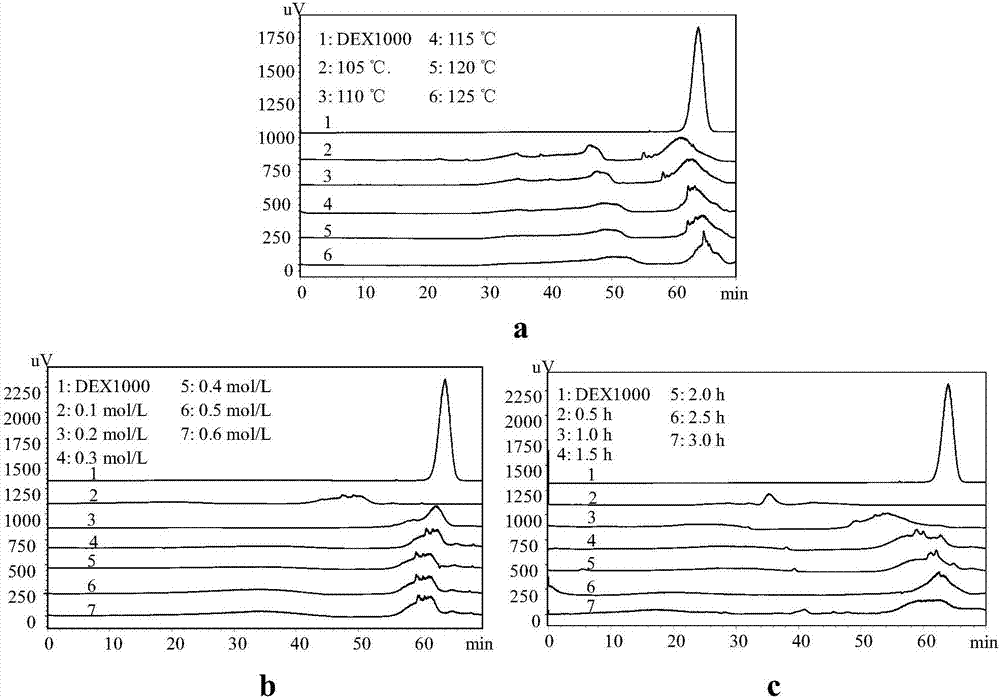
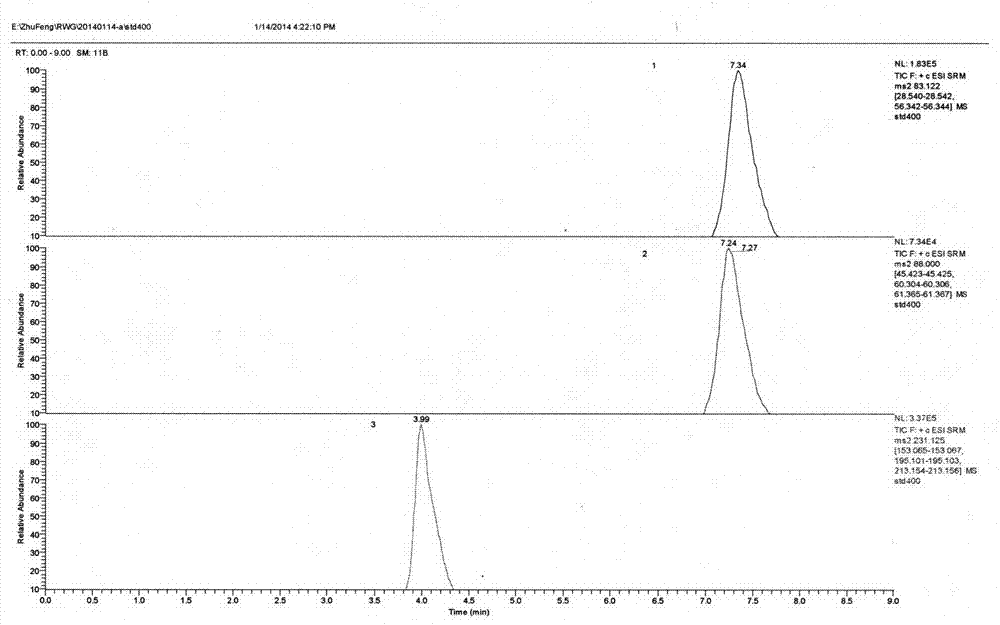
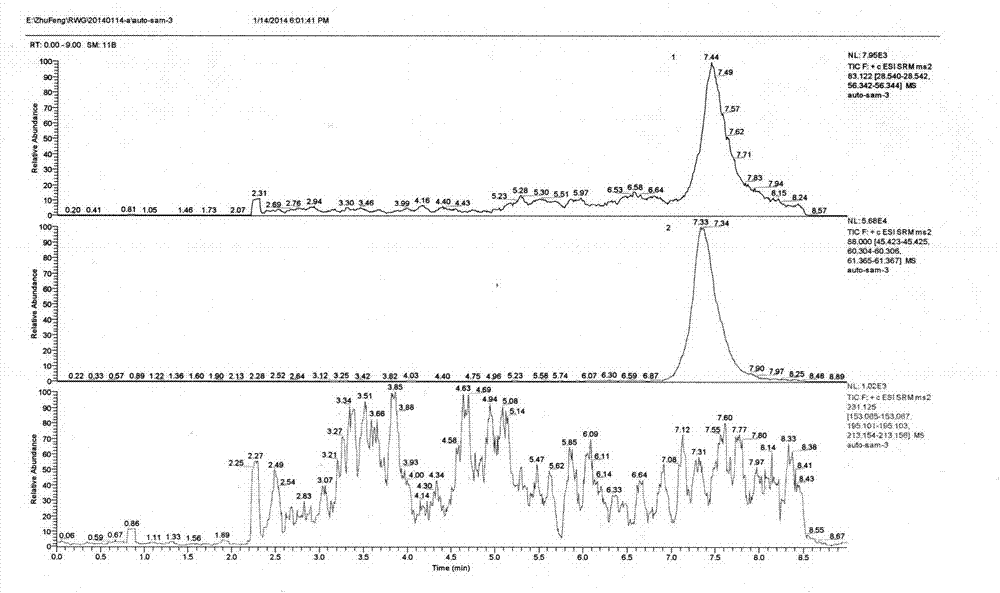
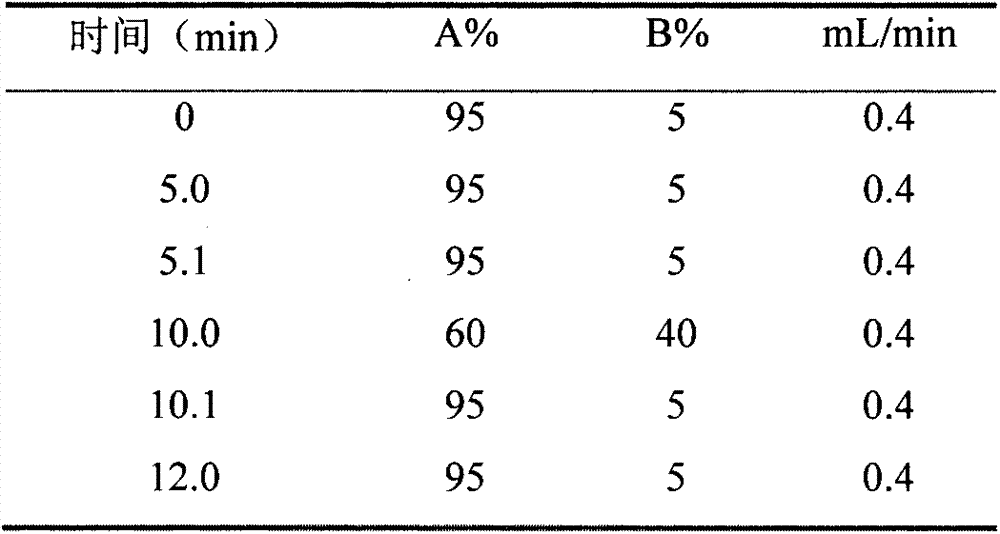
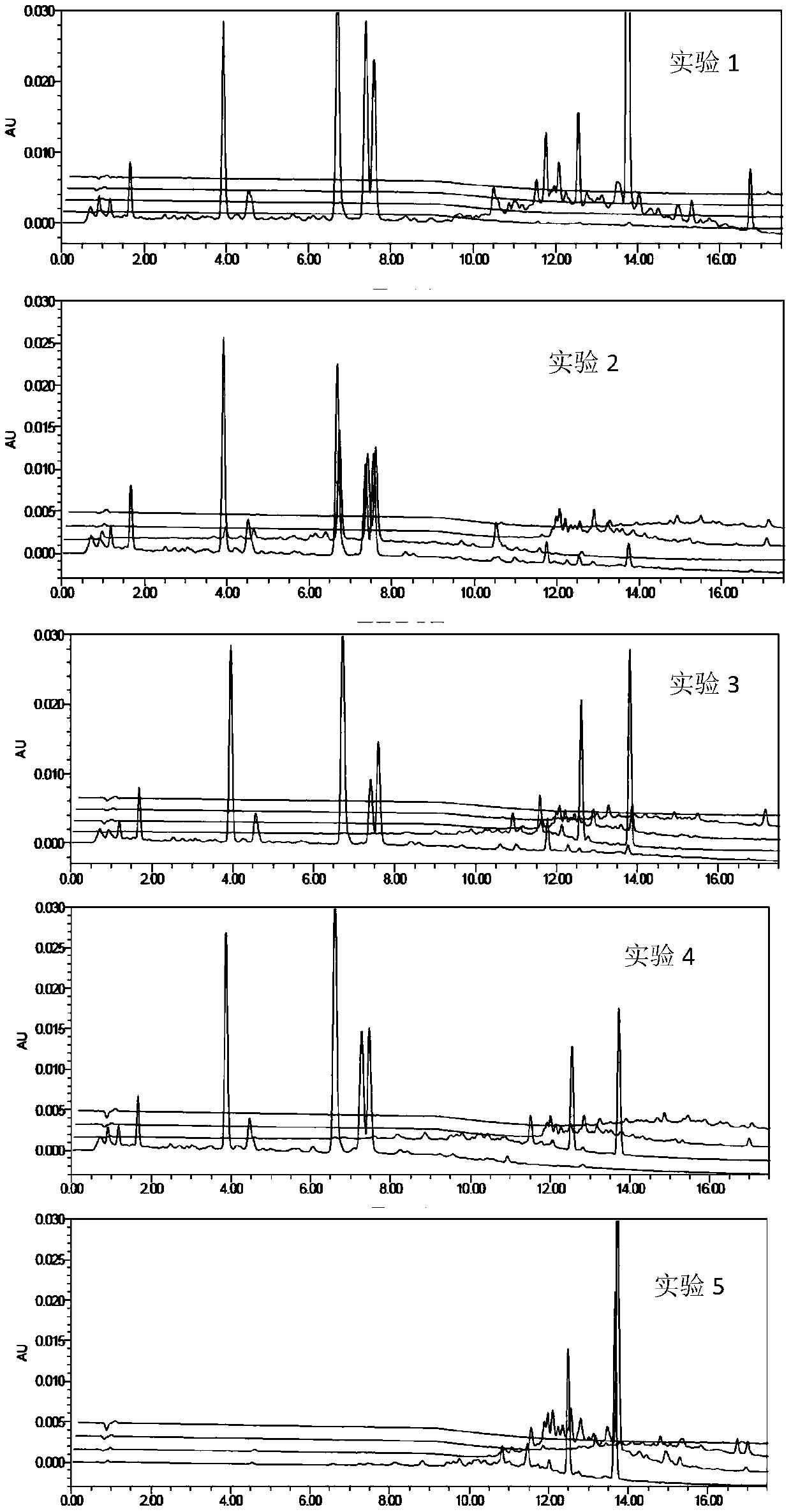
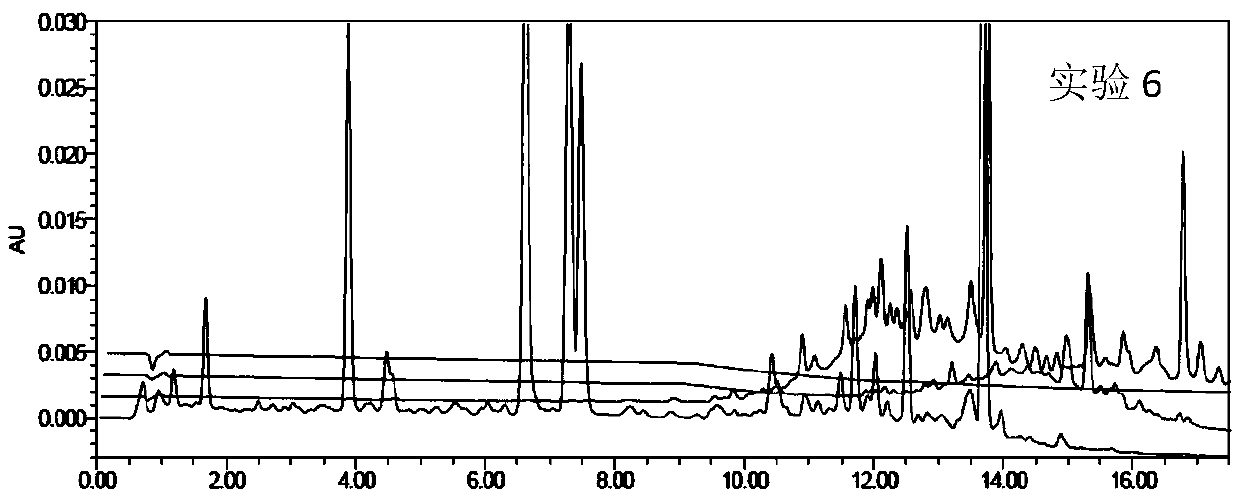
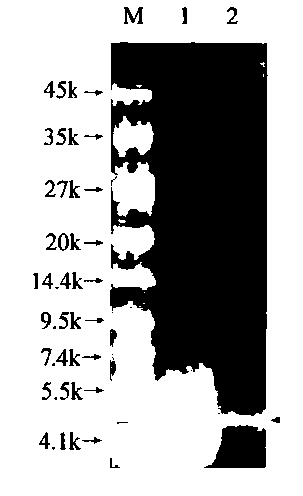
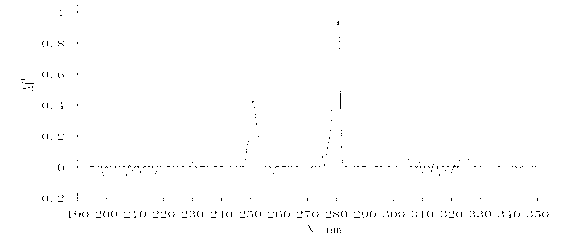Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
38results about How to "Effective separation and purification" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
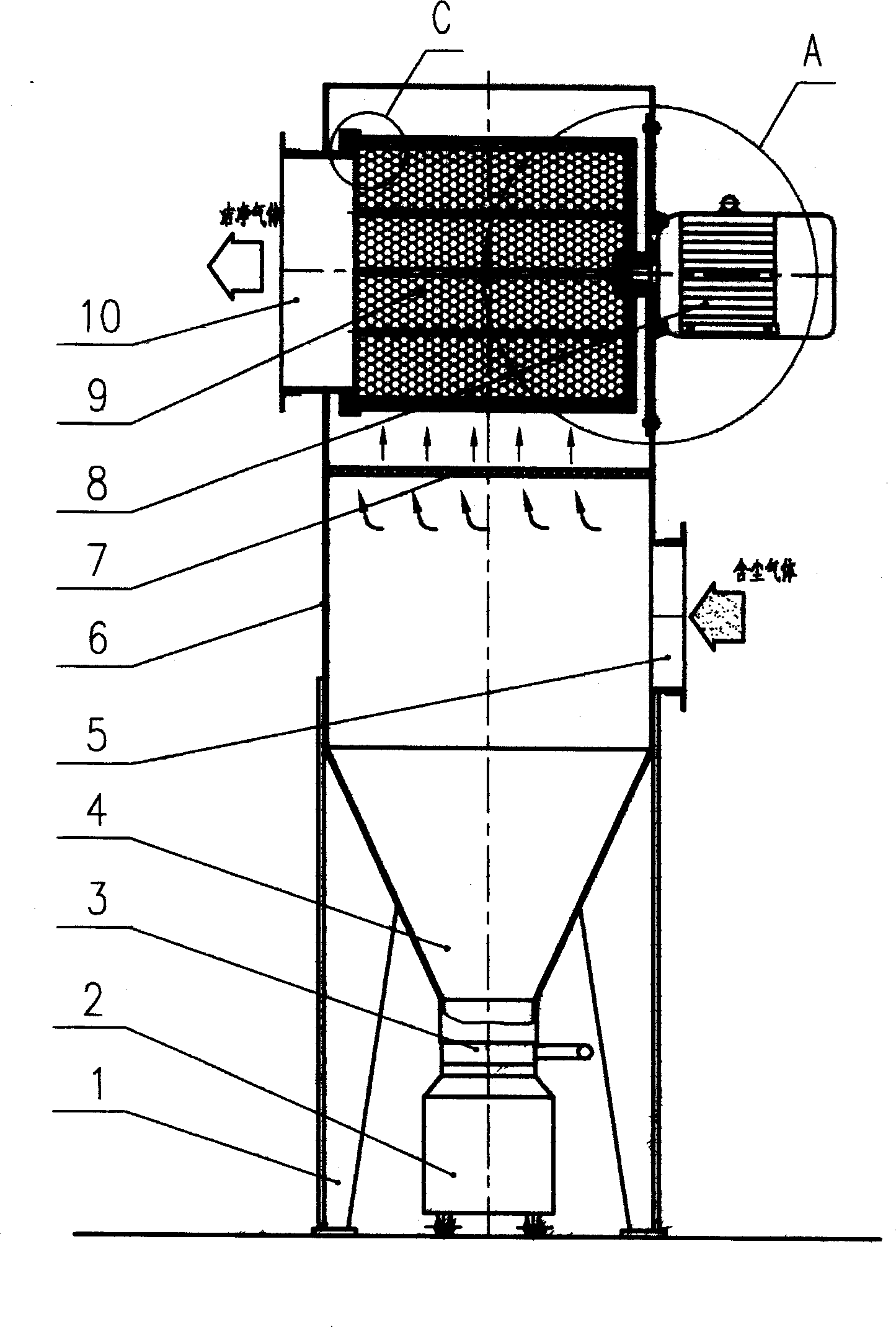
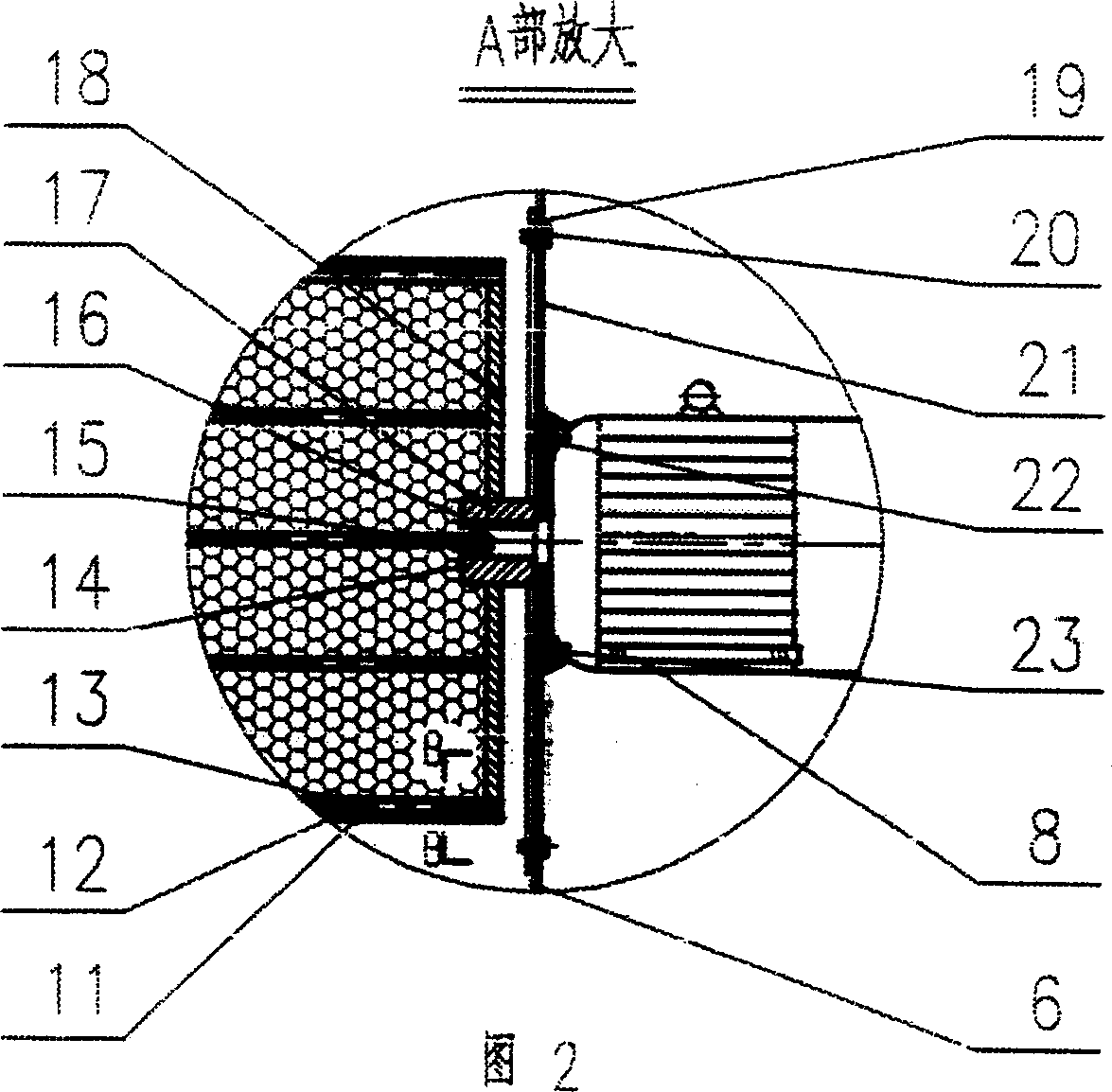
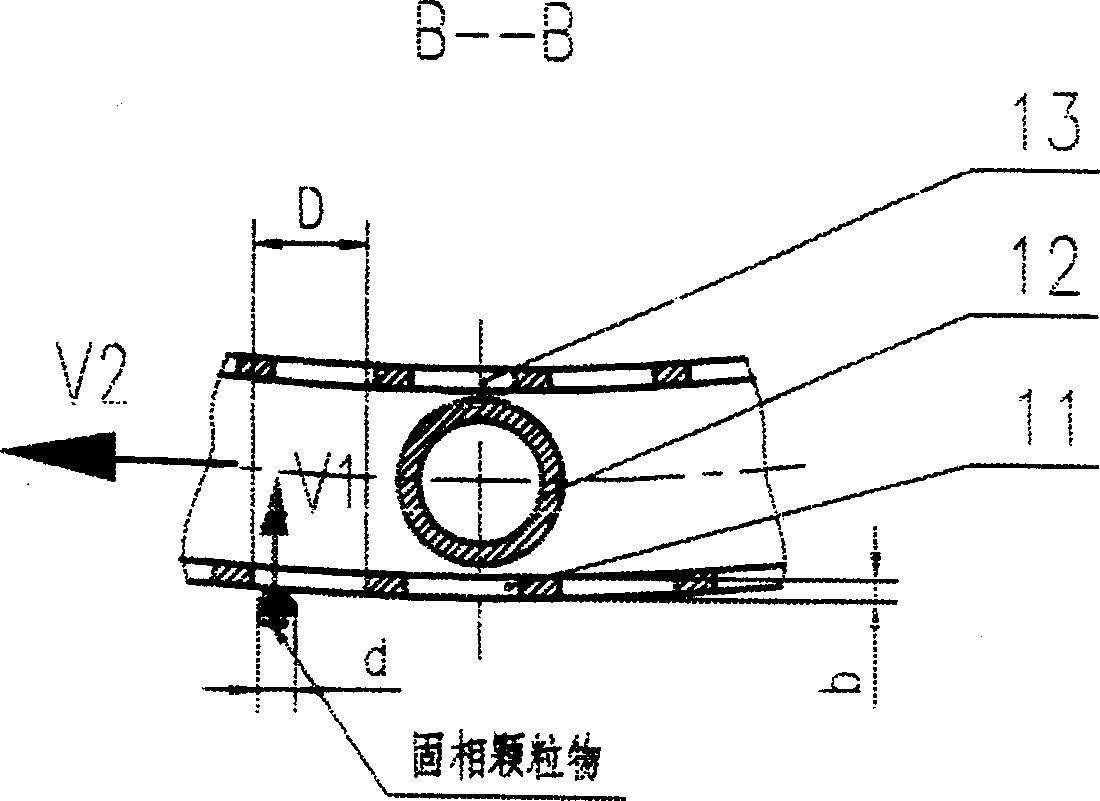
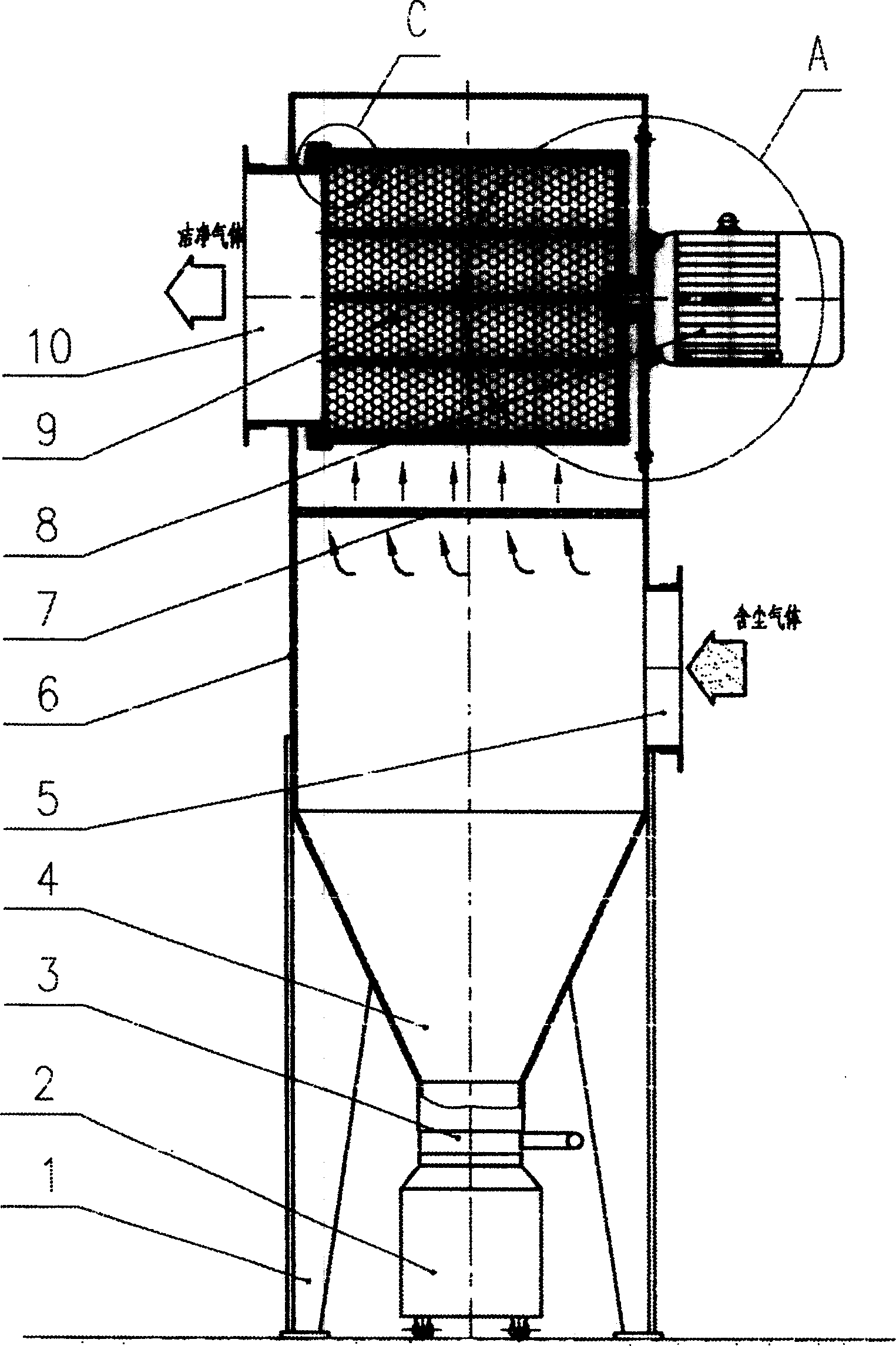
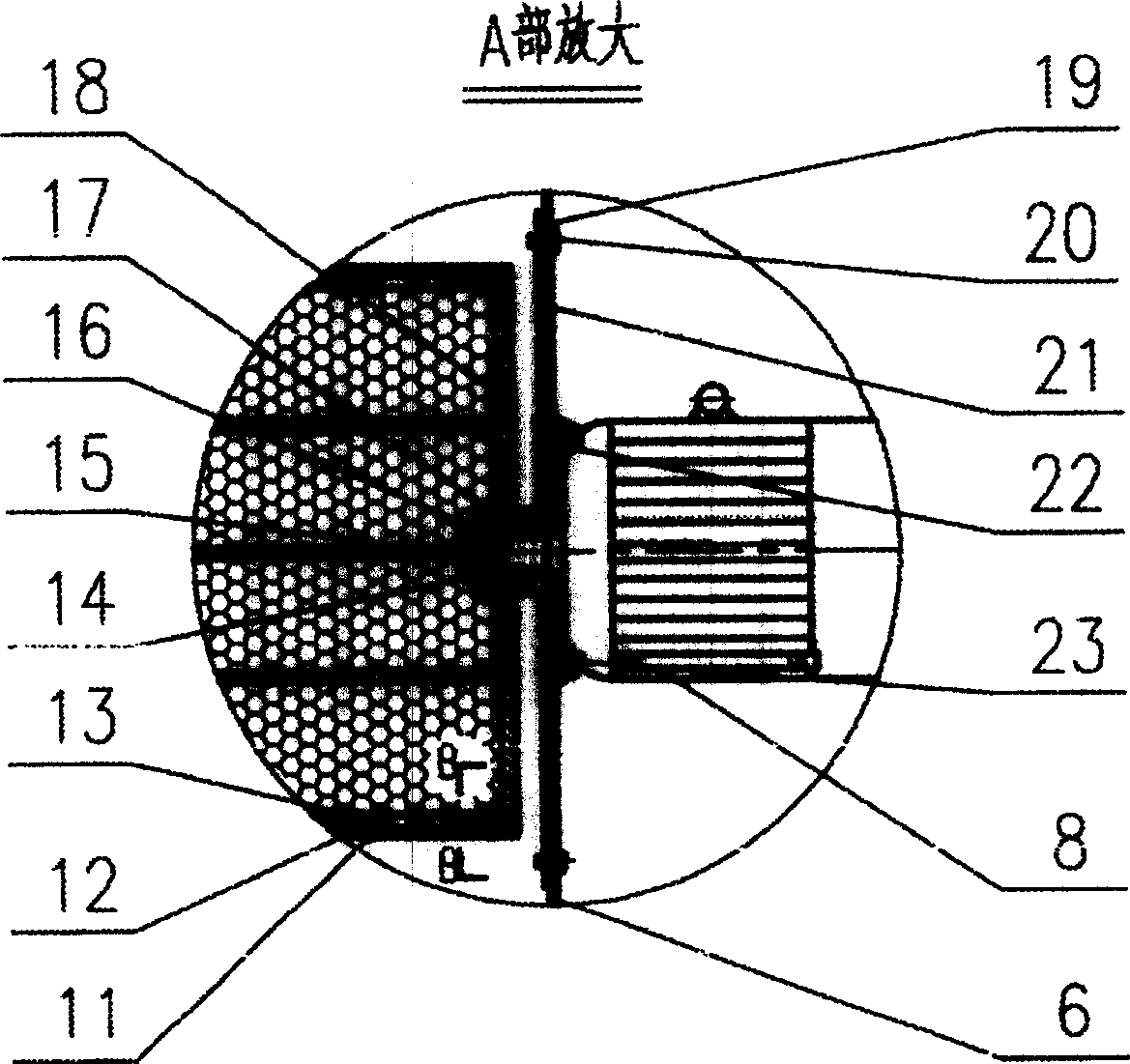
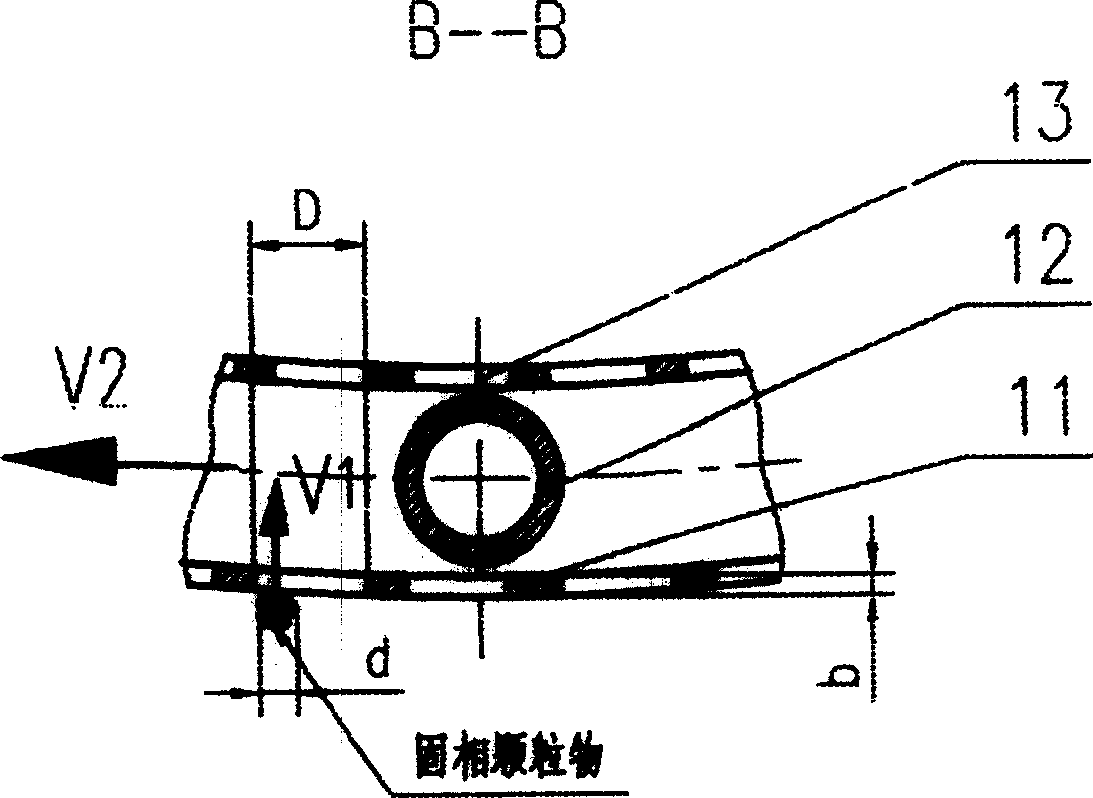
Filtration type gas separation and purification equipment with rapid moving filtering layer
InactiveCN1480239AEfficient separationEffective separation and purificationDispersed particle filtrationFiltrationProduct gas
