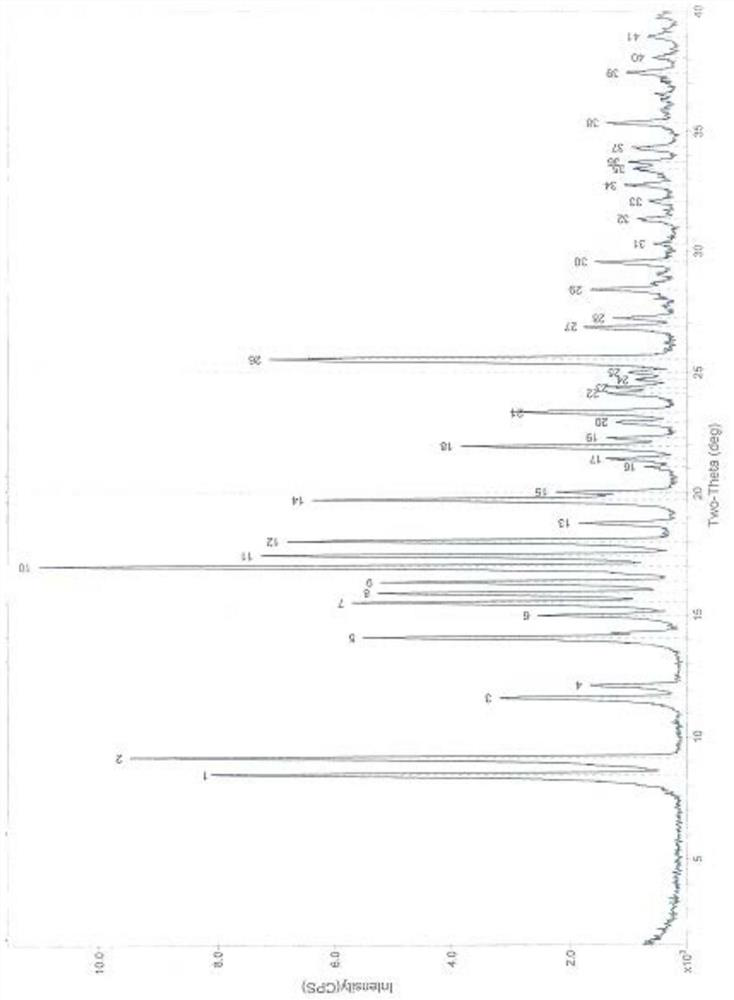
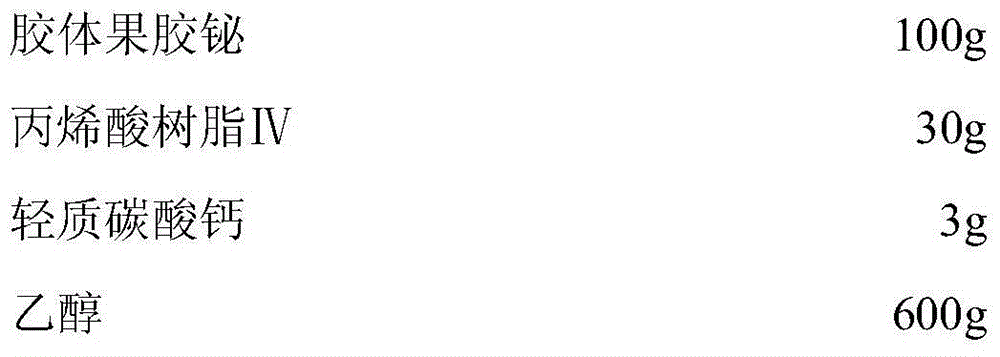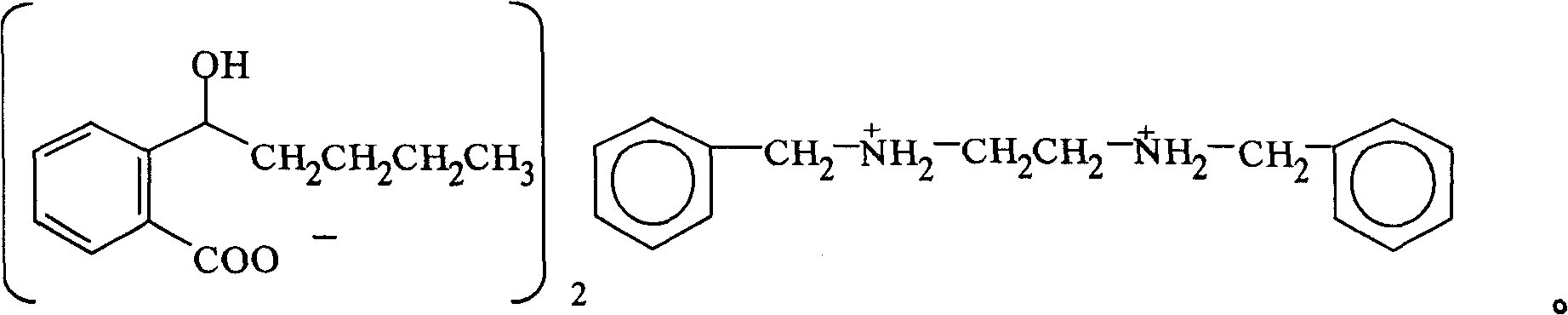Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
35results about How to "No hygroscopicity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
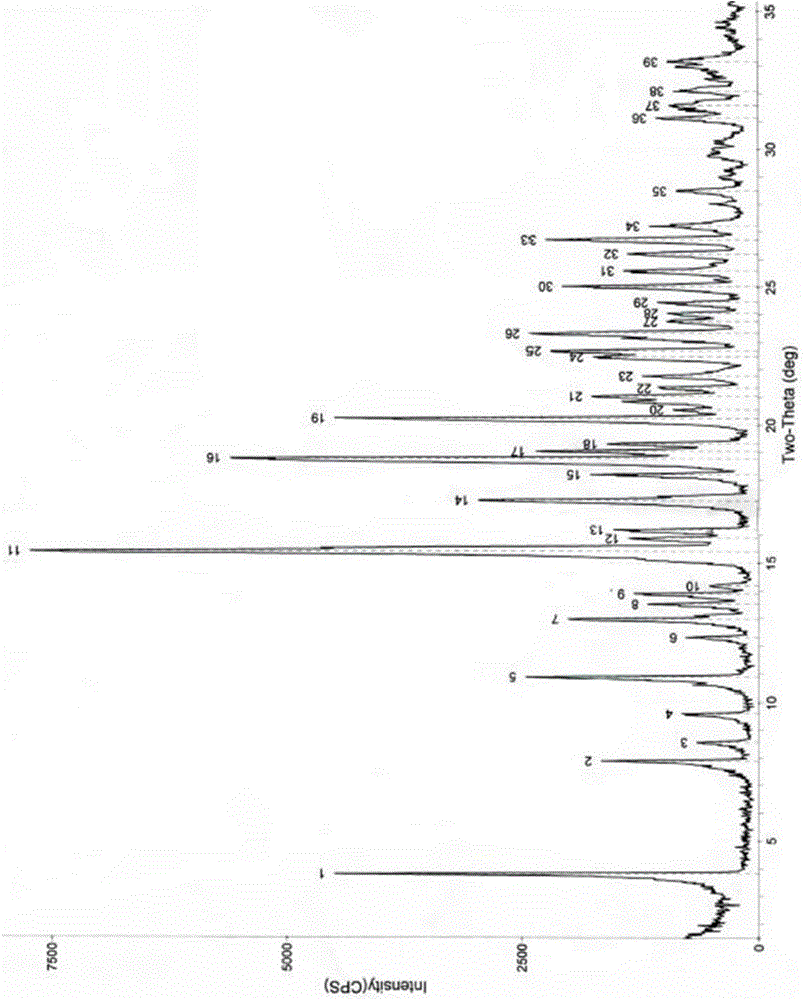
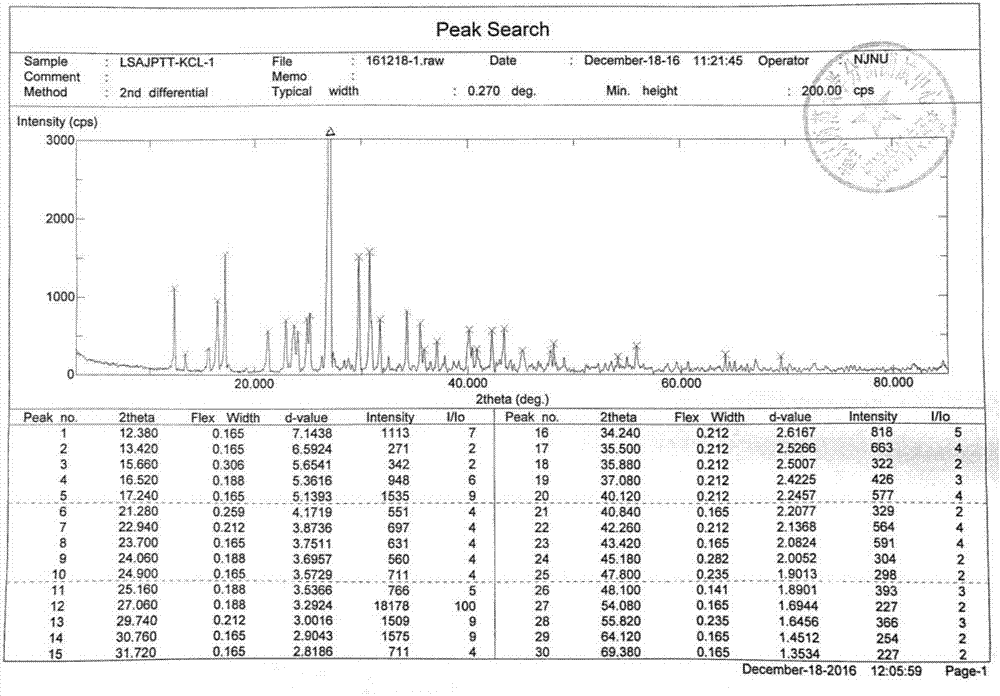
Azithromycin new crystal-form compound and preparation method thereof
ActiveCN104910222AImprove stabilityImprove performanceSugar derivativesSugar derivatives preparationAzithromycinX-ray
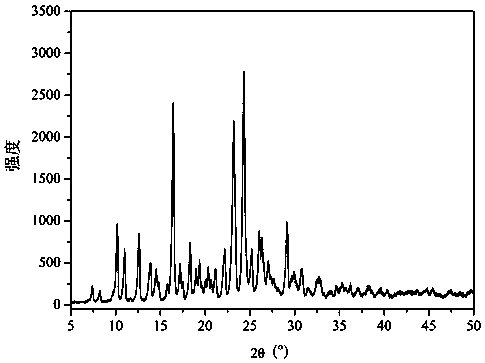
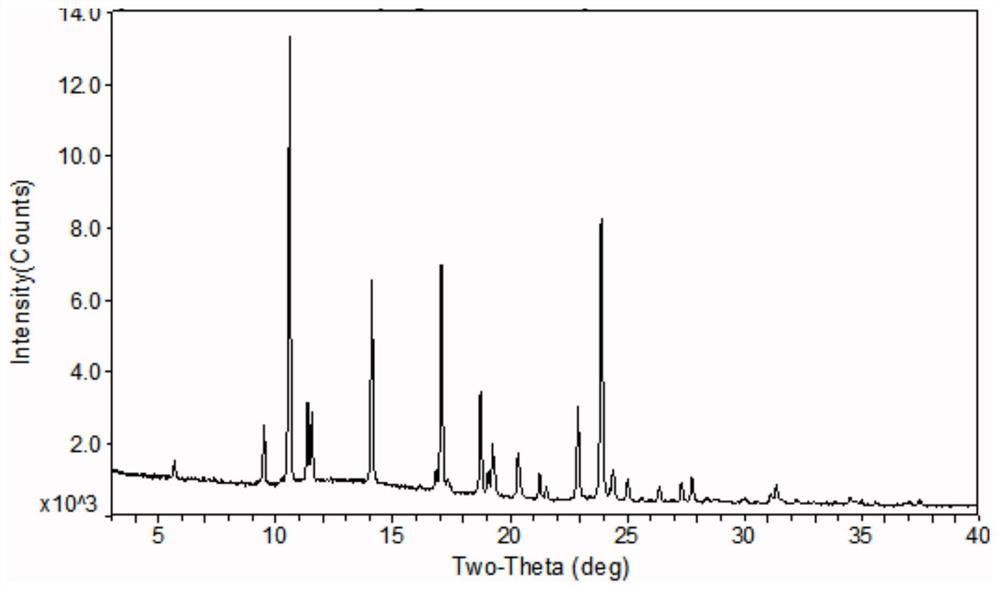
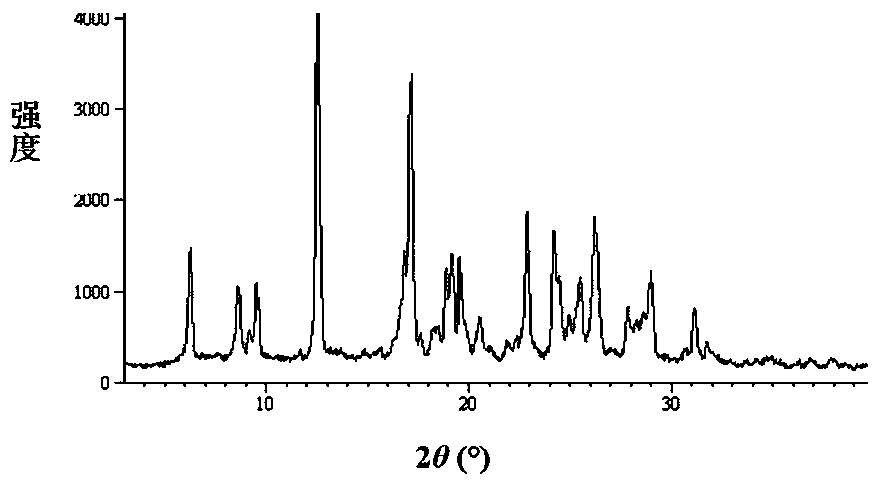
The invention relates to an azithromycin new crystal-form compound and a preparation method thereof, belonging to the technical field of medicine. According to the azithromycin new crystal-form compound provided by the invention, an X-ray powder diffraction diagram has characteristic peaks at the positions that the reflection angle 2theta is 3.8+ / -0.2 degrees, 15.4+ / -0.2 degrees, 17.2+ / -0.2 degrees, 18.7+ / -0.2 degrees, 20.1+ / -0.2 degrees and 23.2+ / -0.2 degrees. The azithromycin new crystal-form compound provided by the invention is subjected to a stability test under the conditions that the temperature is 40+ / -2 DEG C and the relative humidity is 75+ / -5 percent. The test result shows that the