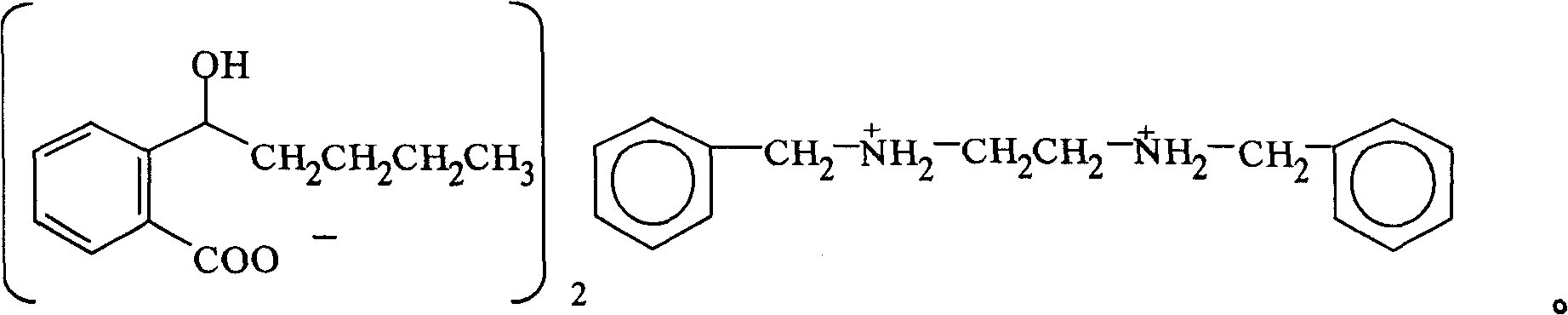Novel 2-(alpha-hydroxyl amyl) and its preparing method and use
A technology of hydroxypentyl and benzoic acid, which is applied in the field of application, can solve the problems of undisclosed preparation methods of zinc salt diethylamine salt, etc., and achieve excellent protective effect, good preventive and therapeutic effect, and low toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1: Preparation of 2-(α-hydroxypentyl)benzoic acid N,N'-dibenzylethylenediamine salt.
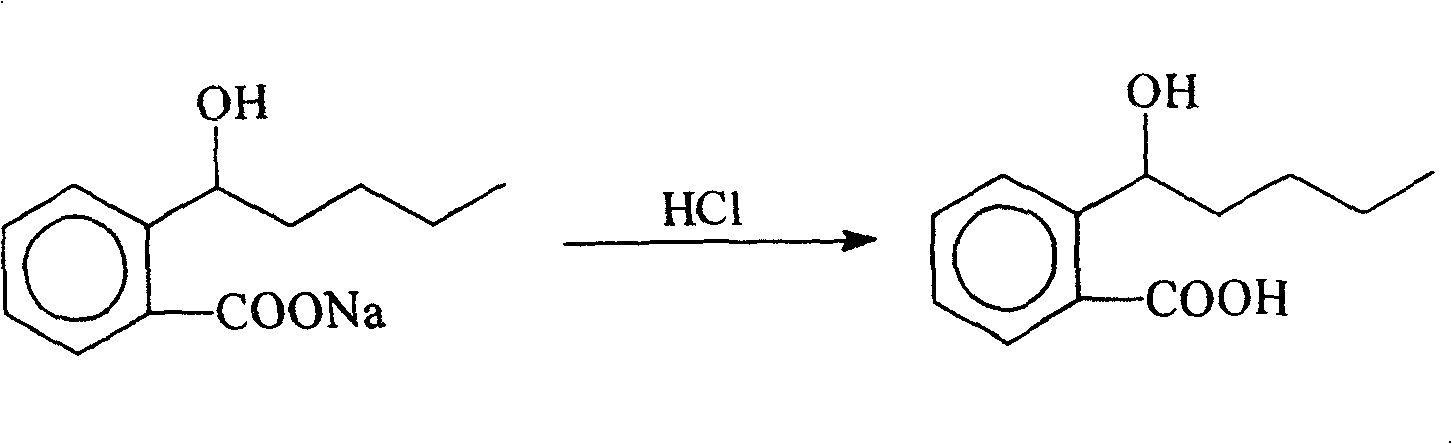
[0031] 1, the preparation of 2-(alpha-hydroxy n-pentyl) benzoic acid
[0032]
[0033] operate:
[0034] Add 80g (0.348mol) of 2-(α-hydroxypentyl)sodium benzoate (prepared according to CN1382682A) and 500ml of water into a 2.5L beaker, stir and dissolve, cool to -10°C, and add cold 5% sodium benzoate in batches Hydrochloric acid, control the internal temperature at 0 to -10°C, adjust the pH to 2 to 3, extract with 500ml×3 ether, wash the ether layer with 300ml×3 water until neutral, dry the ether layer, filter off the desiccant, and depressurize Diethyl ether was removed to obtain 64 g of white solid.
[0035] 2. Preparation of 2-(α-hydroxy n-pentyl)benzoic acid N,N'-dibenzylethylenediamine salt
[0036]
[0037] operate
[0038] (1) Add 37.2 g (0.155 mol) of N, N'-dibenzylethylenediamine and 1.0 L of diethyl ether into the reaction flask, and after fully dissolving ...
Embodiment 2
[0046] Embodiment 2: Appearance character and melting point contrast of various 2-(alpha-hydroxypentyl) benzoates
[0047] Table 1 is a comparison of appearance properties and melting points of various 2-(α-hydroxypentyl)benzoates.
[0048]
[0049] * : Prepared by CN1382682A. ** : Prepared by the calcium salt method in CN1382682A *** : Prepared according to the method of Example 1
[0050] The results showed that lithium salts, sodium salts, magnesium salts, and zinc salts were all foamy or colloidal solids without obvious melting points. Morpholine and diethylamine salts are viscous liquids. None of them meet the requirements of the pharmaceutical industry. Potassium salt, calcium salt, benzylamine salt, N,N'-dibenzylethylenediamine salt, tert-butylamine salt have good physical appearance and fixed melting point. Aniline does not react at all.
Embodiment 3
[0051] Embodiment 3: Wet Stability Comparison
[0052] According to Chinese Pharmacopoeia 2000 edition two appendix XIX C " drug stability guideline " item " high humidity test method " described method test, time is 10 days. The results are shown in Table 2.
[0053] Table Dipotassium salt, calcium salt, benzylamine salt, N, N'-dibenzylethylenediamine salt, tert-butylamine salt wet stability comparison
[0054]
[0055] * The determination of the decomposition product adopts HPLC method, according to Chinese Pharmacopoeia 2000 edition two appendix VD test, C18 column, mobile phase acetonitrile:0.02M sodium dihydrogen phosphate=40:60, detection wavelength 230nm, flow rate 1ml / min.
[0056] The experimental results show that the hygroscopicity of potassium salt and benzylamine salt is more serious, and the decomposition is serious after moisture absorption, while N, N'-dibenzylethylenediamine salt and calcium salt are less hygroscopic and stable. Tert-butylamine salt has ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com