A filter-type gas separating-cleaning apparatus with quickly moving filter layer features that its filter cylinder rotating at high speed is composed of external filter net, supporting posts, internal filter net, shaft sleeve, shaft disk and supporting rings, and the gas containing solid particles and / or liquid drops is filtered by said filter cylinder. Its advantage is use of big-mesh filter material to remove fine solid particles and / or liquid drops, resulting in high filter speed, and easy cleaning.
Owner:暴辰生 +1
Production method and use of ergosterol
InactiveCN105884850AGood chromatographyClear resultOrganic active ingredientsAntinoxious agentsDissolutionSilica gel
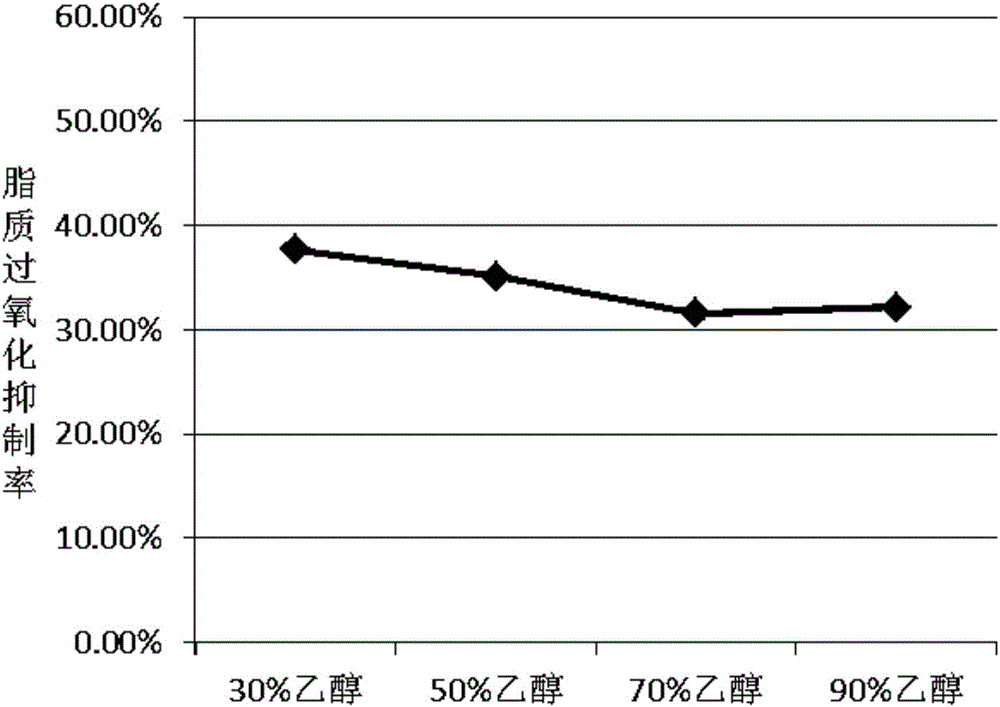
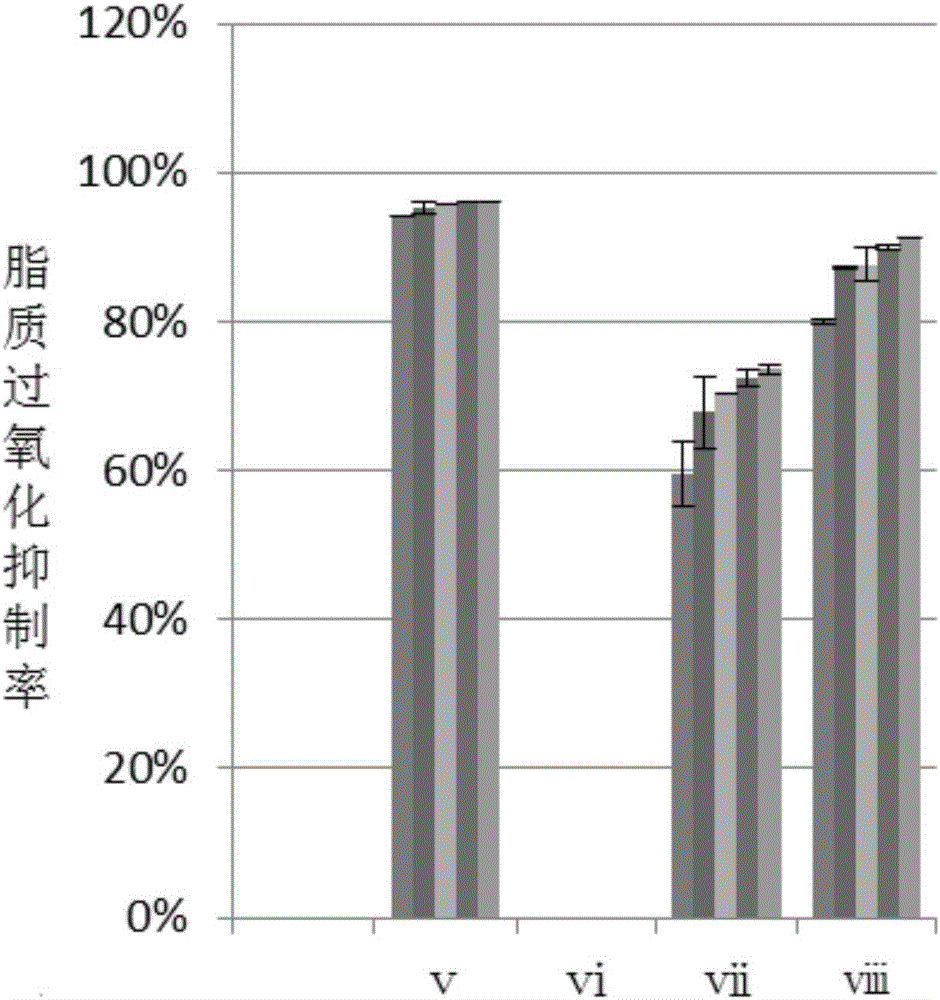
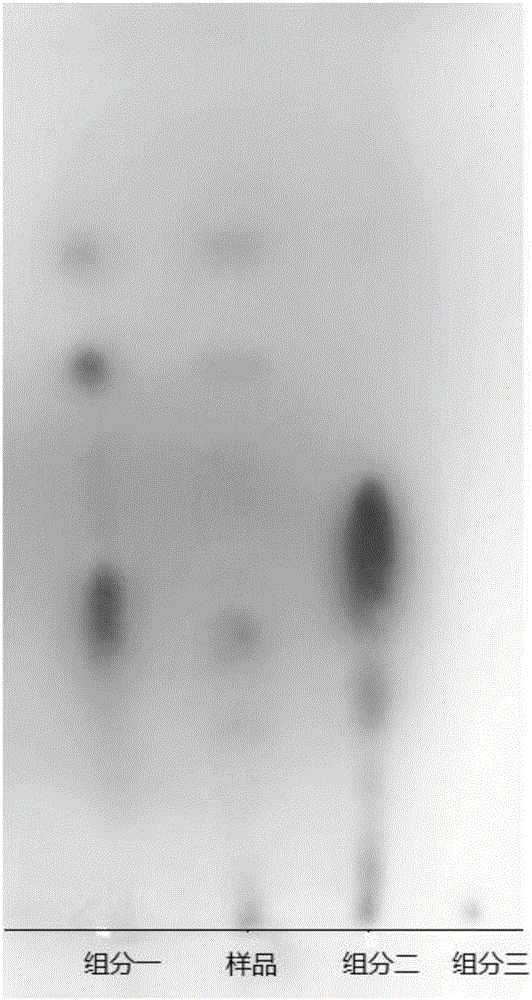
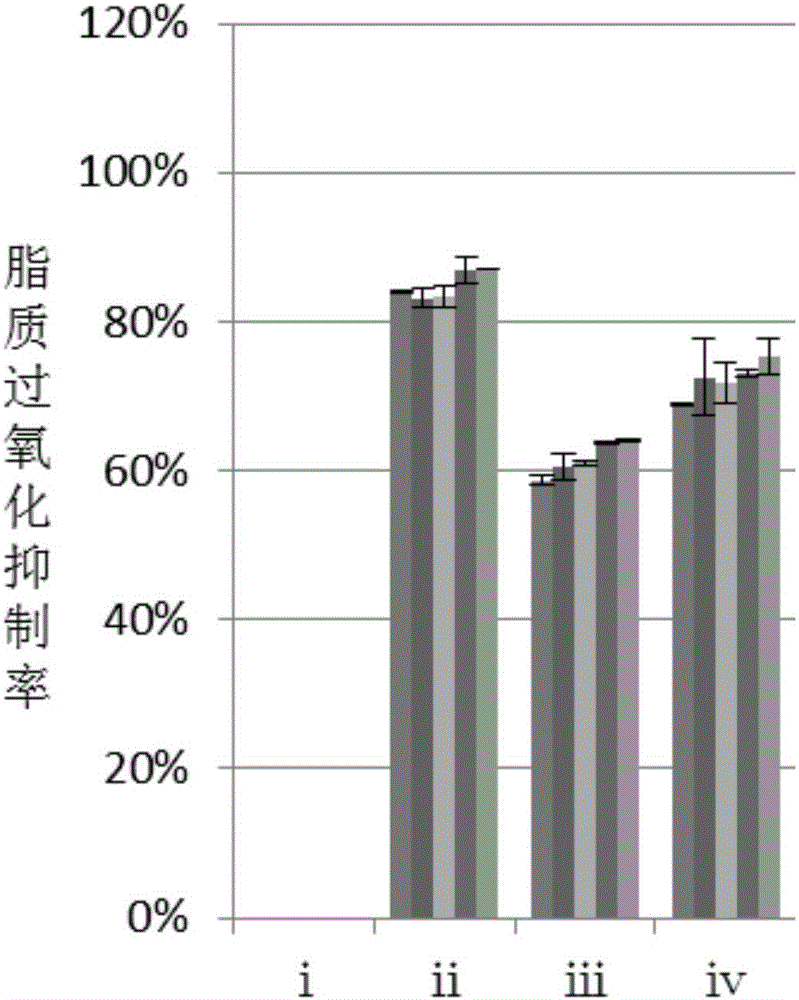