content of each related substance of the azithromycin new crystal-form compound provided by the invention is not changed obviously, and excellent quality stability is achieved. The repose angle measuring result shows that the azithromycin new crystal-form compound has excellent fluidity and is more suitable for medicine preparation and long-time storage.
Owner:CSPC OUYI PHARM CO LTD
Pyrroloquinolinequinone disodium crystal and preparation method thereof
InactiveCN108069962ASimple processGood reproducibilityOrganic chemistry methodsChemistryAqueous solution
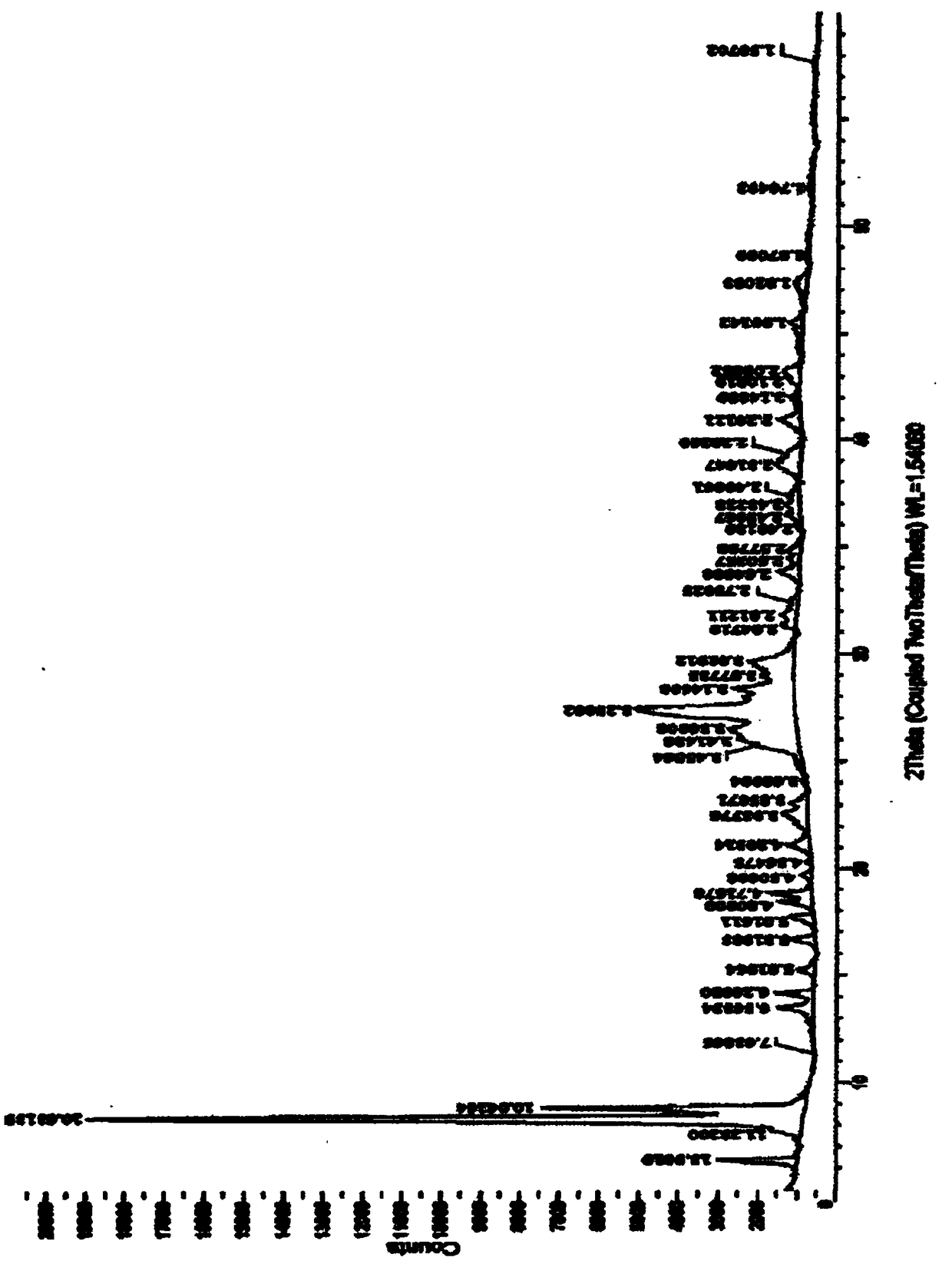
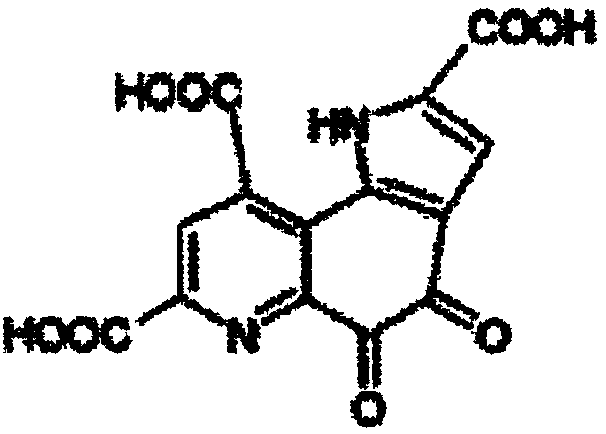
The invention aims at providing a pyrroloquinolinequinone disodium crystal simply suitable for large-scale production and a preparation method thereof. The preparation method of the pyrroloquinolinequinone disodium crystal comprises the following steps of adjusting the pH (potential of hydrogen) value in a water solution containing methoxatin free state or alkali metal salt thereof to 7.5 to 8.5;adding acid to adjust the pH value to 3.0 to 4.0; desalting, crystallizing, and drying, so as to obtain the pyrroloquinolinequinone disodium crystal. The preparation method has the advantages that thepreparation method is simple, the control is easy, and the prepared pyrroloquinolinequinone disodium crystal basically has no hygroscopicity.
