A crystal form of a spirocyclic dihydroisoquinoline carboxamide derivative and a preparation method thereof
A crystal form and drug technology, applied in the crystal form of spirocyclic dihydroisoquinoline carboxamide derivatives and its preparation field, can solve the problems of poor product stability, poor fluidity, fine crystallization, etc., and achieve stable production process , HPLC purity change is small, the effect of high crystal form purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] N-(4-(ethylsulfonyl)benzyl)-2'-(4-(trifluoromethyl)phenyl)-2',3'-dihydro-1'H-spiro[cyclopropane-1, Preparation of 4'-isoquinoline]-7'-carboxamide
[0060]
[0061] first step
[0062] 1-(7'-Bromo-1'H-spiro[cyclopropane-1,4'-isoquinoline]-2'(3'H)-yl)-2,2,2-trifluoroethanone 1b
[0063] N-((1-(4-bromophenyl)cyclopropyl)methyl)-2,2,2-trifluoroacetamide 1a (22g, 68.3mmol, prepared by the method disclosed in the patent application "WO2011124093" (obtained) was dissolved in 150 mL of pre-made mixed solvent of acetic acid and sulfuric acid (V / V=2:3), added paraformaldehyde (7.96 g, 264.99 mmol), and stirred for 12 hours. The reaction solution was poured into 500mL ice water, extracted with ethyl acetate (500mL×2), the organic phases were combined, washed with water, saturated sodium bicarbonate solution and saturated sodium chloride solution successively, dried over anhydrous sodium sulfate, filtered, and the filtrate was decompressed Concentration gave the crude title c...
Embodiment 2
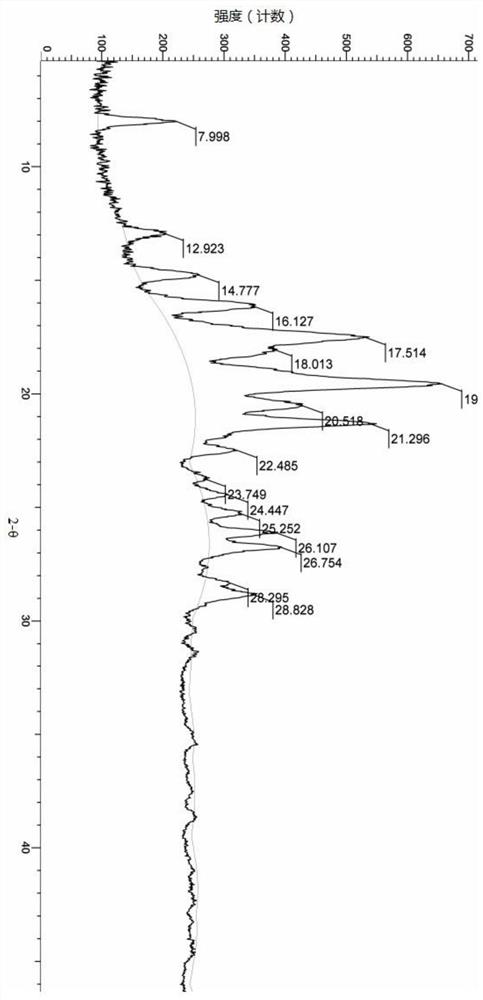
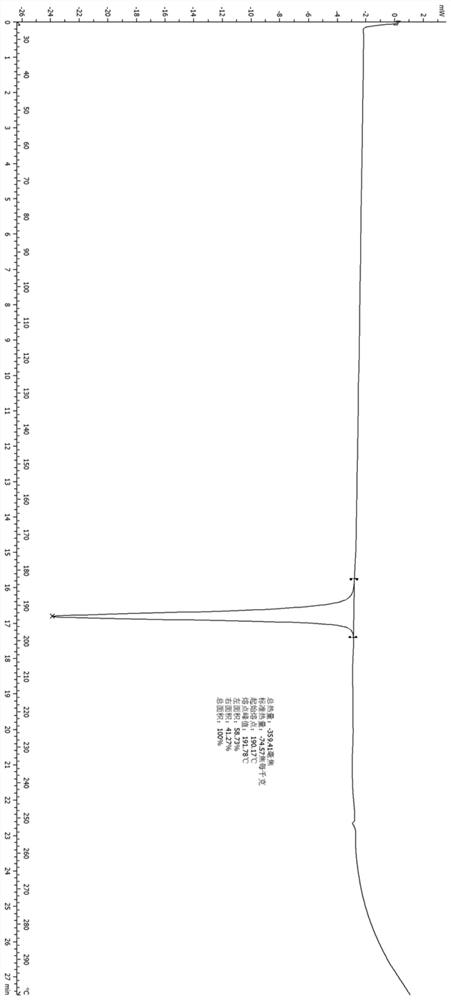
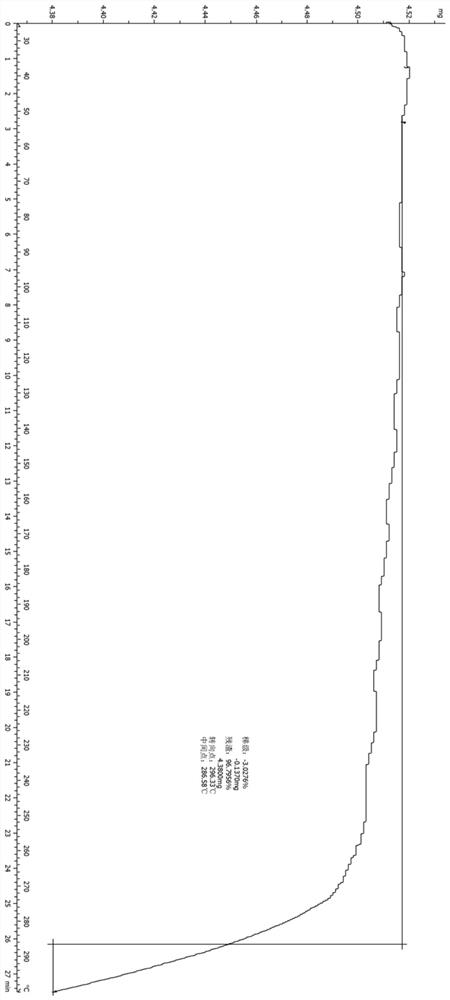
[0082] The compound represented by formula (I) (100 mg, 0.2 mmol) was added into 5 mL of ethyl acetate, heated to reflux, stirred to dissolve, and slowly cooled to room temperature. The reaction solution was filtered, the filter cake was collected, and vacuum-dried to obtain the A crystal form of the compound represented by formula (I). Its X-ray diffraction pattern is shown in figure 1 , whose DSC spectrum is shown in figure 2 , see the TGA spectrum image 3 , and its characteristic peak positions are shown in the table below:
[0083]
Embodiment 3
[0085] The compound represented by formula (I) (100 mg, 0.2 mmol) was added into 5 mL of methanol, heated to reflux, stirred to dissolve, and slowly cooled to room temperature. The reaction solution was filtered, the filter cake was collected, and vacuum-dried to obtain the A crystal form of the compound represented by the formula (I).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com