The invention discloses a production method of ergosterol. The production method includes the following steps: adding an extracting solvent to a dried Monascus purpureus-fermented sample, extracting by an extraction process to obtain crude extract, eluting the crude extract by column chromatography to obtain primary purified eluent, subjecting the primary purified elution to silica gel thin-layer chromatography, combining eluents with same silica gel thin-layer chromatography results and performing column chromatography again to obtain secondary purified eluent, removing the solvent from the secondary purified eluent, and recrystallizing through petroleum ether to obtain ergosterol. The preparation of ergosterol from Monascus purpureus as material is provided for the first time, crude extract of the sample is extracted first, and an optimal column chromatography eluent is screened then according to distribution conditions and dissolutions in different solvents, so that ergosterol is isolated and purified effectively. The production method of the ergosterol is simple, easy to implement and low in time consumption, the material is easy to obtain and low in cost, and batch production of ergosterol is achieved.
Owner:周礼红
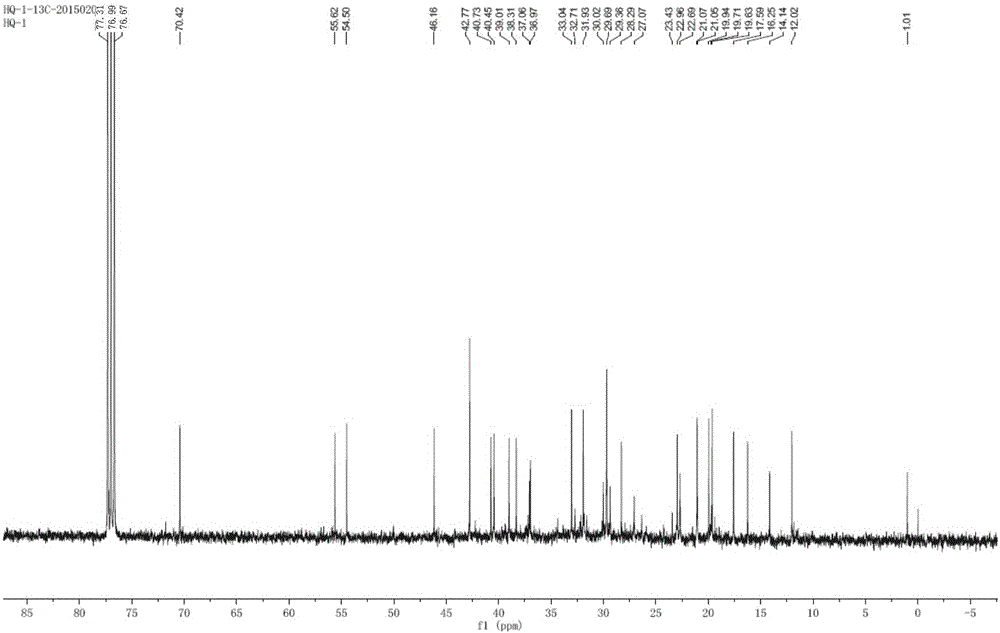
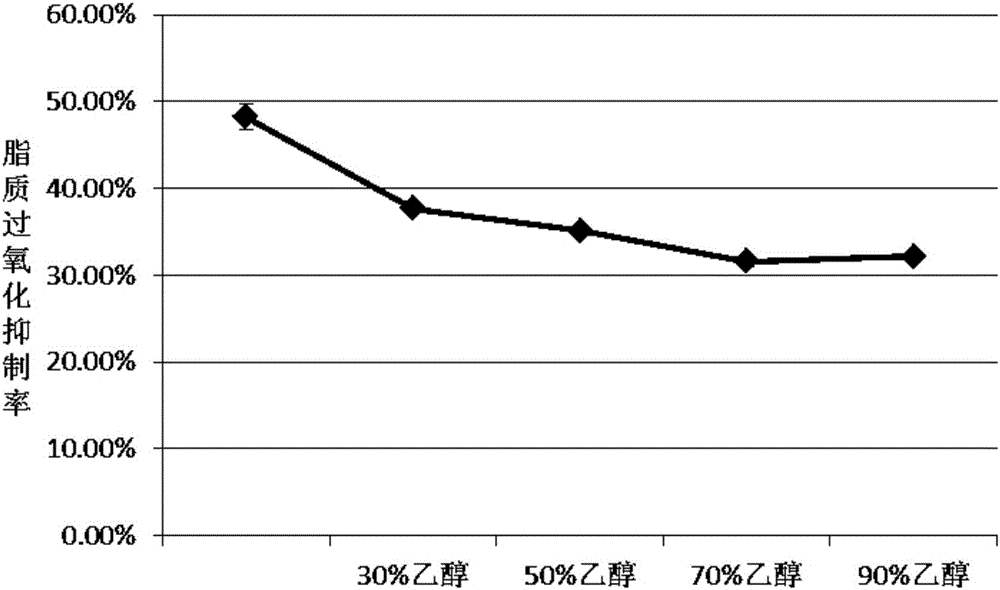
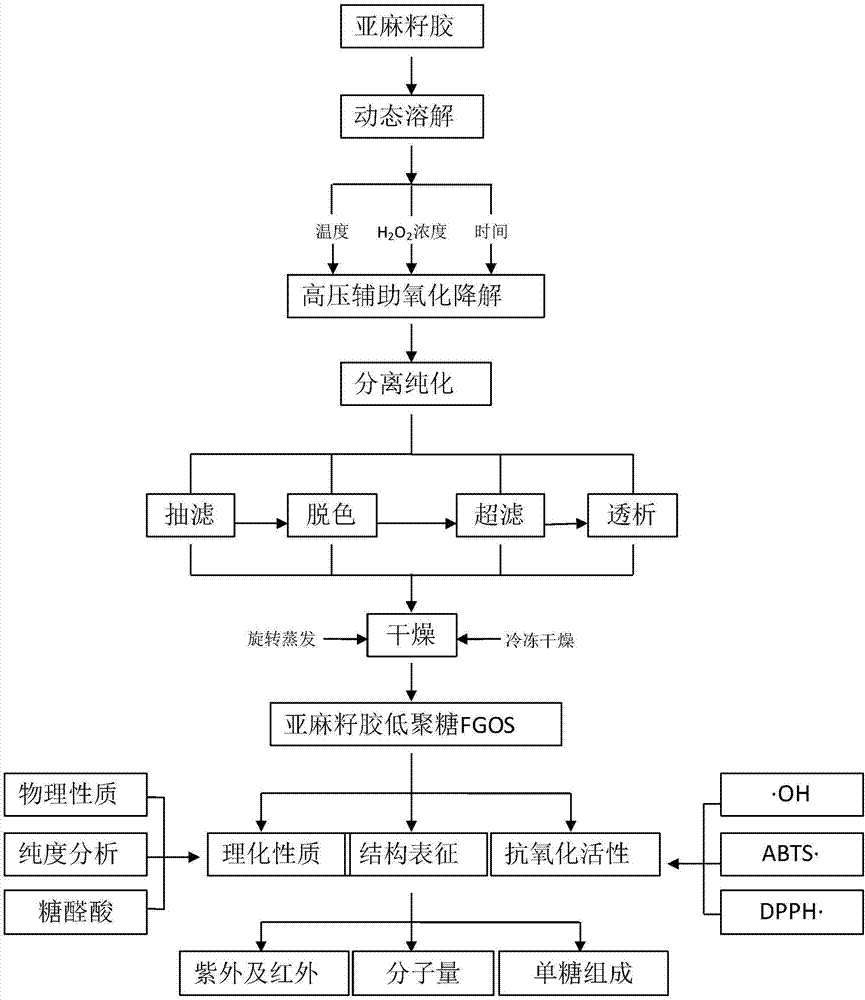
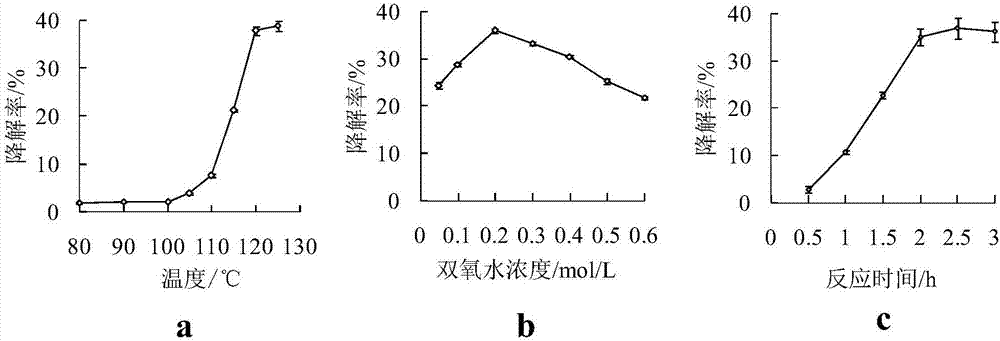
Method for preparing flaxseed gum oligosaccharides by hydrogen peroxide oxidation degradation technology
InactiveCN106977616AEfficient cuttingEffective separation and purificationFood ingredient as antioxidantSugar derivativesMonosaccharide compositionUltrafiltration
The invention discloses a method for preparing flaxseed gum oligosaccharides by a hydrogen peroxide oxidation degradation technology. The flaxseed gum oligosaccharides (FGOSs) are prepared from commercial flaxseed gum by adopting a high pressure assisted hydrogen peroxide oxidation degradation technology through dynamic dissolving, oxidative degradation, suction filtering, decolorizing, ultrafiltration, dialysis, concentration and freeze-drying. The FGOSs obtained in the invention are a semisolid viscous substance, have a light reddish brown color and special fragrance, can be easily dissolved in water, are insoluble in ethanol and other organic solvents, can easily absorb moisture, are a typical carbohydrate substance, and contain uronic acid; the FGOSs have uniform components, the average molecular weight is 1047 Da, the FGOS have a pyranose ring structure, belong to pyranose, and are composed of the following seven monosaccharides: rhamnose, fucose, arabinose, xylose, mannose, galactose and glucose, and the FGOS have certain anti-oxidation ability, and have a high removal rate on hydroxyl free radicals, DPPH free radicals, ABTS free radicals and superoxide anion free radicals.