Owner:SHANDONG JINCHENG BIO PHARMA CO LTD
Low-moisture-absorption colloidal bismuth pectin capsule and preparation technology thereof
InactiveCN104825419AAct as a porogenFast releaseAntibacterial agentsOrganic active ingredientsAcrylic resinBismuth / pectin
The invention discloses a low-moisture-absorption colloidal bismuth pectin capsule and a preparation technology thereof. In a preparation of the low-moisture-absorption colloidal bismuth pectin capsule, acrylic resin IV is taken as a coating material and light calcium carbonate is taken as an anti-sticking agent to coat colloidal bismuth pectin to obtain a coated pellet, and the obtained coated pellet is used to fill a capsule shell. The low-moisture-absorption colloidal bismuth pectin capsule and the preparation technology have the advantages that the problems that the colloidal bismuth pectin preparation is prone to absorbing moisture and clustering and not easy to disperse uniformly are solved, therapeutic efficacy of the colloidal bismuth pectin preparation is improved, less auxiliary materials are used, the preparation technology is simple, and the low-moisture-absorption colloidal bismuth pectin capsule is easy to produce industrially in large scale.
Owner:浙江得恩德制药股份有限公司
Preparation method of glucosamine sulfate compound salt
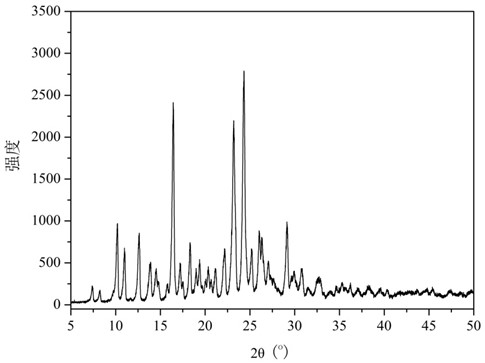
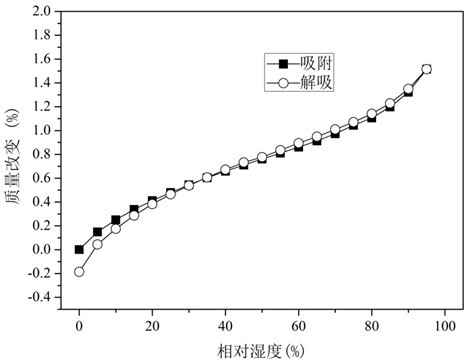
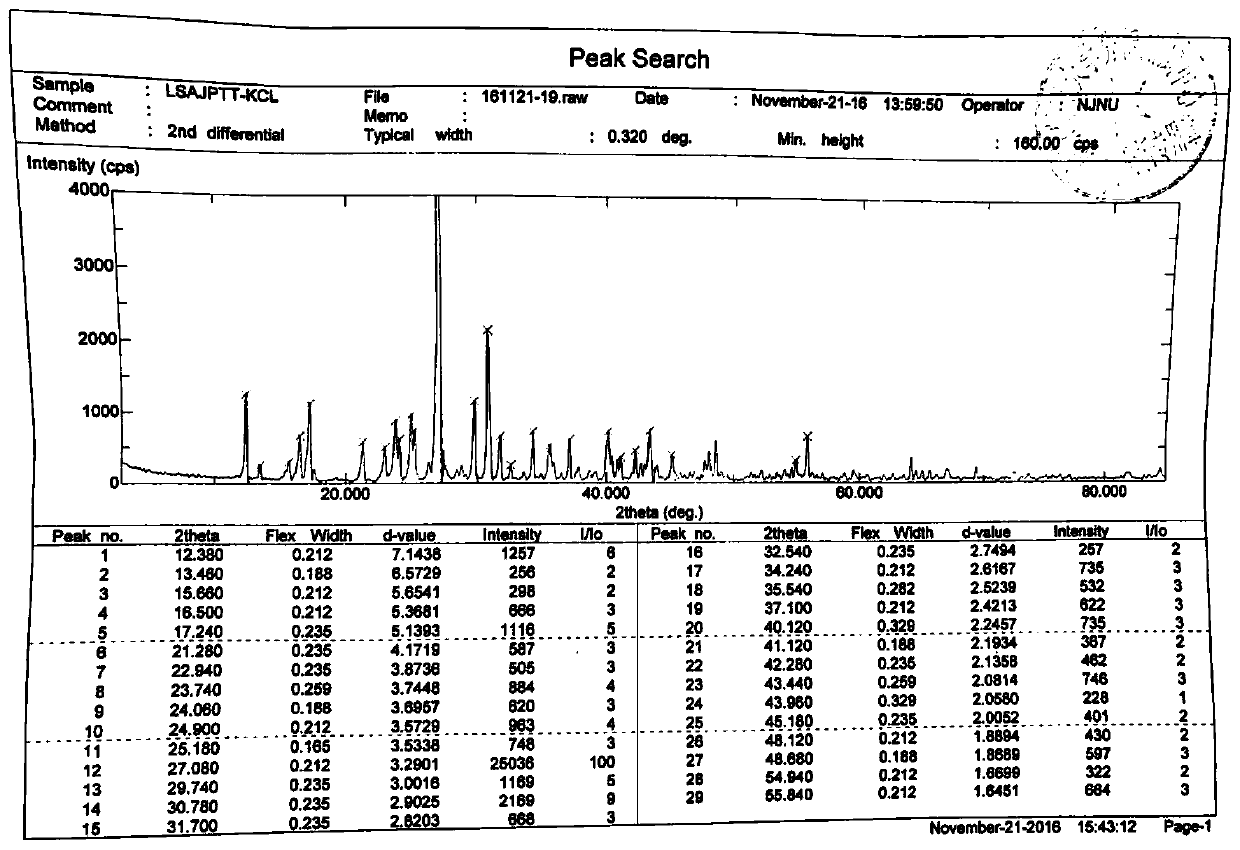
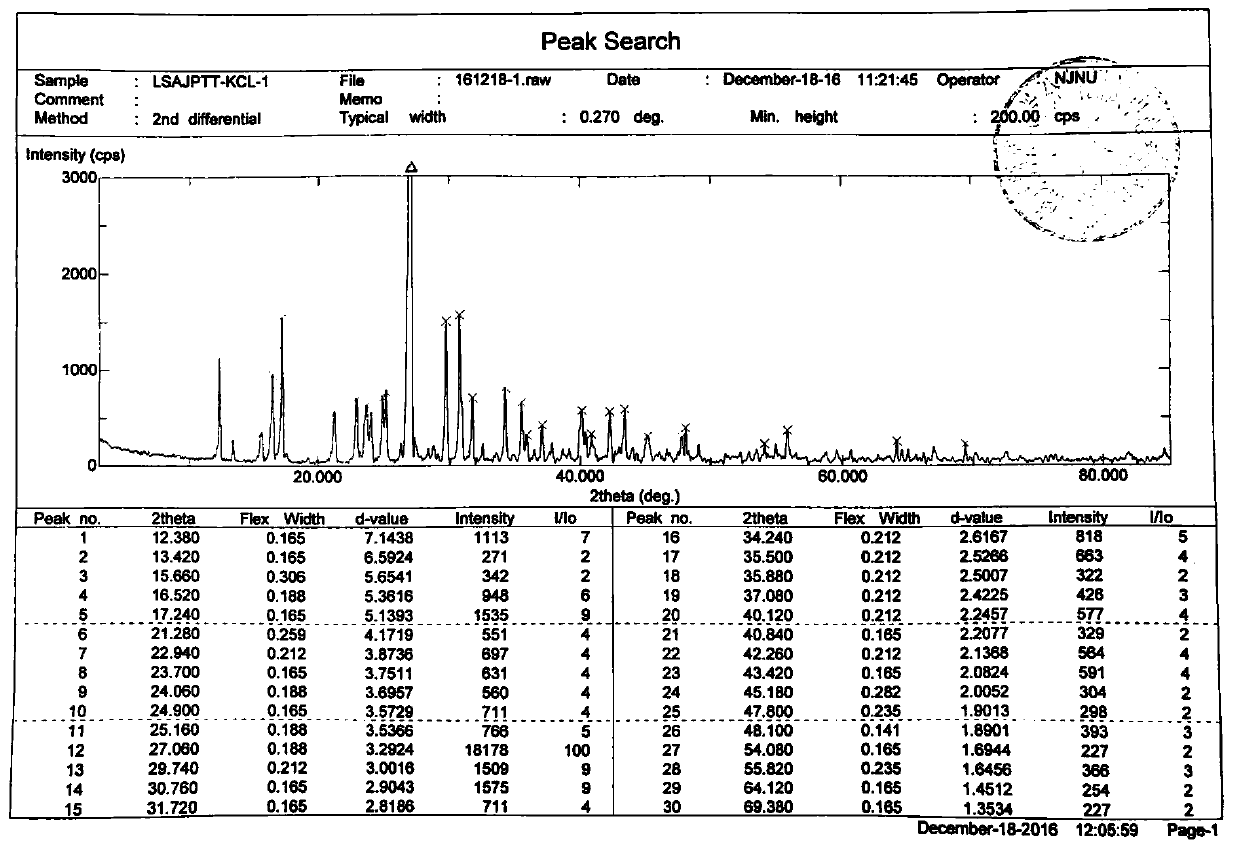
ActiveCN106866751ASmall water absorptionConvenient treatmentSugar derivativesSugar derivatives preparationFreeze-dryingX-ray
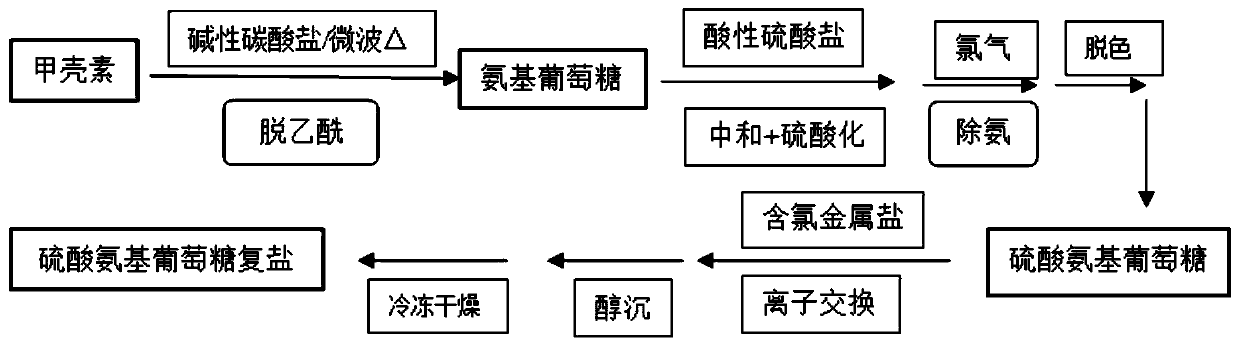
The invention relates to glucosamine sulfate compound salt and a preparation method thereof. The X-ray diffraction of the compound salt shows that the compound salt has a strongest peak at the position of 27.080 degrees. The preparation method comprises the following steps: 1, enabling chitin and a basic carbonate solution to have a deacetylation reaction under the condition of microwave heating, and drying to obtain glucosamine; 2, preparing the obtained glucosamine into a glucosamine solution, adding an excessive acid sulfate solution into the glucosamine solution for neutralizing the glucosamine solution, and enabling the glucosamine solution to be sulfated; removing the generated ammonia by using chlorine; adding activated carbon for carrying out a decolorizing reaction, and filtering the reaction product while the product is hot; 3, adding chlorine-containing metal salt into the solution obtained in the step 2, heating for carrying out an ion exchange reaction, and then carrying out a reflux reaction; 4, adding alcohol as a precipitator into the solution obtained after the reactions of the step 3 are finished, filtering to obtain precipitate, and carrying out freeze drying on the precipitate to obtain glucosamine sulfate compound salt concentrate. The preparation method provided by the invention is simple in production technology, low in cost and high in product purity, enables the quality of the product to reach the pharmacopeia standards, and can be used for large-scale production.
