Emodin-berberine hydrochloride pharmaceutical co-crystal and preparation method thereof
A technology of berberine hydrochloride and emodin, applied in organic chemistry methods, organic chemistry, etc., to achieve the effects of simple and easy preparation methods, increased maximum concentration of drugs, and clear crystal structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] 0.742 g of berberine hydrochloride and 0.540 g of emodin were suspended in 20 mL of acetonitrile, and stirred for 48 hours to obtain a large amount of yellow precipitate, which was filtered with suction. The crystals were recrystallized from acetonitrile to obtain yellow crystals.
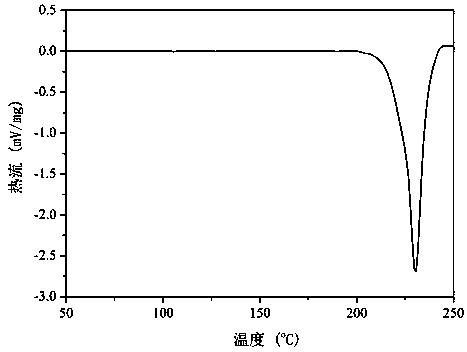
[0028] figure 1 This is the XRD pattern of an emodin-berberine hydrochloride drug co-crystal prepared in Example 1. Depend on figure 1 It can be seen that the prepared crystal has a diffraction angle of 2 θ ° ± 0.1 is expressed as: 8.3°, 9.1°, 10.0°, 10.4°, 12.0,°12.5°, 14.0°, 15.0°, 16.7°, 18.2°, 18.5°, 20.1°, 20.4°, 20.7°, 21.4° , 23.0°, 24.0°, 24.7°, 25.3°, 25.9°, 26.5°, 27.0°, 28.0°, 30.0°, 30.6°, 31.4°, 32.2° have characteristic diffraction peaks.
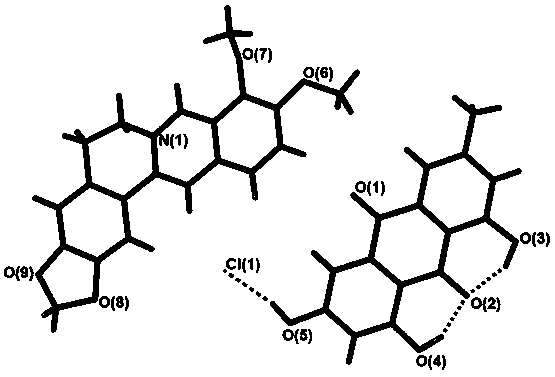
[0029] figure 2 It is the crystal structure unit of an emodin-berberine hydrochloride drug co-crystal prepared in Example 1. Depend on figure 2 It can be known that the prepared drug co-crystal includes berberine cation, chloride...
Embodiment 2
[0033] Pharmacokinetic parameters of emodin-berberine hydrochloride co-crystal in rats:
[0034] The SD male rats weighing 190-210 g were raised in conventional feeding conditions, drinking water freely, and after fasting for 12 hours, press
[0035] 40 mg / kg (emodin dose) was administered by gavage, before and after administration at 0.10, 0.17, 0.35, 0.53, 0.68, 0.87, 1, 1.5, 2, 4, 6, 8, 12, 24, About 0.5 ml of blood was collected from the retrobulbar venous plexus at 36 h, and centrifuged at 4000 rpm for 10 min. Take 200 μl of plasma, add 400 μl of methanol, vortex for 2 min, centrifuge at 10,000 rpm for 10 min, take the supernatant and blow dry with nitrogen. 100 μl of mobile phase (methanol:water=50:50) was added, vortexed for 1 min, centrifuged at 15,000 rpm for 1 min, and 40 μl of the supernatant was taken for HPLC-MS detection. The HPLC-MS detection system is Aligent 1260 LC-6410 MS high performance liquid chromatography system, and the chromatographic column is Ultima...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com