Owner:JINAN UNIVERSITY
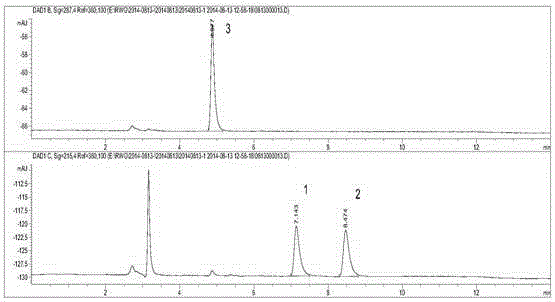
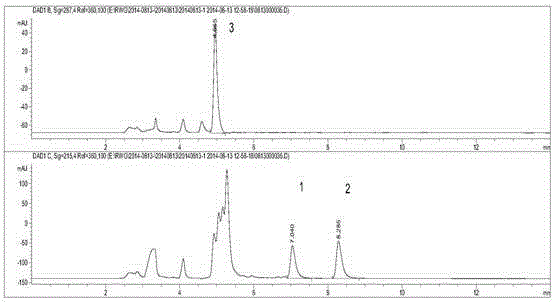
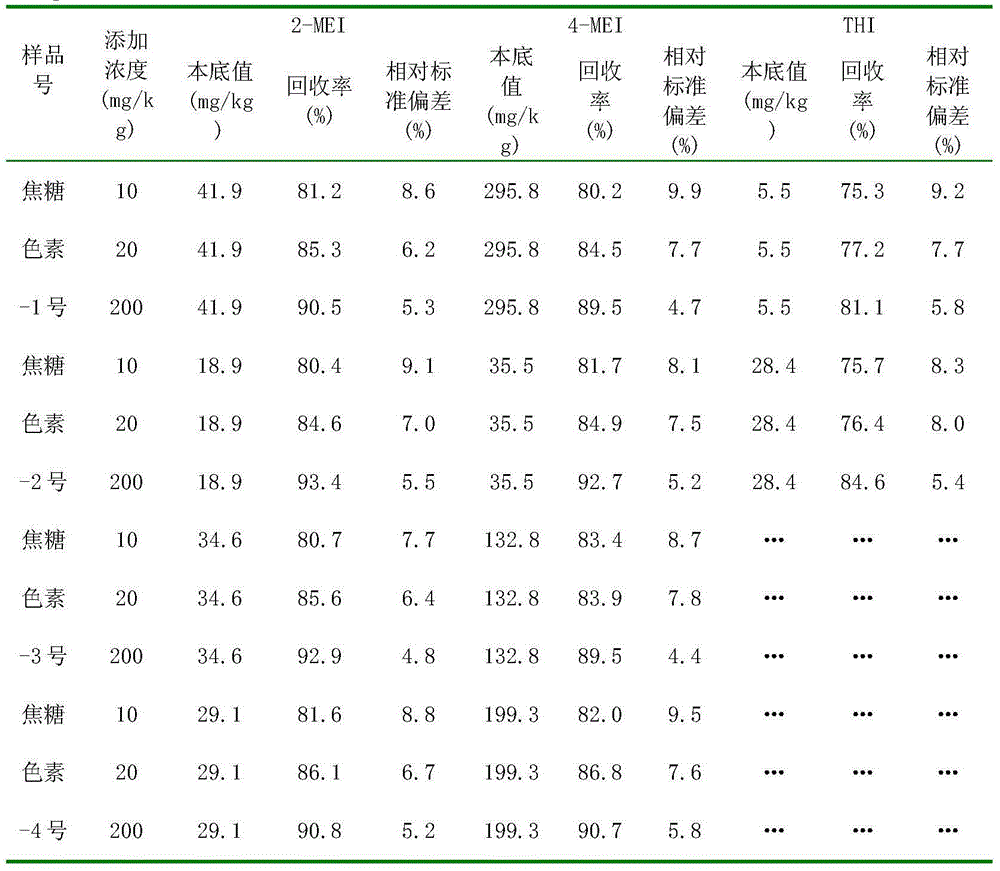
Method for detecting byproducts 4-methylimidazole and 2-acetyl-4-hydroxy-butylimidazole in caramel pigment
InactiveCN104297399AEffective separation and purificationAccurate and reliable measurement resultsComponent separationCaramel Flavor4-Methylimidazole
A method for detecting byproducts 4-methylimidazole and 2-acetyl-4-hydroxy-butylimidazole in a caramel pigment comprises the following steps: 1, preparing a solution of a sample to be detected; 2, detecting the sample; and 3, analyzing result. The method has the advantages of rapid detection, high sensitivity, high accuracy and the like.
Owner:JIANGSU PROVINCIAL CENT FOR DISEASE PREVENTION & CONTROL
Production technology for fermentatively producing proteinase K by utilizing fungi microorganisms
InactiveCN103173428ATo satisfy the market's needsGenetically stableHydrolasesMicroorganism based processesBiotechnologyFungal microorganisms
The invention relates to a production technology for fermentatively producing proteinase K by utilizing fungi microorganisms. The production technology comprises the following steps of: using tritirachium album limber (Tritirachium Album Limber) as an original strain; mutagenizing by means of 60Go Gamma rays, and also mutagenizing by a protoplast in the presence of ultraviolet rays; introducing a special culture medium; and carrying out submerged fermentation to produce the proteinase K. Under the culture conditions that the culture temperature is 22 to 28 DEG C, the pH (Potential Of Hydrogen) of fermentation liquor is maintained within the range of 5.5 to 6.0, and a fermentation tank is at the speed of stirring of 250 to 500rev / min, the output of the proteinase K is greatly increased; and the production technology is suitable for being applied to industrial production. Compared with the prior art, the production technology for fermentatively producing the proteinase K by utilizing the fungi microorganisms, provided by the invention, can be used for filling the gap in production technology of the proteinase K of domestic, greatly increasing the biological expression quantity, and decreasing the production cost at the same time, and therefore, the market competitiveness of products can be greatly improved.
Owner:上海林叶生物科技有限公司
Liquid chromatography detecting method for 2,4-methylimidazole and 2-acetyl-4-tetrahydroxyl-buthylimidazole in caramel pigment
InactiveCN104359989AEffective separation and purificationAccurate and reliable measurement resultsComponent separationPhysical chemistryCombinatorial chemistry
The invention discloses a detecting method for byproducts 2-methylimidazole, 4-methylimidazole and 2-acetyl-4- tetrahydroxyl-buthylimidazole in caramel pigment by combining solid-phase extraction with high-efficiency liquid chromatography. The method comprises the following steps: 1. preparing a to-be-detected sample solution; 2. detecting a sample; and 3. analyzing results. The method disclosed by the invention has the characteristics of quick detection, high sensitivity, high degree of accuracy, low cost, strong universality, and the like.
Owner:JIANGSU PROVINCIAL CENT FOR DISEASE PREVENTION & CONTROL
Preparation method of phenolic aldehyde-based super-hydrophilic carbon nanofiber net film
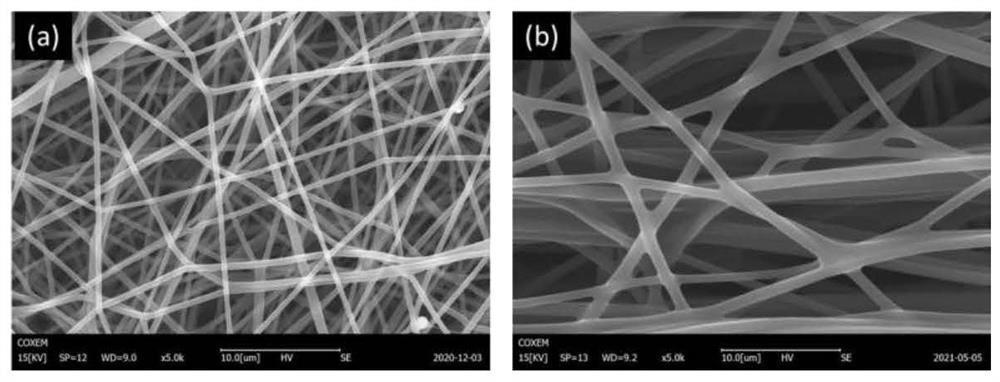
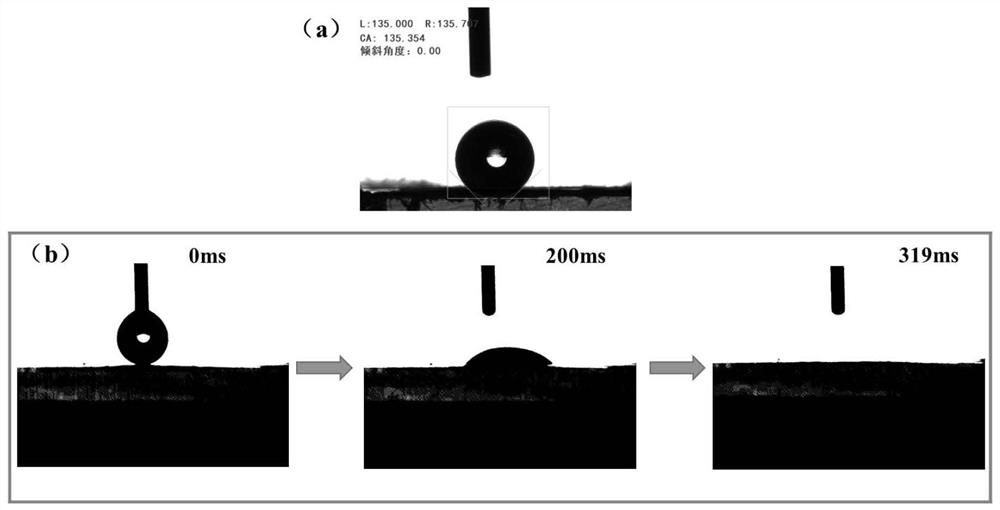
ActiveCN113600033AHas superhydrophilic propertiesSimple processSemi-permeable membranesMembranesFiberPolymer science
The invention discloses a phenolic aldehyde-based super-hydrophilic carbon nanofiber net film and a preparation method thereof, wherein the preparation method comprises the steps: firstly, adding a mixture of thermoplastic phenolic resin and thermosetting phenolic resin, a spinning polymer and hydrophilic nanoparticles or a precursor of the hydrophilic nanoparticles into a solvent, stirring and dissolving to obtain a spinning solution, then carrying out electrostatic spinning, and preparing a composite precursor nano-fiber net film; performing heat treatment on the composite precursor nano-fiber net film, and performing multi-temperature-section carbonization treatment under the protection of high-purity nitrogen to prepare a primary carbon nano-fiber net film; and finally performing surface hydrophilicity enhancement treatment on the primary carbon nano-fiber net film to obtain the super-hydrophilic carbon nano-fiber net film. According to the method disclosed by the invention, a mixture of thermoplastic phenolic resin and thermosetting phenolic resin is used as a main carbon source, the hydrophilic nanoparticles are introduced, and after surface hydrophilicity enhancement treatment, the carbon nanofiber net film with a super-hydrophilic characteristic is obtained and can be used for efficient separation and purification of an oil-in-water type oil-water mixture.