Owner:JIANGSU SHUANGLIN MARINE BIOLOGICAL PHARM CO LTD
Apixaban crystal form and preparation method therefor
ActiveCN110452240AHigh chemical purityReduce production energy consumptionOrganic chemistry methodsBlood disorderAnticoagulation ActivityX-ray
The invention relates to an apixaban crystal form and a preparation method therefor, belongs to the field of medicinal chemicals and particularly relates to an apixaban nicotinate monohydrate eutecticcrystal with anticoagulation activity and a preparation method therefor. Proven by X-ray powder diffraction spectrum analysis on the disclosed crystal, a 2[theta] value of a characteristic absorptionpeak is located at 5.7+ / -0.2 degrees, 6.0+ / -0.2 degrees, 11.5+ / -0.2 degrees, 13.3+ / -0.2 degrees, 15.4+ / -0.2 degrees, 15.8+ / -0.2 degrees, 17.5+ / -0.2 degrees, 17.7+ / -0.2 degrees, 20.0+ / -0.2 degrees, 22.1+ / -0.2 degrees and 23.4+ / -0.2 degrees. The preparation method has the advantages that reagents are cheap, readily available and environmentally friendly, the preparation method is simple, the crystallization conditions are moderate, and the crystal is easy to separate; and the apixaban nicotinate monohydrate eutectic crystal disclosed by the invention can be used for treating thromboembolism.
Owner:GUANGZHOU BAIYUSN TIANXIN PHARMA
Hydroxyl amyl benzoate potassium crystal and preparation method thereof
InactiveCN103113210AStable in natureSuitable for storageOrganic active ingredientsCardiovascular disorderPotassium benzoateMedicinal chemistry
The invention discloses a hydroxyl amyl benzoate potassium crystal and a preparation method thereof. The powder of the hydroxyl amyl benzoate potassium crystal disclosed by the invention has characteristic peaks under X-ray diffraction at 2theta, namely 7.0+ / -0.2 degrees and 20.9+ / -0.2 degrees. The hydroxyl amyl benzoate potassium crystal disclosed by the invention is stable in quality, stable in crystal form and more suitable for storage and use as a crude drug.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD
Non-hygroscopicity protocatechuic acid berberine monohydrate having crystal form
ActiveCN107188890AEasy to prepareClear crystal structureOrganic chemistry methodsCarboxylic compound separation/purificationSolubilityBerberine hydrochloride
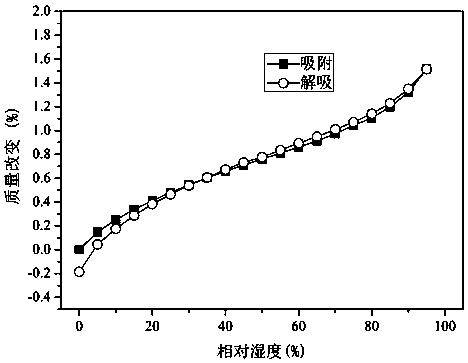
The present invention discloses a non-hygroscopicity protocatechuic acid berberine monohydrate having a crystal form, and belongs to the technical field of drug crystallization. According to the present invention, berberine hydrochloride, protocatechuic acid and sodium hydroxide are dissolved in an ethanol aqueous solution according to a certain ratio to form the protocatechuic acid berberine monohydrate crystal; the preparation method has characteristics of simpleness, easy performing, low cost, and high crystal yield; and the prepared protocatechuic acid berberine monohydrate crystal does not have the hygroscopicity, has the moderate solubility in water, can be rapidly dissolved, and can be used in cardiovascular diseases and anti-cancer.
Owner:MINJIANG UNIV
Citalopram pamoate and crystal form thereof, and preparation method and application thereof
InactiveCN107311968AHigh selectivityLittle side effectsOrganic active ingredientsNervous disorderSolubilityHypodermoclysis
The invention relates to citalopram pamoate and a crystal form thereof, and a preparation method and application thereof, belonging to the technical field of medicine. The citalopram pamoate has a structure as shown in a formula I which is described in the specification. The citalopram pamoate has low solubility, can be used for intramuscular injection or subcutaneous injection when made into a suspension, forms a medicine storehouse in vivo, prolongs in-vivo drug release speed and achieves the purpose of long-acting treatment.
Owner:GUANGZHOU HENOVCOM BIOSCI CO LTD
Emodin-berberine hydrochloride pharmaceutical co-crystal and preparation method thereof
InactiveCN108530440AEasy to prepareClear crystal structureOrganic chemistry methodsAcetonitrileBerberine hydrochloride
The invention discloses an emodin-berberine hydrochloride pharmaceutical co-crystal and a preparation method thereof, and belongs to the technical field of pharmaceutical crystallization. The preparation method is characterized in that the berberine hydrochloride and the emodin are dissolved into acetonitrile according to the ratio of 1 to 1 to form the emodin-berberine hydrochloride pharmaceutical co-crystal; a co-crystal structural unit is prepared from berberine hydrochloride molecules and emodin molecules, wherein the molar ratio of the berberine hydrochloride molecules to the emodin molecules is 1 to 1 and the molecular formula is [C15H10O5][C20H18ClNO4]. The preparation method disclosed by the invention is simple and feasible and the cost is low. In addition, the relative bioavailability of the emodin in the prepared pharmaceutical co-crystal in rats is significantly improved compared with that of pure emodin, and the relative bioavailability of the emodin in the prepared pharmaceutical co-crystal is 1.7 times that of the pure emodin.
Owner:MINJIANG UNIV
A kind of low hygroscopic colloid bismuth pectin capsule and its preparation process
InactiveCN104825419BAct as a porogenFast releaseAntibacterial agentsOrganic active ingredientsAcrylic resinBismuth / pectin
The invention discloses a colloidal bismuth pectin capsule with low hygroscopicity and a preparation process thereof. The preparation uses acrylic resin IV as a coating material and light calcium carbonate as an anti-sticking agent to coat the colloidal bismuth pectin. The obtained Coated pellets are filled with capsule shells, which greatly improves the problem of easy moisture absorption and difficult dispersion of colloidal pectin bismuth preparations in the storage process, and improves the curative effect of the drug; at the same time, there are fewer types of excipients used, and the preparation process is simple and easy for industrialization Big production.
Owner:浙江得恩德制药股份有限公司
Dihydromyricetin-berberine hydrochloride pharmaceutical co-crystals and preparation method thereof
ActiveCN110054606AEasy to prepareClear crystal structureOrganic chemistryNatural extract food ingredientsBerberine hydrochlorideInhibitory effect
The invention discloses dihydromyricetin-berberine hydrochloride pharmaceutical co-crystals and belongs to the technical field of pharmaceutical crystals. The pharmaceutical co-crystals are formed bycombining dihydromyricetin with berberine hydrochloride through intermolecular hydrogen bond action. A preparation method of the co-crystals is simple, convenient and easy to implement and low in cost. The pharmaceutical co-crystals prepared by the method are clear in structure and free of hygroscopicity; dihydromyricetin and berberine hydrochloride in water are similar in dissolution rates and can be dissolved synchronously, and the pharmaceutical co-crystals show synergistic enhanced inhibition effect on specific tumor cells.
