Citalopram pamoate and crystal form thereof, and preparation method and application thereof
A technology of pamolate and citalopram, which is applied in the field of medicine, can solve problems such as poor patient compliance, interruption of treatment, and serious side effects of patients, and achieve the effects of strong selectivity, small side effects, and high purity of crystal forms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
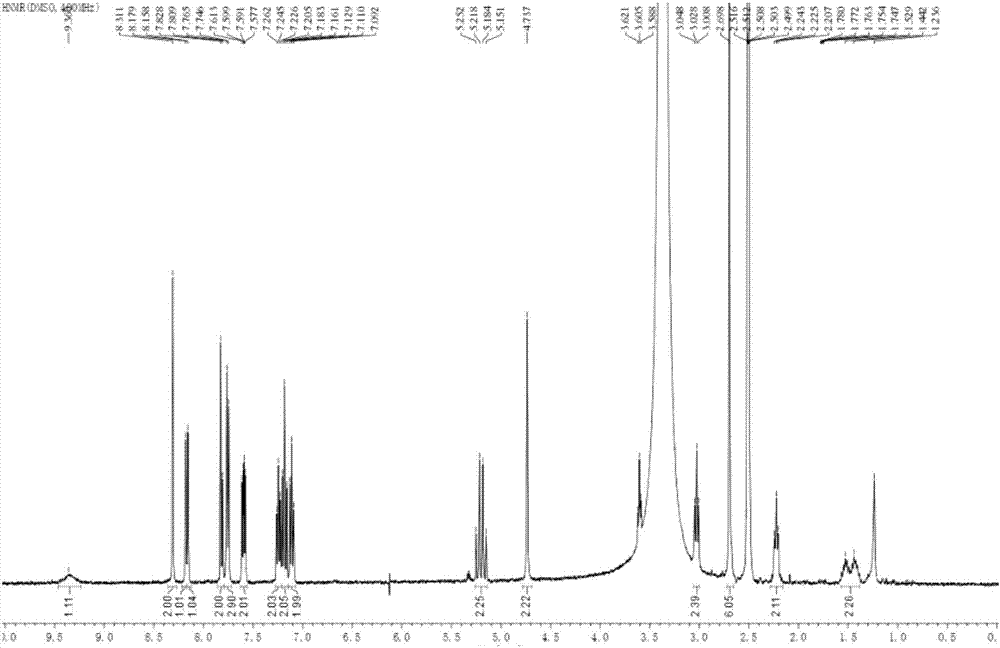
[0067] A citalopram pamolate, prepared by the following method:
[0068] Add 1.0 g of D-citalopram free base (3.08 mmol) and 1.14 g of pamoic acid (2.93 mol) to 50 ml of tetrahydrofuran, stir at room temperature, filter after 12 hours of reaction, and dry the solid under reduced pressure at 50°C to obtain 1.70 g of D-citalopram Citalopram pamolate is light yellow solid powder.
[0069] The above-mentioned obtained solid is subjected to XRPD powder diffraction analysis and infrared analysis, and it is confirmed that the obtained citalopram pamolate crystalline form is a crystal form I with the following characteristics:
[0070] Measured by Cu-Kα radiation, in the X-ray powder diffraction spectrum expressed by diffraction angle 2θ, at 8.93±0.2゜, 11.29±0.2゜, 13.21±0.2゜, 18.44±0.2゜, 20.63±0.2゜, 21.90±0.2 There is a diffraction peak at ゜.
[0071] Among them, the detection equipment and detection conditions of XRPD are:
[0072] Testing instrument: Empyrean X-ray diffractometer...
Embodiment 2
[0089] A citalopram pamolate, prepared by the following method:
[0090]Add 1.0g (3.08mmol) D-citalopram free base and 0.60g (1.54mmol) pamolic acid to 50ml tetrahydrofuran, the molar ratio of citalopram to pamoic acid is 2:1, that is, X=1 / 2. Stir at room temperature. After reacting for 5 hours, stop the reaction, spin the reaction solution to dryness, and dry under reduced pressure at 50° C. to obtain 1.6 g of D-citalopram hemipamoic acid salt as a light yellow solid powder.
[0091] D-citalopram hemipamolate was tested for solubility in different solvents, and the results were as follows:
[0092] Table 3 Solubility of D-citalopram hemipamolate in different solvents
[0093] solvent
[0094] It can be seen from the above results that when X is 1 / 2, that is, two molecules of D-citalopram and one part of pamoic acid form a salt, the solubility is relatively large, and the long-term release performance is not as good as that of citalopram with X being 1. molybdenum...
Embodiment 3
[0096] A citalopram pamolate, prepared by the following method:
[0097] Add 1.0g (3.08mmol) of D-citalopram free base and 1.14g (2.93mmol) of pamoic acid to 50ml of tetrahydrofuran, stir at 50°C, react for 3 hours, stop the reaction, cool to room temperature, filter, solid 50 °C and dried under reduced pressure to obtain 1.72 g of D-citalopram pamolate as a light yellow solid powder, which was verified to be a crystal form I product.
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com