Owner:NANTONG UNIVERSITY
Bivalent egg yolk antibody against DVH (duck virus hepatitis) as well as preparation method and application of bivalent egg yolk antibody
ActiveCN106854647AImprove securityEffective separation and purificationEgg immunoglobulinsSsRNA viruses positive-senseDuck hepatitis A virusYolk
The invention provides a bivalent egg yolk antibody against DVH (duck virus hepatitis) as well as a preparation method and an application of bivalent egg yolk antibody. The bivalent egg yolk antibody contains a DHAV (duck hepatitis A virus)-1 type egg yolk antibody against DVH and a DHAV-3 type egg yolk antibody against DVH. The preparation method comprises steps as follows: (1) a DHAV-1 type strain against DVH and a DHAV-3 type strain against DVH are inoculated with an SPF chick embryo and a susceptible duck embryo respectively, an allantoic fluid is obtained, obtained virus fluids are mixed in proportion and inactivated with formalin, and a vaccine is prepared; (2) laying hens are immunized with the vaccine, sampling is performed after immunization for measuring whether the neutralizing titer of DHAV-1 type and DHAV-3 type antigens and antibodies in hyperimmune egg yolk of chickens is larger than or equal to 1:8192, and later, hyperimmune eggs of the chickens are collected; (3) eggshells of the hyperimmune eggs are disinfected, isovolumetric distilled water is added after the egg yolk is collected, and the mixture is stirred and mixed uniformly and then is subjected to pasteurization at the low temperature; purification with an acidified distilled water method and purification with a caprylic acid method are performed; microfiltration and ultrafiltration are performed. The provided bivalent egg yolk antibody is low in cost and high in titer, DVH caused by DHAV-1 and DHAV-3 can be effectively controlled, and remarkable social benefits can be obtained.
Owner:PU LIKE BIO ENG
Duck viral hepatitis bivalent yolk antibody, preparation method and application thereof
InactiveCN103865884AImprove securityEffective separation and purificationEgg immunoglobulinsSsRNA viruses positive-senseYolkOctanoic Acids
The present invention provides a duck viral hepatitis bivalent yolk antibody and a preparation method thereof, wherein the bivalent yolk antibody comprises a duck viral hepatitis DHAV-1 type antibody and a duck viral hepatitis DHAV-3 type antibody. The preparation method comprises: (1) adopting a duck viral hepatitis DHAV-1 type strain and a duck viral hepatitis DHAV-3 type strain to respectively vaccinate SPF chicken embryo and susceptible duck embryo, harvesting allantoic fluid, mixing the harvested virus liquids according to a certain ratio, carrying out formaldehyde inactivation, and preparing a vaccine; (2) adopting the vaccine to immunize laying hens, sampling after immunization to determine whether the neutralizing titer of the anti-DHAV-1 type antigen antibody and the anti-DHAV-3 type antigen antibody in the chicken hyperimmune egg yolk is more than or equal to 1:8192, and collecting the hyperimmune egg of the chicken; and (3) disinfecting the eggshell of the hyperimmune egg, collecting the egg yolk, adding the equal volume of distilled water, uniformly stirring and mixing, carrying out low temperature pasteurization inactivation, adopting an acidification distilled water method to purify, adopting an octanoic acid method to purify, and carrying out micro-filtration and ultra-filtration. The duck viral hepatitis bivalent yolk antibody has characteristics of low cost and high titer, and can be provided for effectively controlling duck viral hepatitis caused by DHAV-1 and DHAV-3 so as to obtain significant social benefits.
Owner:PU LIKE BIO ENG
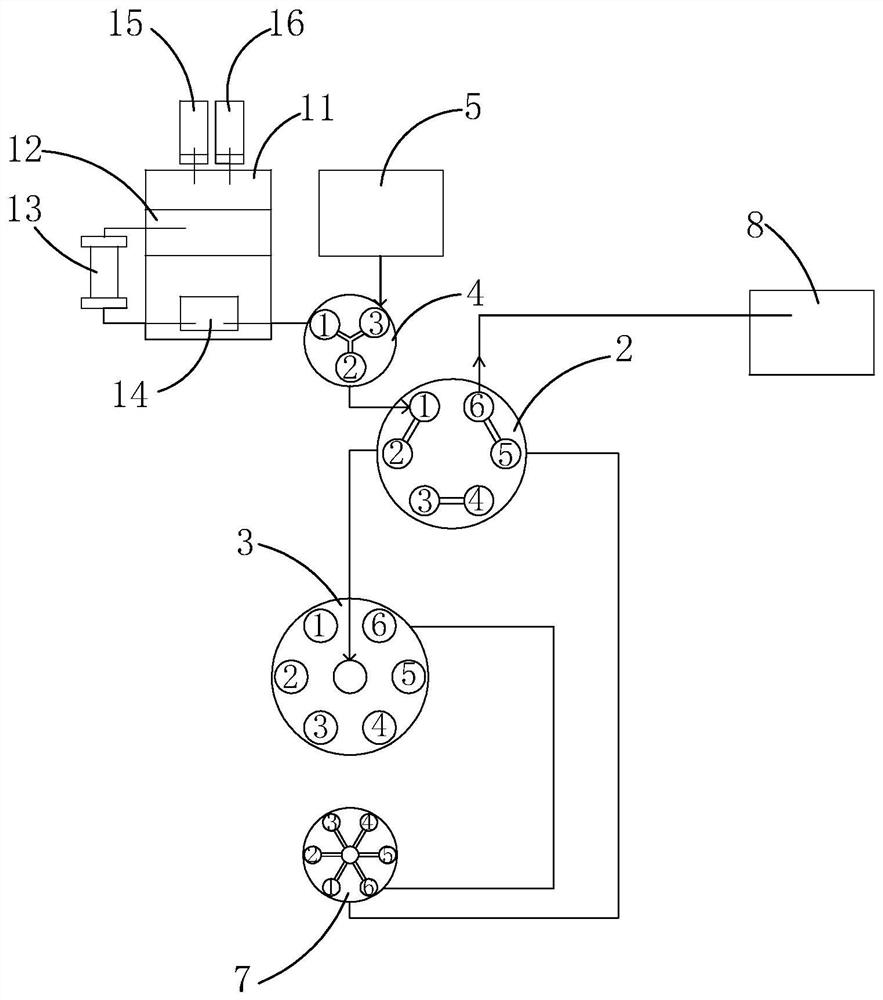
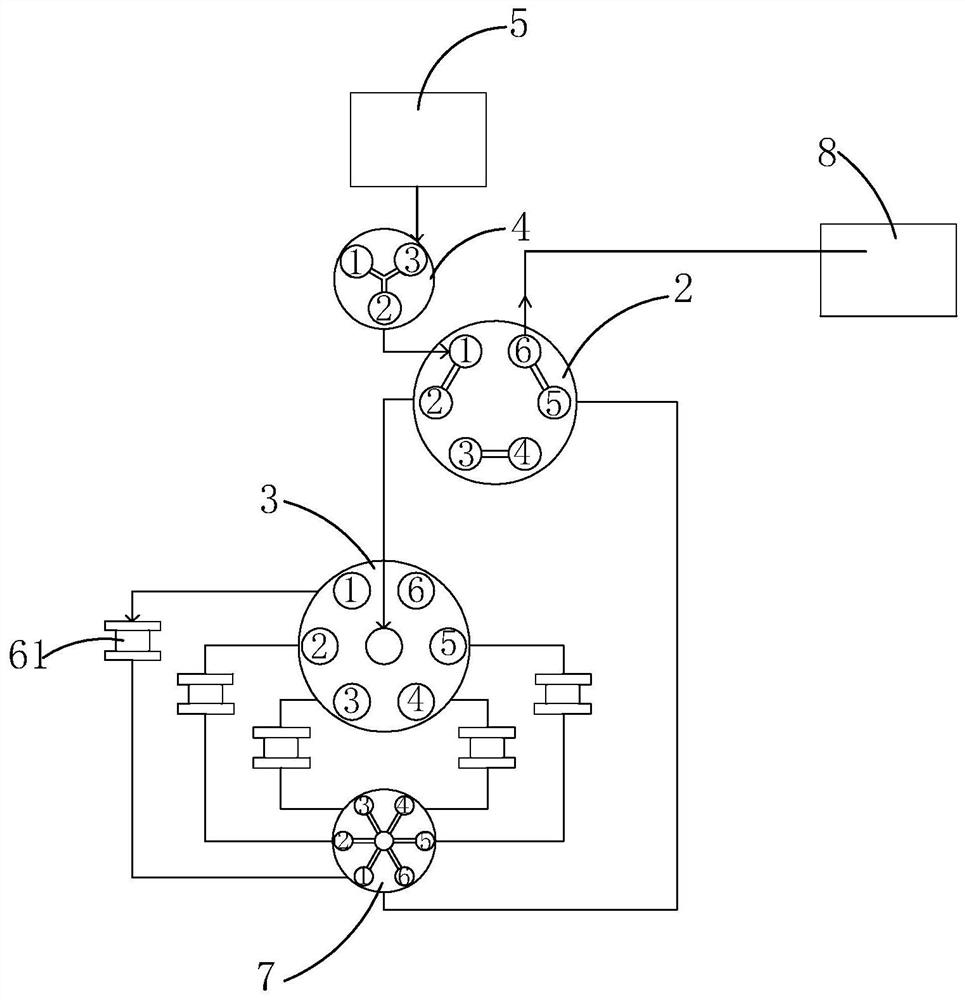
Two-dimensional high-pressure preparative liquid chromatography system and separation and purification method for low-content target components in medicine
ActiveCN113219112AEffective separation and purificationEfficient separationComponent separationFluid phaseSeparation column
The invention relates to the technical field of preparative liquid chromatography separation, and particularly discloses a two-dimensional high-pressure preparative liquid chromatography system and a separation and purification method for low-content target components in a medicine. The system comprises a first-dimensional high-pressure preparative liquid chromatography system, a two-position six-way switching valve, a multi-position selection valve, a trapping column and a second-dimensional high-pressure preparative liquid chromatography system, the second-dimensional high-pressure preparative liquid chromatography system comprises a second mixer and a two-dimensional separation column; the first-dimensional high-pressure preparative liquid chromatography system is sequentially communicated with the two-position six-way switching valve, the multi-position selection valve and the trapping column, and the outlet end of the trapping column is communicated with the two-position six-way switching valve again; and the second mixer is sequentially communicated with the two-position six-way switching valve and the two-dimensional separation column. The method comprises the following steps: detecting a sample by using a one-dimensional system, and trapping a target component to a trapping column; and switching the two-position six-way switching valve, back-washing out captured components, and carrying out two-dimensional detection. The method disclosed by the invention has the advantage of efficiently separating and purifying impurity components with extremely low content in the medicine.