Owner:MINJIANG UNIV
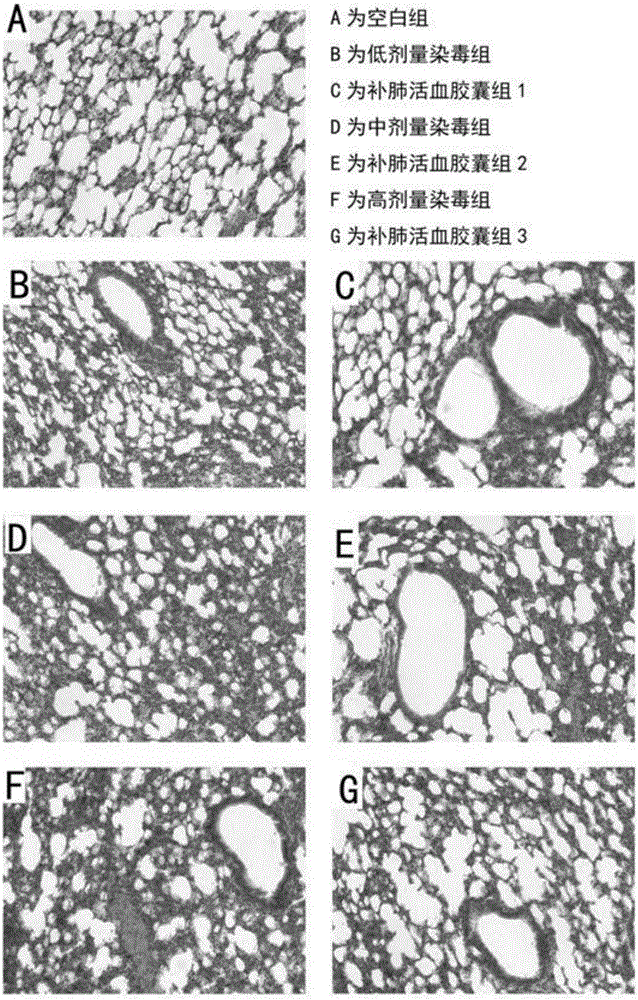
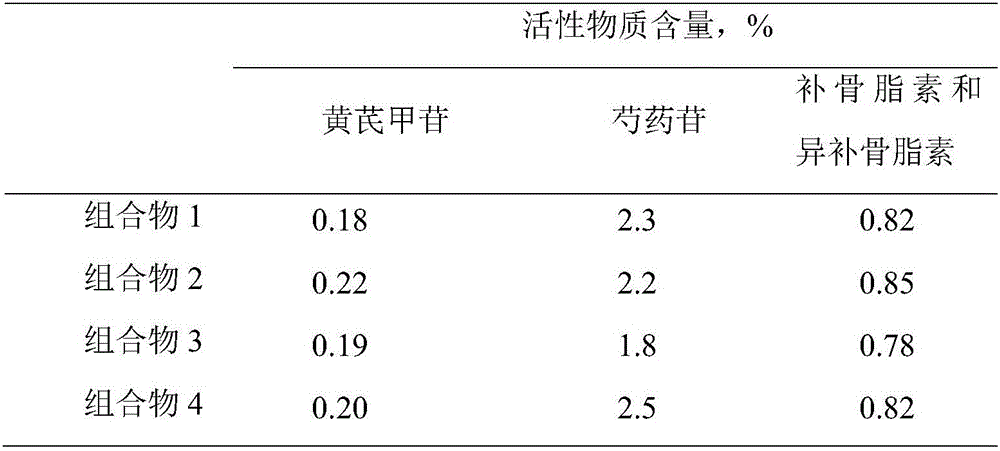
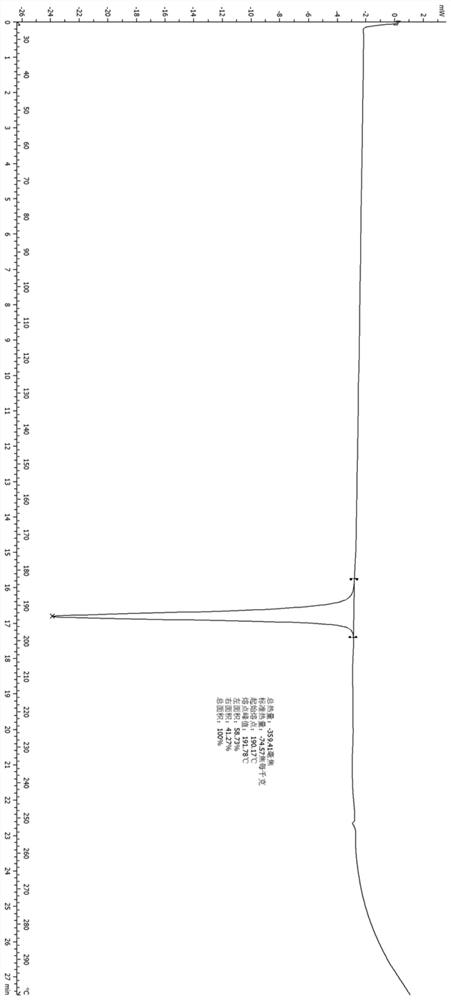
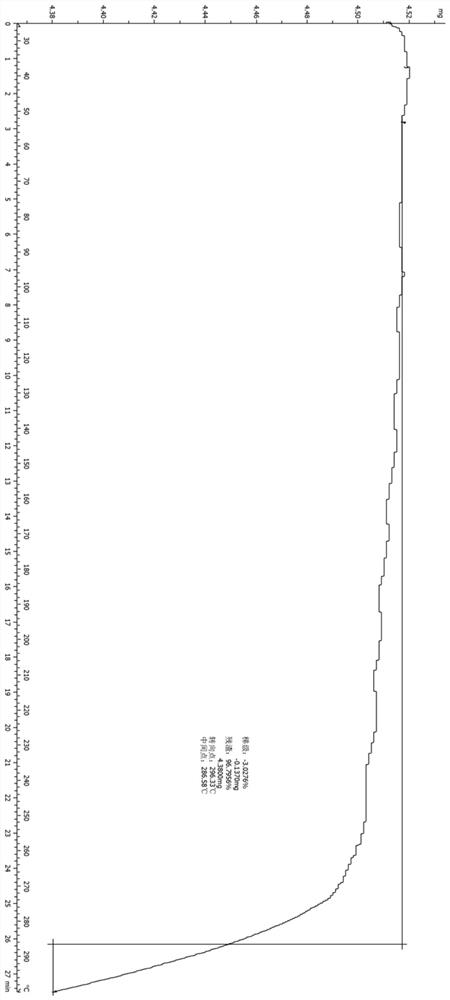
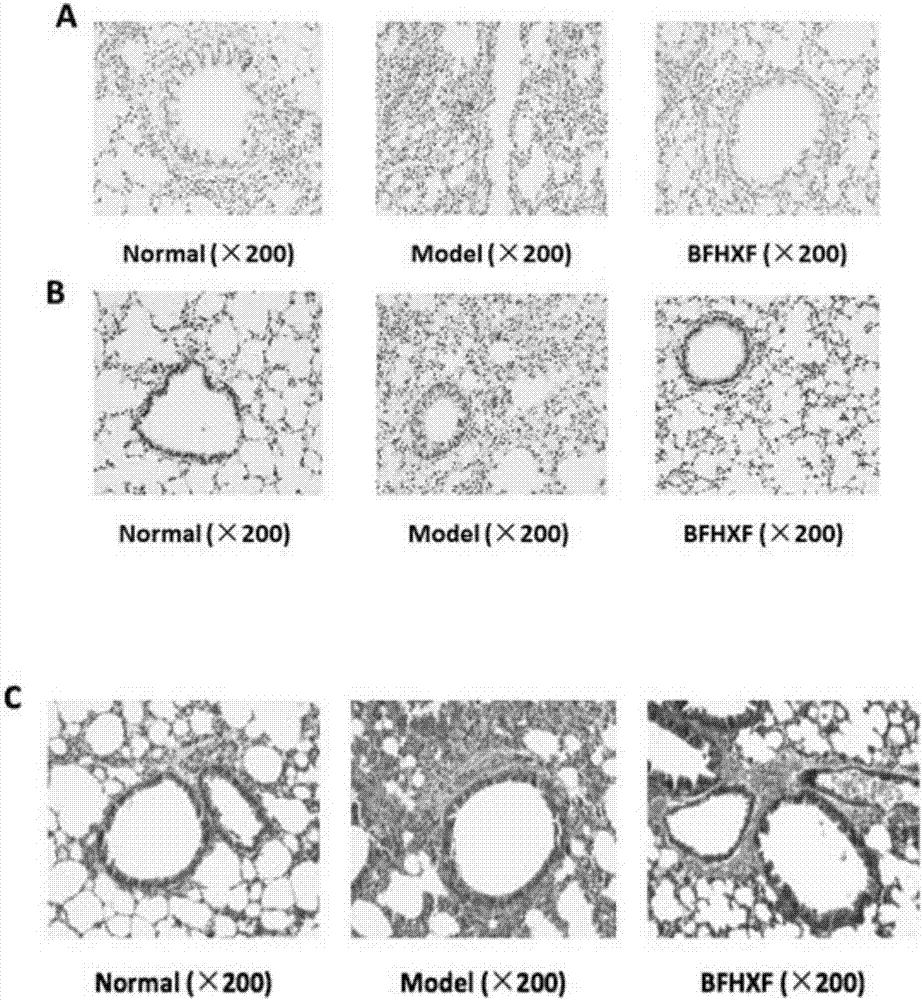
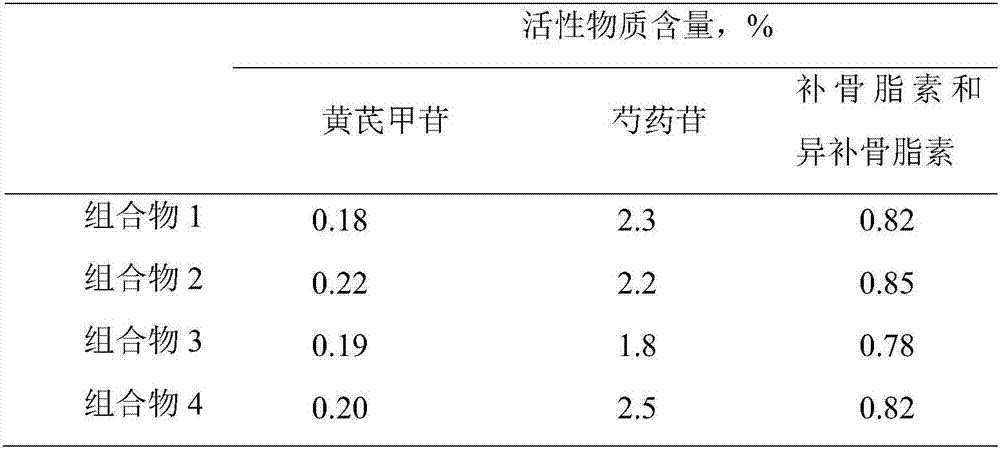
Application of lung tonifying and blood circulation promoting capsule in treating or preventing PM2.5 induced pulmonary injury
ActiveCN106474207ASafety and little side effectsLow costCapsule deliveryRespiratory disorderModern medicineSide effect
The invention belongs to the technical field of traditional Chinese medicine, and in particular relates to application of a lung tonifying and blood circulation promoting capsule for treating or preventing pulmonary injury. The traditional Chinese medicine composition selects medicinal materials with appropriate compatibility, conforms to research theories of traditional Chinese medicine and modern medicine, and has good drug effects and low toxic side effects. The traditional Chinese medicine composition is mainly prepared from astragalus root, red peony root and fructus psoraleae.
Owner:GUANGDONG LEIYUNSHANG PHARM CO LTD
Crystal form of macrocyclic compound and preparation method and application thereof
ActiveCN113754657AGood chemical stabilityGood physical stabilityNervous disorderAntipyreticChemical compoundMethyl palmoxirate
The invention belongs to the technical field of medicine synthesis, and discloses a crystal form of a macrocyclic compound and a preparation method and application thereof. The macrocyclic compound is (6R, 16R)-9-fluoro-16-methyl-13-oxa-2, 17, 21, 25-tetraazapentane [16.6.2. 02, 6.07, 12.022, 26] hexacosan-1 (25), 7, 9, 11, 18 (26), 19, 21, 23-octane-19-nitrile, and the X-ray diffraction pattern of the crystal form has characteristic peaks at the positions where the 2 theta values are 9.49 + / -0.2, 10.60 + / -0.2, 11.54 + / -0.2, 14.10 + / -0.2, 17.09 + / -0.2, 19.15 + / -0.2, 20.30 + / -0.2, 22.85 + / -0.2, 23.89 + / -0.2 and 27.74 + / -0.2. The crystal form has no hygroscopicity, and has good stability and pharmacokinetic properties.
