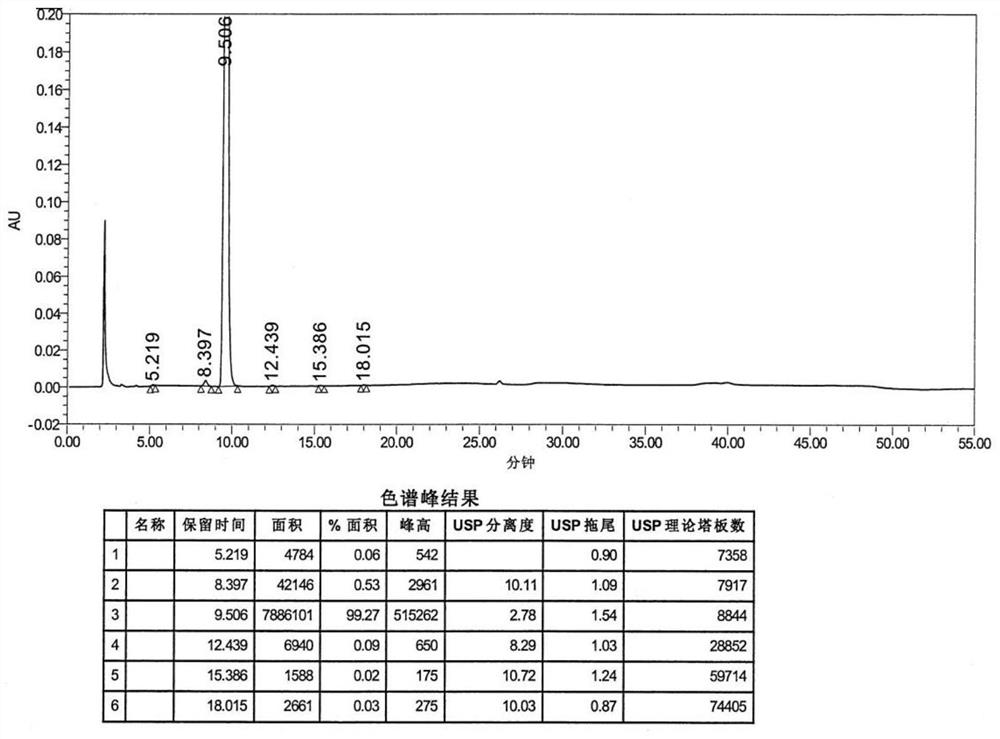
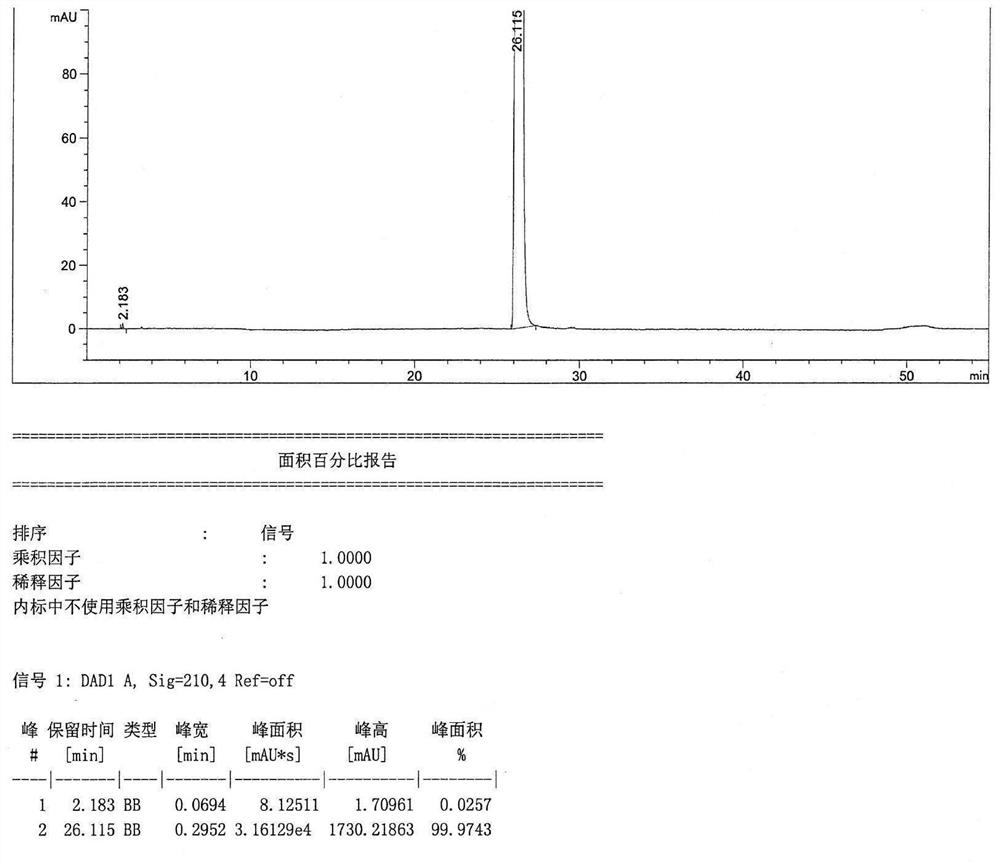
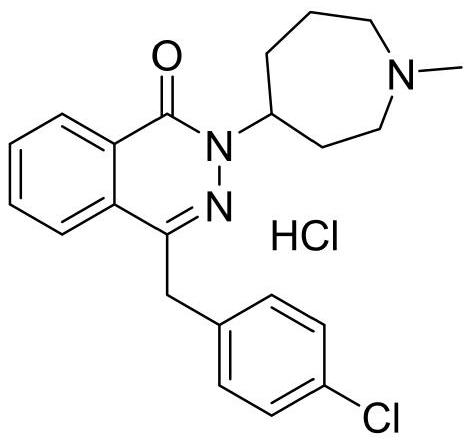
Preparation method of azelastine hydrochloride
A technology of azelastine hydrochloride and hydrochloric acid, which is applied in the field of synthesis of raw materials, can solve the problems of unqualified raw material content, loss on drying, difficult to reach pharmaceutical standards, poor stability of raw materials, etc., and the product quality is easier to control and reduce The effect of quality risk and stability improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] A preparation method of azelastine hydrochloride, the steps are as follows:
[0041] 1) Put 1.7g of potassium hydroxide and 50mL of drinking water into the reaction flask at room temperature, stir until it dissolves, then add 3.9g of 1-methylhexahydroazepin-4-one and 4.2g of benzohydrazide, 25~ React at 35°C for 1 hour. After the reaction is complete, cool down to 0-10°C. Add 3g of sodium borohydride in batches. After the addition, keep the temperature at 0-10°C for 2 hours. Potassium carbonate solution to adjust the pH to 10-11, add 50mL of dichloromethane, separate the liquid to take the organic phase, extract the water phase with 50mL of dichloromethane once, combine the organic phase, wash twice with 50mL*2 drinking water, and then use 50mL After washing once with saturated sodium chloride solution, the organic phase was dried over anhydrous sodium sulfate, filtered with suction, and the filtrate was concentrated to dryness under reduced pressure to obtain 7.4 g of ...
Embodiment 2
[0046] A preparation method of azelastine hydrochloride, the steps are as follows:
