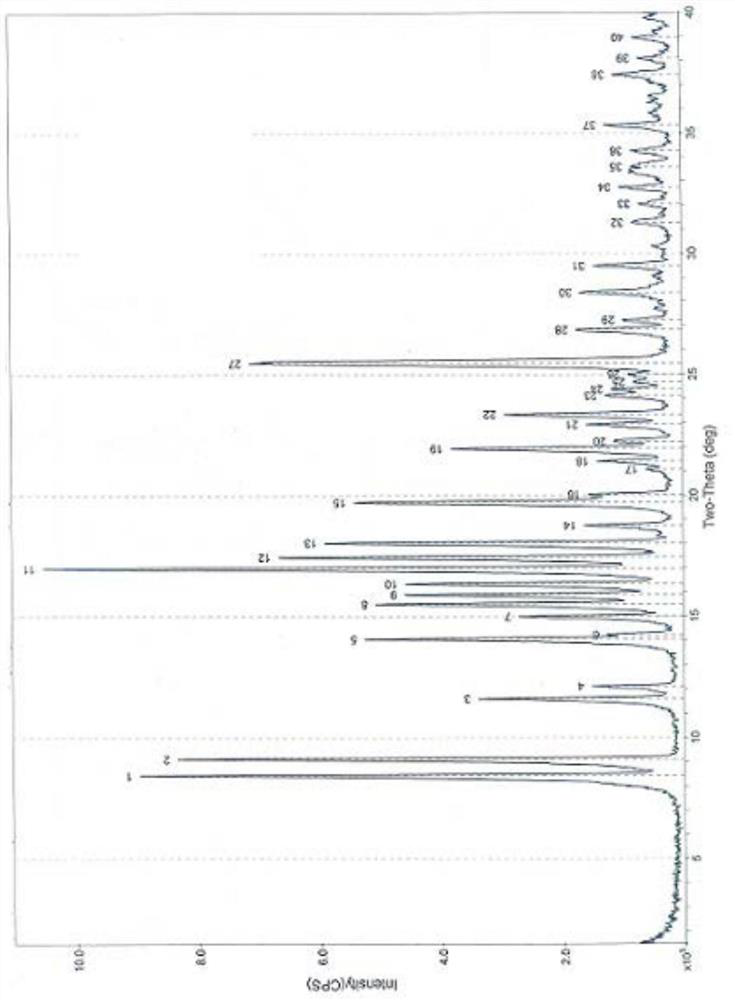
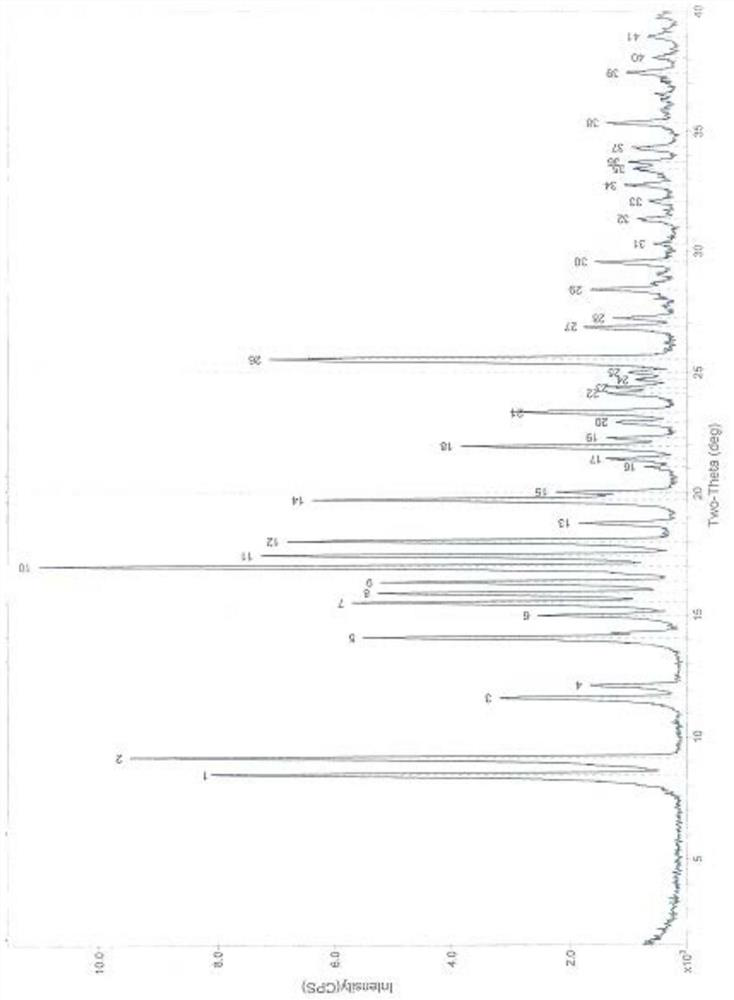
A new crystal form of sofosbuvir and its preparation method
A technology of febuvir crystal form and crystal form, which is applied in the field of medicine, can solve the problems of unstable crystal form A, poor stability of crystal form A, poor fluidity, etc., and achieve excellent stability, good properties, good fluidity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0055] Preparation Example 1: Preparation of Sofosbuvir Form 1 (refer to CN102858790A)
[0056] 2kg of sofosbuvir (prepared with reference to the disclosed method of CN102858790A, the same below) was dissolved in 10L of dichloromethane, then the solution was evaporated at 5°C, and after drying, 1.8kg of sofosbuvir in form 1 was obtained, with an HPLC purity of 99.4%.
preparation example 2
[0057] Preparation Example 2: Preparation of Sofosbuvir Form 6 (Refer to CN102858790A)
[0058] 500 g of sofosbuvir Form 1 was added to 20 L of deionized water, heated to 50 °C, and stirred vigorously for 1 h, during which time solids began to precipitate out of solution, forming a thin slurry. The slurry was cooled to 20 °C over 1.5 h and held at 20 °C for 16 h, then further cooled to 0-5 °C over 0.5 h and held at 0-5 °C for 2.5 h. The slurry was filtered on a medium porosity glass fritted funnel and washed with 5 L of ice-cold water. The wet cake was suction dried for 2 h, then vacuum dried at 50 °C for 23 h, and 440 g of Form 6 was isolated with an HPLC purity of 99.5%.
preparation example 3
[0059] Preparation Example 3: Preparation of Sofosbuvir Form A (refer to CN104130302A)
[0060] Weigh 570g of sofosbuvir, add 5L of acetone to dissolve, add 30L of n-pentane, and then leave it at room temperature for 16 hours in a closed room, and solids precipitate out. After filtration, the obtained solid was vacuum-dried at room temperature for 24 hours to obtain 500 g of solid, which was crystal form A, and the HPLC purity was 99.3%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| weight ratio | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com