Owner:GUANGZHOU BAIYUNSHAN PHARMA HLDG CO LTD BAIYUNSHAN PHARMA GENERAL FACTORY
Preparation method of azelastine hydrochloride
ActiveCN113045547ALarge operating spaceReduce quality riskOrganic chemistryAgainst vector-borne diseasesAzelastineHydrolysis
The invention relates to the technical field of synthesis of raw material medicines, and particularly discloses a preparation method of azelastine hydrochloride. The method comprises the following steps: condensing benzoyl hydrazine and 1-methylhexahydroazepine-4-one serving as raw materials, reducing with sodium borohydride, and reacting to obtain a compound 1; preparing a solid intermediate compound 2 from the compound 1 and organic binary weak acid; hydrolyzing the compound 2, and cyclizing with a compound 3 to form a compound 4; and salifying the compound 4 by hydrochloric acid, and separating water by toluene to obtain a compound 5, namely azelastine hydrochloride. The intermediate compound 2 prepared by the invention is a solid, the stability of the intermediate compound 2 is greatly improved compared with that of hydrochloride obtained in other literatures, the intermediate compound 2 is free of hygroscopicity, the purity can reach 99% or above, the quality risk of subsequent bulk drugs can be greatly reduced, and the process production operation space is larger; and the obtained azelastine hydrochloride does not contain water and meets related quality standards, the yield is increased to 80% or above compared with other literatures, and the purity can reach 99% or above.
Owner:WUHAN LEADPHARM TECH CO LTD
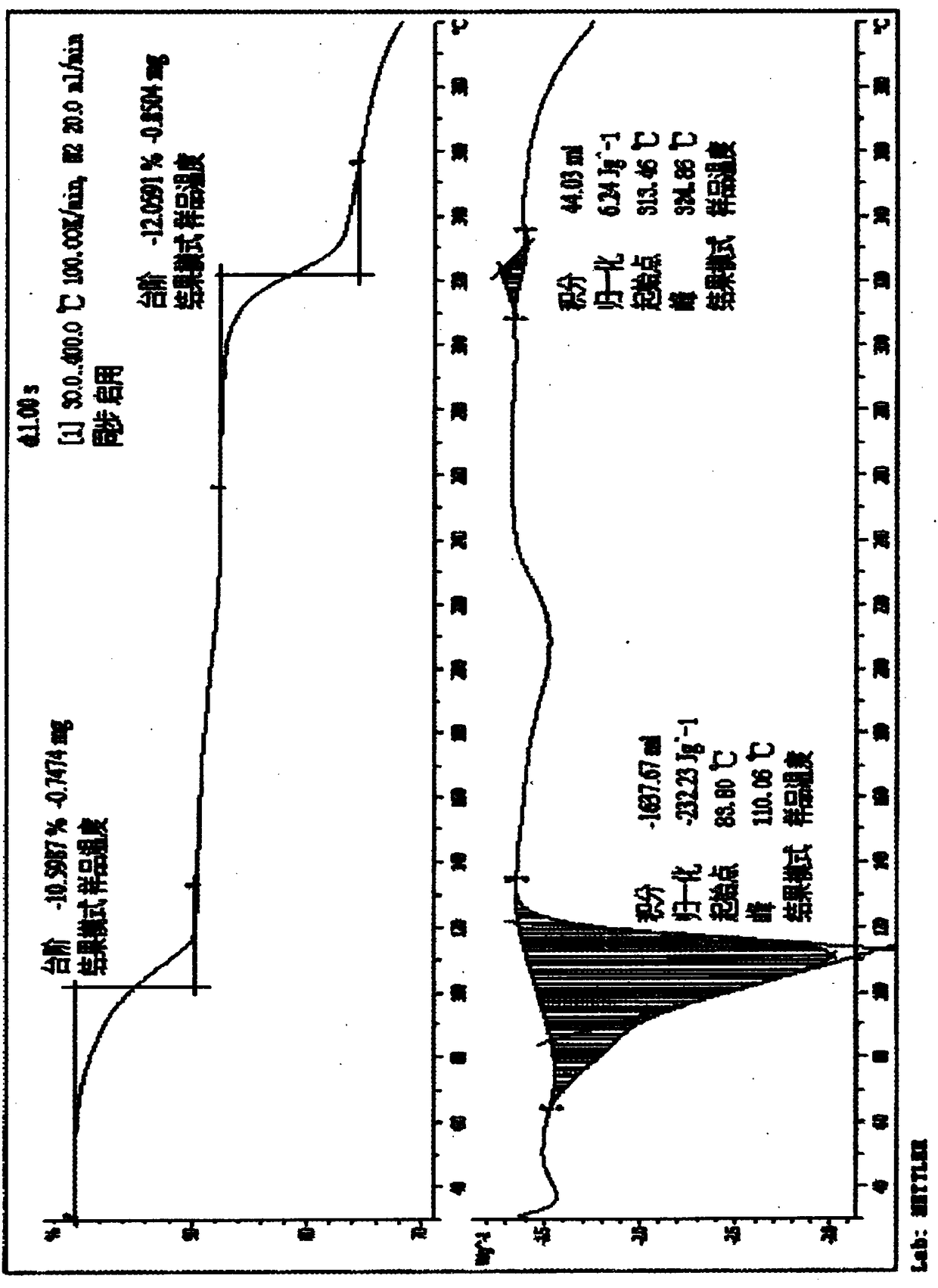
Valnemulin hydrochloride hydrate crystal form and preparation method thereof and pharmaceutical composition containing crystal form
ActiveCN110294697AEasy to operateEasy to makeAntibacterial agentsOrganic compound preparationValnemulin HydrochlorideX-ray
The invention relates to a valnemulin hydrochloride hydrate crystal form and a preparation method thereof and a pharmaceutical composition containing the crystal form. Specifically, the valnemulin hydrochloride hydrate crystal form shows characteristic peaks 2 Theta at 9.2+ / -0.2 degrees, 9.5+ / -0.2 degrees, 10.3+ / -0.2 degrees, 11.8+ / -0.2 degrees, 12.3+ / -0.2 degrees, 12.8+ / -0.2 degrees, 15.1+ / -0.2 degrees, 17.5+ / -0.2 degrees and 18.2+ / -0.2 degrees in an X-ray powder diffraction pattern. The valnemulin hydrochloride hydrate crystal form has high bulk density, no hygroscopicity and good stability.The invention also provides the preparation method of the valnemulin hydrochloride hydrate crystal form and the pharmaceutical composition containing the crystal form. The composition can be used forpreparing various preparations, such as dosage forms of soluble powder, oral liquids, sustained-release particles, injections and the like, and has wide clinical application prospect.
Owner:TIANJIN RINGPU BIO TECH +1
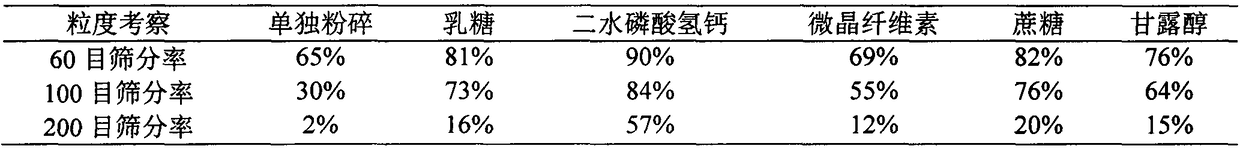
Thyroid tablets produced by direct whole-powder tabletting and preparation process thereof
ActiveCN109464411AHigh densityLow densityPowder deliveryInorganic non-active ingredientsHydrogen phosphateMedicine
The invention belongs to the technical field of pharmaceutical preparations, and relates to thyroid tablets produced by direct whole-powder tabletting and a preparation process of the thyroid tabletsproduced by the direct whole-powder tabletting, in particular to synergetic application of calcium hydrogen phosphate-assisted optimization of powder properties of thyroid raw materials and freeze-drying assisted grinding technology. More than 98% of the particle sizes of thyroid mixed ground materials prepared by the invention are smaller than 250 microns, and the thyroid mixed ground materials are difficultly reaggregated, and uniformly mixed with other auxiliary materials; RSD of the T3 and T4 contents in thyroid powder is not greater than 4%; the angle of repose of the mixed powder is smaller than 35 degrees, so that requirements of powder direct tabletting are met; the content uniformity (A+2.2S) of T3 and T4 in the thyroid tablets is not greater than 20, each tablet is disintegratedwithin 15min, and the disintegration time limit difference is small (RSD is not greater than 5%). The thyroid tablets produced by the direct whole-powder tabletting and the preparation process of thethyroid tablets produced by the direct whole-powder tabletting disclosed by the invention effectively solve the problems that the thyroid raw materials are not easily ground, and not uniformly mixed with the auxiliary materials and the like, optimize the grinding effect of the thyroid raw materials, improve the powder properties of the thyroid raw materials, and greatly enhance the content uniformity of T3 and T4 in the thyroid tablets.
Owner:CHINA PHARM UNIV
Soluble powder of crystalline valnemulin hydrochloride and application thereof
The invention discloses soluble powder of crystalline valnemulin hydrochloride and application thereof. The soluble powder consists of valnemulin hydrochloride hydrate crystal, a flavoring agent and auxiliary materials. The soluble powder made of the crystalline valnemulin hydrochloride does not induce dampness, has good thermal stability and low production cost, has no pungent smell, does not need to be coated, has better water solubility, and is beneficial to take by drinking water for livestock and poultry groups. Meanwhile, the soluble powder medicine has good palatability, improves the compliance of livestock and poultry medicines, increases the water drinking amount and the feed intake of animals, ensures the medicine dosage of livestock and poultry, and further improves the curativeeffect of valnemulin hydrochloride in prevention and treatment of mycoplasma infection and sensitive bacterial infection diseases of livestock and poultry.
