Apixaban crystal form and preparation method thereof
A technology of apixaban and crystal form, applied in the field of anticoagulant drugs, co-crystals and their preparation, can solve the problems of chemical stability and hygroscopicity and other physical and chemical druggability characteristics without further relevant reports, and achieve shortening The production cycle, the reduction of production energy consumption, and the effect of facilitating the processing and production of preparations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Preparation of Apixaban Nicotinic Acid Monohydrate Cocrystal
[0041] Apixaban N-1 crystal form is prepared by the method disclosed in the patent US2006 / 0160841.
[0042] Add 0.182g of nicotinic acid and 0.68g of apixaban into 7ml of 0.5% water-containing trifluoroethanol, stir at 30°C for 24 hours, then add 15ml of ethyl acetate dropwise, stir at the same temperature for 30 minutes, filter to obtain white crystals, and place in a drying oven Vacuum-dried at 40°C for 4 hours to obtain 0.66 g of white crystals. The molar yield is 74%. Melting point: 191.0~197℃. 1 H NMR (500MHz, DMSO-d 6 )δ: 13.42(s, 1H), 9.06(s, 1H), 8.77(d, J=3.8Hz, 1H), 8.25(d, J=7.9Hz, 1H), 7.70(s, 1H), 7.53( dd,J=7.8,4.9Hz,1H),7.49(d,J=8.8Hz,2H),7.43(s,1H),7.33(d,J=8.6Hz,2H),7.26(d,J=8.6 Hz, 2H), 6.98(d, J=8.9Hz, 2H), 4.03(t, J=6.5Hz, 2H), 3.78(s, 3H), 3.57(t, J=5.5Hz, 2H), 3.19( t, J = 6.5Hz, 2H), 2.37 (t, J = 6.2Hz, 2H), 1.82 (dd, J = 12.4, 7.1Hz, 4H). 13 C NMR (125MHz, DMSO-d 6 )δ: 168.9, ...
Embodiment 2
[0055] Apixaban N-1 crystal form is prepared by the method disclosed in the patent US2006 / 0160841.
[0056] Add 0.182g of nicotinic acid and 0.68g of apixaban into 7ml of 0.5% water-containing trifluoroethanol, stir at 20°C for 24 hours, then add 15ml of acetone dropwise, stir at the same temperature for 30 minutes, filter to obtain white crystals, and place them in a drying oven for 40 °C and vacuum-dried for 4 hours to obtain 0.75 g of white crystals. The molar yield is 84%. Melting point: 191.0~197℃.
[0057] The obtained crystals were analyzed by X-ray powder diffraction spectrum, and the 2θ values of the characteristic absorption peaks were located at 5.7±0.2゜, 6.0±0.2゜, 11.5±0.2゜, 13.3±0.2゜, 15.4±0.2゜, 15.8±0.2゜, 17.5± 0.2°, 17.7±0.2°, 20.0±0.2°, 22.1±0.2°, 23.4±0.2°. and figure 1 The result is almost the same.
