Crystal form of macrocyclic compound and preparation method and application thereof
A technology of compounds and macrocycles, which is applied in the field of drug synthesis, can solve problems such as inability of drugs to function, affect clinical efficacy and safety, and differences in stability, and achieve good chemical stability, good physical stability, and good drug stability. Effects of Kinetic Properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] A method for preparing a crystal form of a macrocyclic compound, comprising the following steps:
[0053] 1 mg of the macrocyclic compound (6R,16R)-9-fluoro-16-methyl-13-oxa-2,17,21,25-tetraazaolane[16.6 .2.0 2 , 6 .0 7 , 12 .0 22 , 26 ] Hexacane-1(25), 7,9,11,18(26), 19,21,23-octane-19-carbonitrile was added into 1.0mL methanol, stirred at room temperature (25±5°C) to dissolve After clearing, slowly add 0.8 mL of water dropwise, after the solid precipitates, continue to stir for a period of time, filter, and dry the filter residue to obtain a crystalline powder.
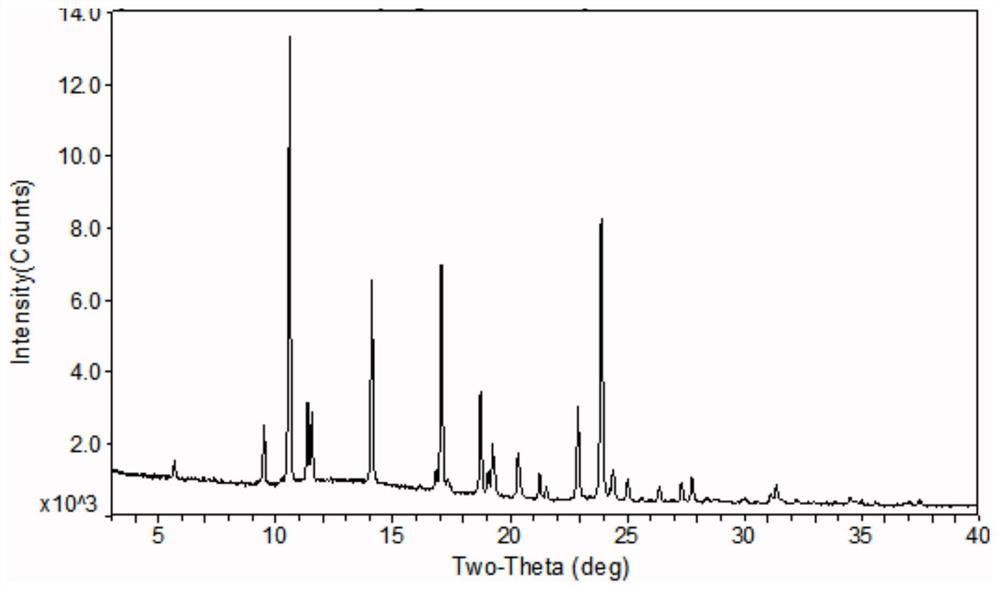
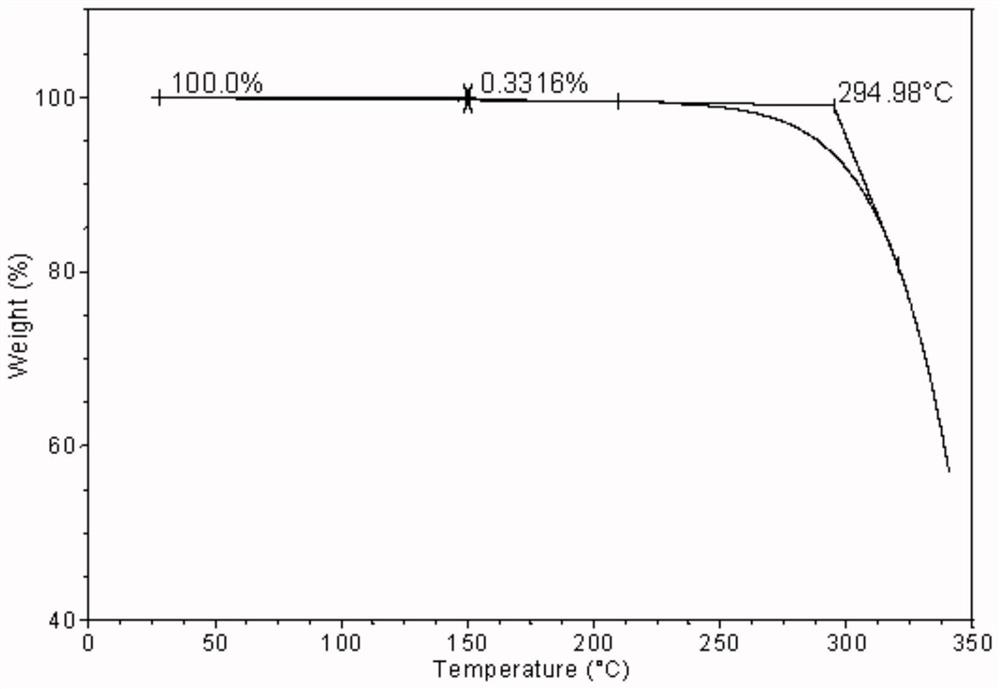
[0054] The obtained crystalline powder was subjected to X-ray powder diffraction, differential scanning calorimetry and thermogravimetric analysis. After the crystalline samples were characterized, they were named crystal forms. Its PXRD spectrum (X-ray powder diffraction spectrum) see figure 1 , figure 1 It shows that the 2θ values of the samples are 5.69, 9.49, 10.60, 11.54, 14.10, 16.11, 17.09,...
Embodiment 2
[0056] A method for preparing a crystal form of a macrocyclic compound, comprising the following steps:
[0057] 15 mg of (6R,16R)-9-fluoro-16-methyl-13-oxa-2,17,21,25-tetraazolane[16.6.2.0 2 , 6 .0 7 , 12 .0 22 , 26 ] Hexacane-1(25), 7,9,11,18(26), 19,21,23-octane-19-carbonitrile was added to 0.4mL methanol, stirred at room temperature to dissolve, then slowly added the solution In 0.8mL of water, after the solid precipitated, continue to stir for a period of time, filter and dry to obtain light yellow crystalline powder. After comparing the PXRD spectrum and DSC spectrum of the crystalline sample, it was determined to be the same crystal form as in Example 1.
Embodiment 3
[0059] A method for preparing a crystal form of a macrocyclic compound, comprising the following steps:
[0060] 15 mg of (6R,16R)-9-fluoro-16-methyl-13-oxa-2,17,21,25-tetraazolane[16.6.2.0 2, 6 .0 7 , 12 .0 22,26 ] Hexacane-1(25), 7,9,11,18(26), 19,21,23-octane-19-carbonitrile was added into 0.2mL of dichloromethane, stirred at room temperature to dissolve, then slowly drop Add 3.6 mL of n-heptane, after the solid precipitates, continue stirring for a period of time, filter and dry to obtain a crystalline powder. After comparing the PXRD spectrum and DSC spectrum of the crystalline sample, it was determined to be the same crystal form as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com