Owner:RINGPU TIANJIN BIOLOGICAL PHARMA
Pyrroloquinoline quinone disodium salt crystal and preparation method thereof
ActiveCN109134459AEasy to manufactureMorphological rulesOrganic chemistry methodsMoisture absorptionPyrroloquinoline quinone
The purpose of the invention is to provide a pyrroloquinoline quinone disodium salt crystal which is simple and suitable for large-scale production and a preparation method thereof, wherein the preparation method of the pyrroloquinoline quinone disodium salt crystal comprises the following steps: the pH of an aqueous solution containing a pyrroloquinoline quinone disodium salt or an alkali metal salt thereof is adjusted to 7.5-8.5, then the acid is added to adjust the pH to 3.0-4.0, the pyrroloquinoline quinone disodium salt crystal is obtained by desalting, crystallizing and drying. The preparation method has the advantages of simple and easy to control, and the obtained pyrroloquinoline quinone disodium salt crystal is almost free of moisture absorption.
Owner:SHANDONG JINCHENG BIO PHARMA CO LTD
A kind of thyroid tablet produced by full powder direct compression and its preparation process
ActiveCN109464411BHigh densityLow densityPowder deliveryInorganic non-active ingredientsMetallurgyFreeze-drying
Owner:CHINA PHARM UNIV
A crystalline form of non-hygroscopic protocatechin berberine monohydrate
ActiveCN107188890BEasy to prepareClear crystal structureOrganic chemistry methodsCarboxylic compound separation/purificationAqueous ethanolBerberine hydrochloride
The present invention discloses a non-hygroscopicity protocatechuic acid berberine monohydrate having a crystal form, and belongs to the technical field of drug crystallization. According to the present invention, berberine hydrochloride, protocatechuic acid and sodium hydroxide are dissolved in an ethanol aqueous solution according to a certain ratio to form the protocatechuic acid berberine monohydrate crystal; the preparation method has characteristics of simpleness, easy performing, low cost, and high crystal yield; and the prepared protocatechuic acid berberine monohydrate crystal does not have the hygroscopicity, has the moderate solubility in water, can be rapidly dissolved, and can be used in cardiovascular diseases and anti-cancer.
Owner:MINJIANG UNIV
Apixaban crystal form and preparation method thereof
ActiveCN110452240BContent unchangedImprove stabilityOrganic chemistry methodsBlood disorderChemical industryMedicinal chemistry
The invention relates to an apixaban crystal form and a preparation method thereof, belonging to the field of medicine and chemical industry, in particular to an apixaban nicotinic acid monohydrate eutectic with anticoagulant activity and a preparation method thereof. It is disclosed that the obtained crystals are analyzed by X-ray powder diffraction spectrum, and the 2θ values of the characteristic absorption peaks are located at 5.7±0.2゜, 6.0±0.2゜, 11.5±0.2゜, 13.3±0.2゜, 15.4±0.2゜, 15.8±0.2゜, 17.5±0.2゜, 17.7±0.2゜, 20.0±0.2゜, 22.1±0.2゜, 23.4±0.2゜. The preparation method has the advantages of cheap and easy-to-obtain reagents, environmental friendliness, simple preparation method, mild crystallization conditions and easy crystal separation. The apixaban nicotinic acid monohydrate cocrystal of the invention can be used for treating thromboembolic diseases.
Owner:GUANGZHOU BAIYUSN TIANXIN PHARMA
A new crystal form of sofosbuvir and its preparation method
ActiveCN105985394BImprove stabilityGood crystal formOrganic active ingredientsSugar derivativesPhysical chemistryPharmaceutical drug
The invention discloses a new crystal form of sofosbuvir and a preparation method thereof. The powder X-ray diffraction of the new crystal form of sofosbuvir of the present invention has characteristic peaks at 2θ: 8.4±0.2°, 9.1±0.2°, 16.9±0.2°, 19.7±0.2°, 25.5±0.2°. The new sofosbuvir crystal form of the present invention has stable quality, stable crystal form, no hygroscopicity, and good fluidity. The preparation method is simple and suitable for industrialization, and is more suitable for storage and use as a raw material drug, and provides a method for the preparation of sofosbuvir medicine. new way.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD
Vernemulin hydrochloride hydrate crystal form, preparation method thereof, and pharmaceutical composition containing the crystal form
ActiveCN110294697BEasy to operateEasy to makeAntibacterial agentsOrganic compound preparationMedicinePharmaceutical drug
The invention relates to a crystal form of warnemuline hydrochloride hydrate, a preparation method thereof and a pharmaceutical composition containing the crystal form. Specifically, the present invention relates to the crystalline form of warnimulin hydrochloride hydrate, the characteristic peaks 2θ of the crystalline form in the X-ray powder diffraction spectrum are at 9.2±0.2°, 9.5±0.2°, 10.3±0.2°, 11.8±0.2 °, 12.3±0.2°, 12.8±0.2°, 15.1±0.2°, 17.5±0.2°, 18.2±0.2° display. The warnemulin hydrochloride hydrate crystal form has a large bulk density, no hygroscopicity and good stability. The present invention also provides a method for preparing the above-mentioned warnemuline hydrochloride hydrate crystal form and a pharmaceutical composition containing the crystal form, which can be used in the preparation of various preparations, such as soluble powder, oral liquid, sustained release Granules, injections and other dosage forms have broad prospects for clinical use.
Owner:TIANJIN RINGPU BIO TECH +1
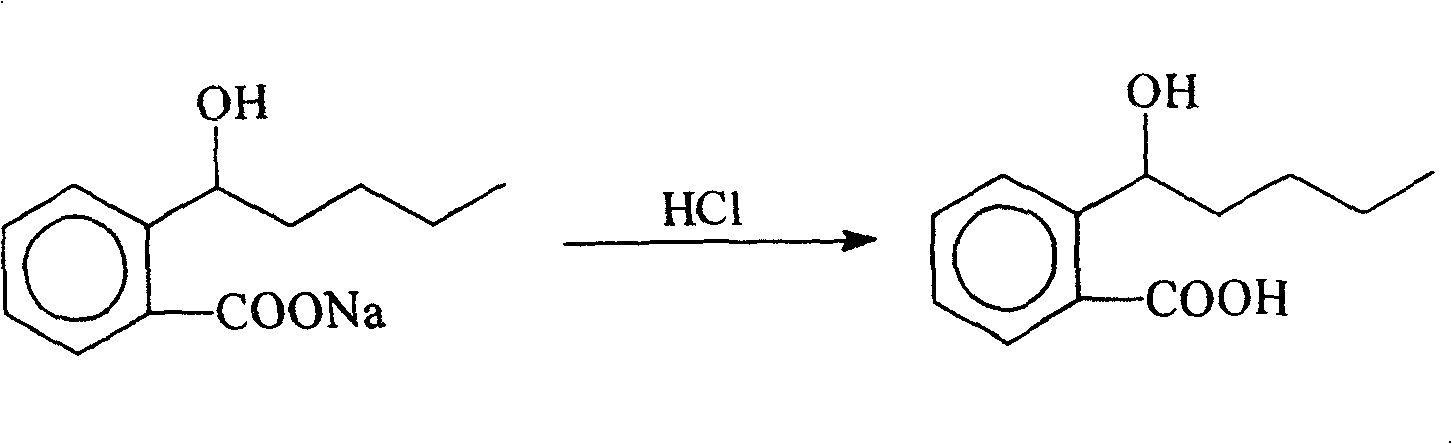
Novel 2-(alpha-hydroxyl amyl) and its preparing method and use
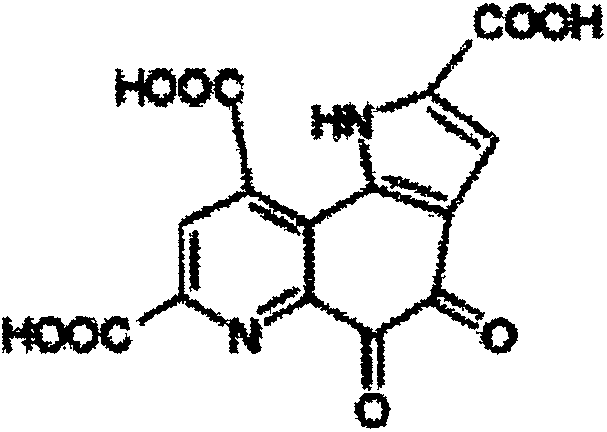
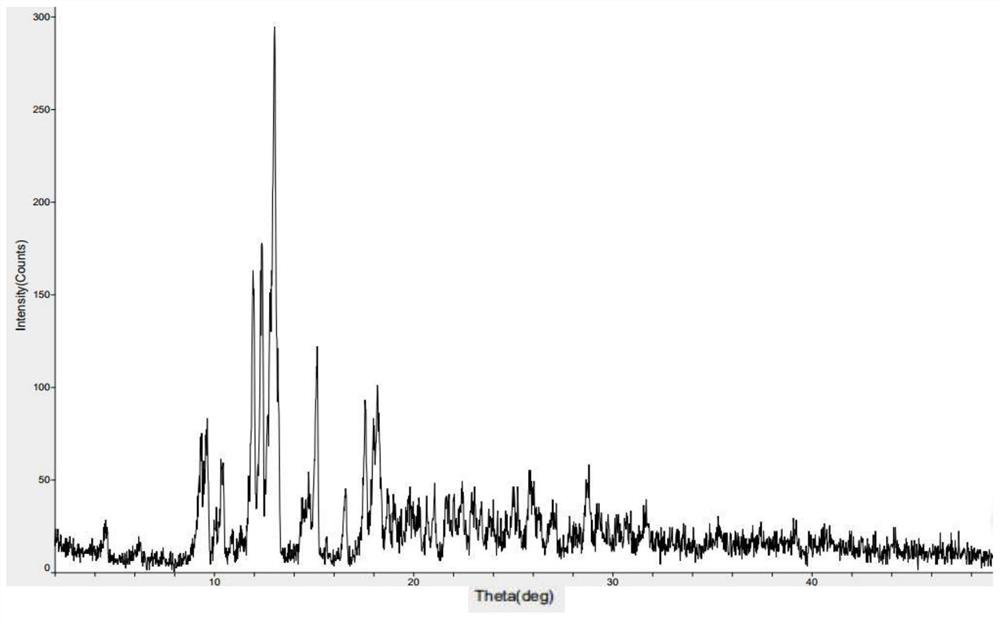
InactiveCN100422133CSuitable for storageNo hygroscopicityOrganic compound preparationPill deliveryMedicineActive component
The present invention relates to a new 2-(alpha-hydroxyamyl) benzoate and its preparation method, and medicine composition using said compound as active component, and also relates to its application for curing cardio-cerebral ischemia and cardio-cerebral arterial infarction.