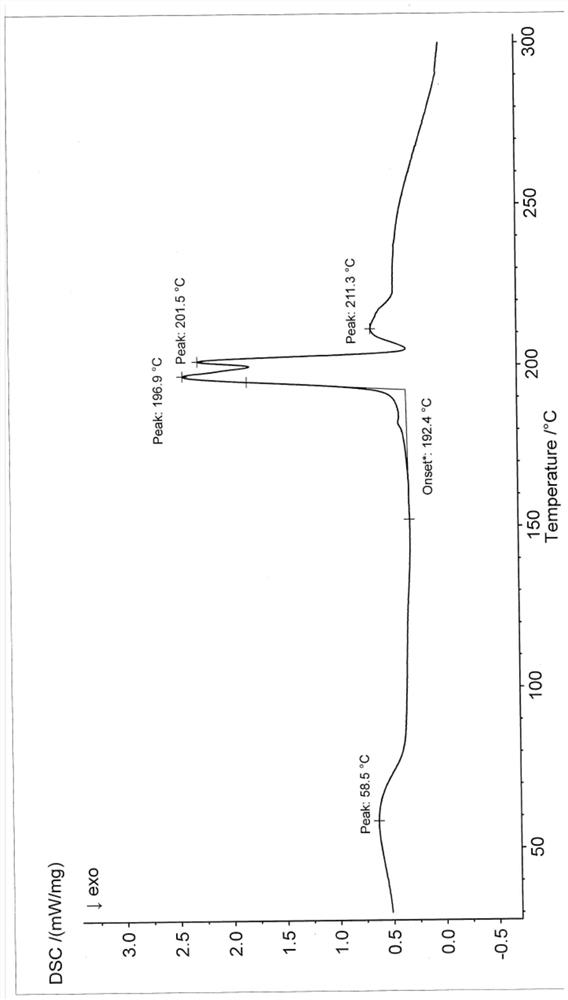
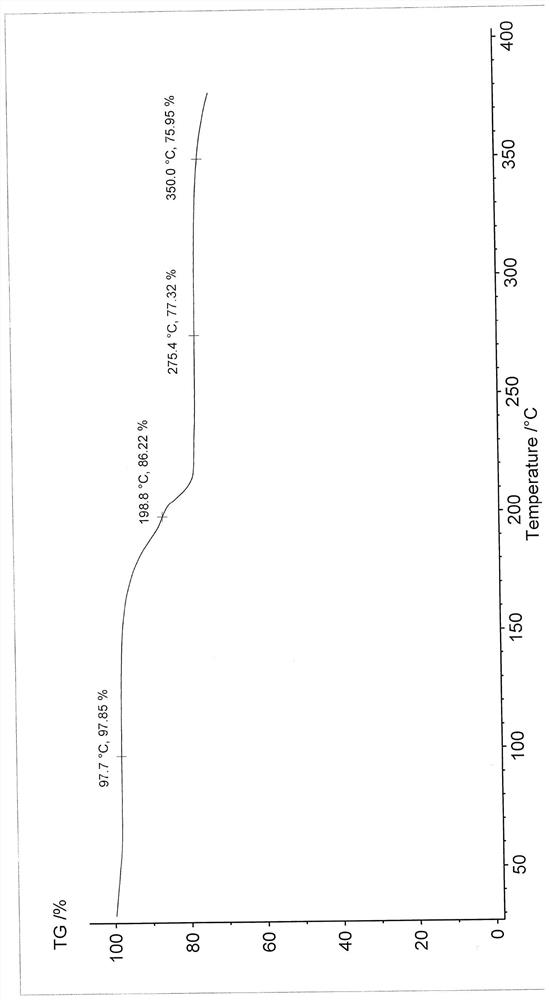
[0058] Differential scanning calorimetry (DSC) test analysis showed that the first endothermic peak was the loss of crystallization water peak at 60±2°C...
Embodiment 3
[0060] Apixaban N-1 crystal form is prepared by the method disclosed in Chinese patent ZL200580040778.4.
[0061] Add 0.182g of nicotinic acid and 0.68g of apixaban into 4ml of 0.5% water-containing trifluoroethanol, stir at 40°C for 24 hours, then add 6ml of acetone, stir at the same temperature for 2 hours, filter to obtain white crystals, and place in a drying oven Vacuum-dried at 40°C for 4 hours to obtain 0.79 g of white crystals. The molar yield is 89%. Melting point: 191.0~197℃.
[0062] The obtained crystals were analyzed by X-ray powder diffraction spectrum, and the 2θ values of the characteristic absorption peaks were located at 5.7±0.2゜, 6.0±0.2゜, 11.5±0.2゜, 13.3±0.2゜, 15.4±0.2゜, 15.8±0.2゜, 17.5± 0.2°, 17.7±0.2°, 20.0±0.2°, 22.1±0.2°, 23.4±0.2°. and figure 1 The result is almost the same.
[0063] Differential scanning calorimetry (DSC) test analysis showed that the first endothermic peak was the loss of crystallization water peak at 60±2°C. There is a char...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com