[0047] 1) Put 1.7g of potassium hydroxide and 50mL of drinking water into the reaction bottle at room temperature, stir until it dissolves, then add 3.9g of 1-methylhexahydroazepine-4-one and 4.2g of benzohydrazide, 25 React at ~35°C for 1 hour. After the reaction is complete, cool down to 0-10°C. Add 3g of sodium borohydride in batches. After the addition, keep the temperature at 0-10°C for 2 hours. After the reaction is complete, add hydrochloric acid to quench the excess sodium borohydride, and then add 50 % potassium carbonate solution to adjust the pH to 10-11, add 50mL of dichloromethane, separate the liquid to take the organic phase, extract the water phase with 50mL of dichloromethane once, combine the organic phase, wash twice with 50mL*2 drinking water, and then use 50 mL of saturated sodium chloride solution was washed once, the organic phase was dried over anhydrous sodium sulfate,...
Embodiment 3
[0052] A preparation method of azelastine hydrochloride, the steps are as follows:
[0053] 1) Put 1.7g of potassium hydroxide and 50mL of drinking water into the reaction bottle at room temperature, stir until dissolved, then add 3.9g of 1-methylhexahydroazepine-4-one and 4.2g of benzohydrazide, 25 React at ~35°C for 1 hour. After the reaction is complete, cool down to 0-10°C. Add 3g of sodium borohydride in batches. After the addition, keep the temperature at 0-10°C for 2 hours. After the reaction is complete, add hydrochloric acid to quench the excess sodium borohydride, and then add 50 % potassium carbonate solution to adjust the pH to 10-11, add 50mL of dichloromethane, separate the liquid to take the organic phase, extract the water phase with 50mL of dichloromethane once, combine the organic phase, wash twice with 50mL*2 drinking water, and then use 50 mL of saturated sodium chloride solution was washed once, the organic phase was dried over anhydrous sodium sulfate, fi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com