Owner:北京天衡药物研究院有限公司
Novel crystal form of 2-aminopyridine derivative and preparation method thereof
PendingCN114437036AHigh purityEasy to prepareOrganic active ingredientsOrganic chemistry methodsPerylene derivativesBipyridine
The invention belongs to the field of medicinal chemistry, and relates to a novel crystal form of a 2-aminopyridine derivative and a preparation method thereof, in particular to a citrate crystal of 5-((R)-1-(2, 6-dichloro-3-fluorophenyl) ethyoxyl)-4 '-methoxy-6'-((S)-2-methylpiperazine-1-yl)-3, 3 '-bipyridine-6-amine and a preparation method thereof, and more particularly relates to a citrate crystal of 5-((R)-1-(2, 6-dichloro-3-fluorophenyl) ethyoxyl)-4'-methoxy-6 '-((S)-2-methylpiperazine-1-yl)-3, 3'-bipyridine-6-amine and a preparation method thereof. The present invention relates to H-type, NH-type and D-type crystals of a citrate of (2, 6-dichloro-3-fluorophenyl) ethyoxyl)-4 '-methoxy-6'-((S)-2-methylpiperazine-1-yl)-3, 3 '-bipyridine-6-amine, and to a method for preparing the same.
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD
A kind of dihydromyricetin-berberine hydrochloride pharmaceutical co-crystal and its preparation method
ActiveCN110054606BEasy to prepareClear crystal structureOrganic chemistryNatural extract food ingredientsPharmaceutical drugBerberine hydrochloride
The invention discloses a drug co-crystal of dihydromyricetin-berberine hydrochloride, which belongs to the technical field of drug crystallization. The present invention combines dihydromyricetin and berberine hydrochloride through intermolecular hydrogen bonding to form the dihydromyricetin-berberine hydrochloride drug eutectic. The preparation method of the invention is simple and easy, and the cost is low. The prepared dihydromyricetin-berberine hydrochloride drug eutectic structure is clear, without hygroscopicity, and the dissolution rate of dihydromyricetin and berberine hydrochloride in water is similar, and can be dissolved simultaneously, showing a synergistically enhanced effect on specific tumor cells. inhibition.
Owner:MINJIANG UNIV
A kind of preparation method of glucosamine sulfate double salt
ActiveCN106866751BSmall water absorptionConvenient treatmentSugar derivativesSugar derivatives preparationFreeze-dryingIon exchange
The invention relates to glucosamine sulfate compound salt and a preparation method thereof. The X-ray diffraction of the compound salt shows that the compound salt has a strongest peak at the position of 27.080 degrees. The preparation method comprises the following steps: 1, enabling chitin and a basic carbonate solution to have a deacetylation reaction under the condition of microwave heating, and drying to obtain glucosamine; 2, preparing the obtained glucosamine into a glucosamine solution, adding an excessive acid sulfate solution into the glucosamine solution for neutralizing the glucosamine solution, and enabling the glucosamine solution to be sulfated; removing the generated ammonia by using chlorine; adding activated carbon for carrying out a decolorizing reaction, and filtering the reaction product while the product is hot; 3, adding chlorine-containing metal salt into the solution obtained in the step 2, heating for carrying out an ion exchange reaction, and then carrying out a reflux reaction; 4, adding alcohol as a precipitator into the solution obtained after the reactions of the step 3 are finished, filtering to obtain precipitate, and carrying out freeze drying on the precipitate to obtain glucosamine sulfate compound salt concentrate. The preparation method provided by the invention is simple in production technology, low in cost and high in product purity, enables the quality of the product to reach the pharmacopeia standards, and can be used for large-scale production.
Owner:JIANGSU SHUANGLIN MARINE BIOLOGICAL PHARM CO LTD
Novel crystal form of polydatin and method for preparing same
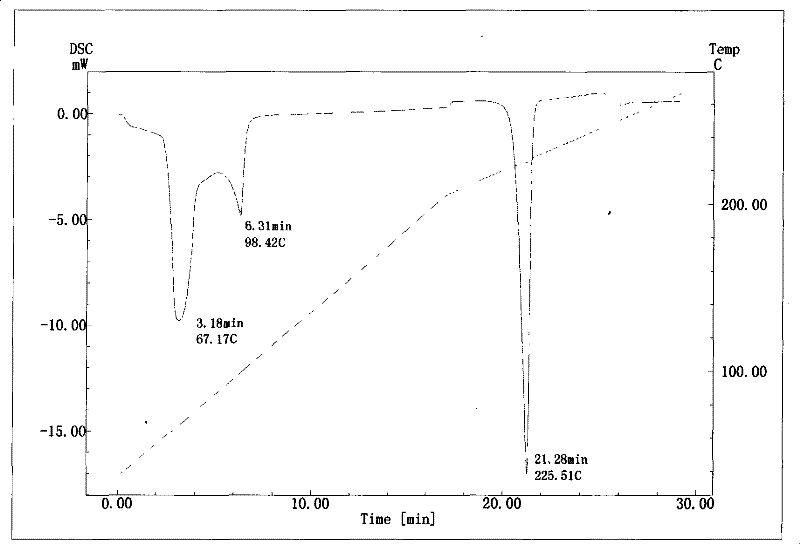
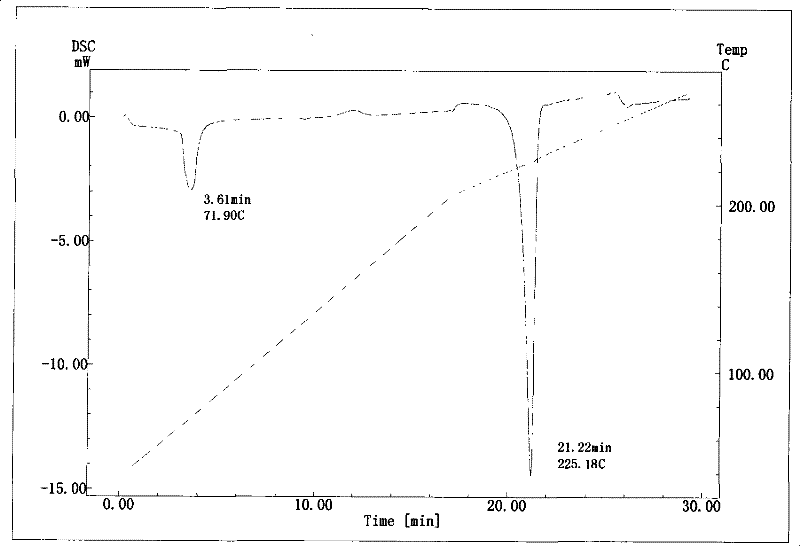
InactiveCN101633677BNot easy to changeHigh dissolution rateSugar derivativesGlucosideDifferential scanning calorimetry
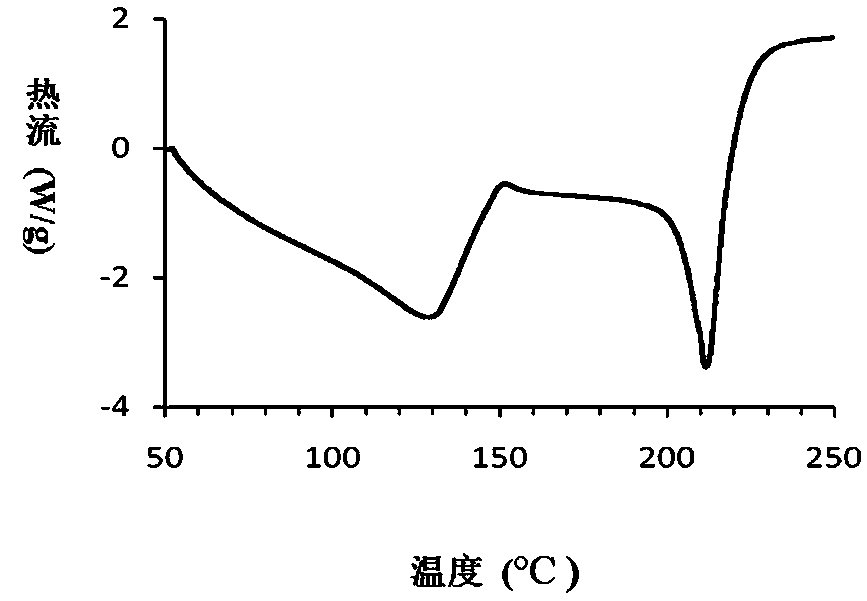
The invention relates to a compound polydatin, in particular to a crystal form of 3,4',5-trihydroxy-stilbene-3-beta-D-glucoside (polydatin) and a method for preparing the same, which belong to the field of medicinal and health products. On a differential scanning calorimetry spectrogram, the crystal form of the polydatin displays that a single sharp endothermic peak occurs at a temperature of 2252.0 DEG C, and one to two dehydration endothermic peaks occur at a temperature of between 50 and 100 DEG C. The crystal form of the polydatin has the advantages that: the novel crystal form of the polydatin contains 0.5-3 H2O crystallization water, is stable, and is difficultly converted into other crystal forms. The process for preparing the crystal form adopts water as a crystallization solvent, and has the advantages of simple process, easy control, stable obtained crystal form, good repeatability, and no problems of environmental pollution and residual solvents.
Owner:SHENYANG WANJIA INST OF BIOLOGICAL TECH RES
A crystal form of a spirocyclic dihydroisoquinoline carboxamide derivative and a preparation method thereof
ActiveCN111217748BHigh crystal purityGood crystal stabilityOrganic active ingredientsAntipyreticIsoquinolineQuinoline
The invention relates to a crystal form of a spirocyclic dihydroisoquinoline carboxamide derivative and a preparation method thereof. Specifically, the present invention relates to the crystal form A of the compound represented by formula (I), which has good stability and can be better used in clinical treatment.
Owner:JIANGSU HENGRUI MEDICINE CO LTD +1
Use of Bufei Huoxue Capsules for the treatment or prevention of lung injury caused by PM2.5
ActiveCN106474207BSafety and little side effectsLow costCapsule deliveryRespiratory disorderSide effectModern medicine
The invention belongs to the technical field of traditional Chinese medicine, and in particular relates to application of a lung tonifying and blood circulation promoting capsule for treating or preventing pulmonary injury. The traditional Chinese medicine composition selects medicinal materials with appropriate compatibility, conforms to research theories of traditional Chinese medicine and modern medicine, and has good drug effects and low toxic side effects. The traditional Chinese medicine composition is mainly prepared from astragalus root, red peony root and fructus psoraleae.
Owner:GUANGDONG LEIYUNSHANG PHARM CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com