Owner:PHARMARON BEIJING
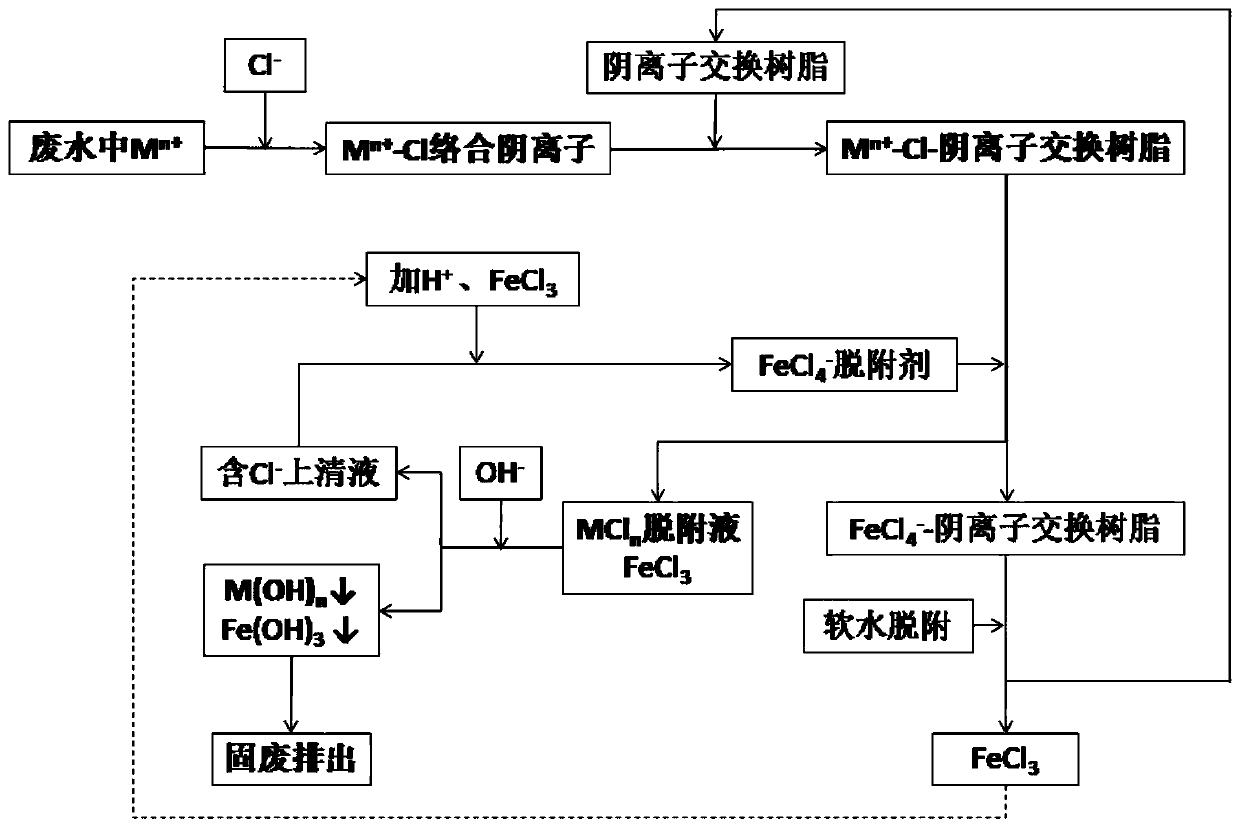
Method for removing heavy metal ions in wastewater through ion exchange resin and resin regeneration method
ActiveCN110117043AEffective separation and purificationAchieve deep removalWater contaminantsWater/sewage treatment by ion-exchangeDesorptionIon exchange
The invention discloses a method for removing heavy metal ions in wastewater through ion exchange resin and a resin regeneration method and belongs to the technical field of environmental protection.The method comprises the following steps that 1, the concentration of Cl- in wastewater with heavy metal ions Mn+ is adjusted, so that the heavy metal ions are transformed into Mn+-Cl type stable complexation negative ions; 2, negative ion exchange resin is adopted for adsorbing the Mn+-Cl type complexation negative ions in the transformed wastewater; 3, a FeCl4- solution is adopted for desorbingthe negative ion exchange resin adsorbing the Mn+-Cl complexation negative ions; 4, the pH value of a desorption solution generated in the third step is adjusted until a metal hydroxide precipitate isformed through Mn+, and through solid-liquid separation, solids are taken as dangerous solid waste for treatment; ferric chloride is added to a supernate, the pH is adjusted, and the FeCl4- solutionis prepared for application in the third step. According to the method, the aim is achieved successfully that heavy metal ions in the wastewater with interference ions are removed with low cost underthe industrial scale. Regeneration is complete, and the service life of the resin is effectively prolonged.
Owner:JIANGSU NJU ENVIRONMENTAL TECH
Separating and purifying method of Arctic marine rhodococcus B7740 produced isoprenoid
ActiveCN107021894AEffective separation and purificationUnique activityOrganic chemistryChromatographic separationMass Spectrometry-Mass Spectrometry
The invention discloses a separating and purifying method of Arctic marine rhodococcus B7740 produced isoprenoid. The method utilizes high-speed counter-current chromatography to separate and purify the Arctic marine rhodococcus B7740 produced isoprenoid. The method has the advantages that the isoprenoid produced by the Arctic marine rhodococcus B7740 is effectively separated and purified by the high-speed counter-current chromatography, so as to obtain three types of rare marine-derived carotenoids; the three types of rare marine-derived carotenoids are further identified by high-precision mass spectrometry.
Owner:HUAZHONG AGRI UNIV +1
Method for determining verbascoside in blood-nourishing and brain-refreshing water extract
ActiveCN110967415AImprove detection efficiencyReduce testing costsComponent separationSolid phase extractionPharmacology
The invention relates to a method for determining verbascoside in blood nourishing and brain-refreshing water extract. The method comprises the following steps: 1) reference substance preparation method: weighing a verbascoside reference substance, and adding methanol to prepare a standard reference solution; 2) a preparation method of a test solution: weighing the blood nourishing and brain refreshing water extract, adding methanol to dissolve, loading on a solid phase extraction column, eluting with a methanol solution, collecting eluent, and fixing the volume with methanol; and 3) a determination method: respectively taking the reference substance solution and the test solution, injecting into an ultra-high performance liquid chromatograph, recording a chromatogram, calculating the verbascoside content of the test solution by using an external standard method according to the peak area, and converting to obtain the verbascoside content in the blood nourishing and brain refreshing water extract.
Owner:TIANJIN TASLY MORDEN TCM RESOURCES
Method for industrially purifying sheep placenta polypeptides
InactiveCN103263441ACompletely brokenAvoid difficultiesPeptide/protein ingredientsMammal material medical ingredientsOrganic solventUltrafiltration
The invention provides a method for industrially purifying sheep placenta polypeptides. The method comprises the following steps: preprocessing and homogenating sheep placentas, continuously carrying out three-time refrigeration under a vacuum condition at different temperatures, filtering, refrigerating, drying, and crushing to prepare lyophilized sheep placenta powder; carrying out a supersonic wave and microwave heating compounding process of the lyophilized sheep placenta powder for dispersion to form a tissue homogenate; cooling the tissue homogenate, centrifuging, and taking the obtained supernatant; adding a mixed organic solvent into the supernatant, fully reacting, centrifuging, taking the obtained new supernatant, adding acetone to the new supernatant for extraction, and taking the obtained precipitate; and dissolving the precipitate, letting the obtained precipitate solution in an ultrafiltration membrane assembly separator and a gel column combined separator for combined purification, and carrying out concentration lyophilizing to obtain purified sheep placenta polypeptides. The method has a low production cost and is suitable for the industrialized production, and a detection result shows that the purity of the sheep placenta polypeptides prepared through the method reaches above 90%.
Owner:LANZHOU DADE AGRI & ANIMAL HUSBANDRY SCI & TECH
Preparation method and application of anti-lipid peroxide
ActiveCN105853470BEffective separation and purificationSimple ingredientsSteroidsPlant ingredientsSolubilityElution
The invention discloses a preparation method of anti-lipid peroxide. The preparation method comprises the following steps that a dry monascus fermentation sample is taken, an extraction solvent is added into the sample, extracting is carried out through a lixiviating method so that a rough extraction product can be obtained, then, the rough extraction product is eluted through column chromatography so that purified eluant can be obtained, silica gel thin layer chromatography is conducted on the purified eluant, eluant with the same silica gel thin layer chromatography results is combined, column chromatography elution is carried out again, and then the anti-lipid peroxide is obtained. Monascus serves as a raw material to prepare the anti-lipid peroxide for the first time, the rough extraction product of the monascus sample is extracted firstly, optimal column chromatography eluant is screened out according to the activity distribution situation and the solubility in different solvents, and the anti-lipid peroxide is effectively separated and purified. The preparation method is simple and easy to realize, the consumed time is short, the raw material is easy to get and low in cost, and batch production of the anti-lipid peroxide is achieved.
Owner:周礼红
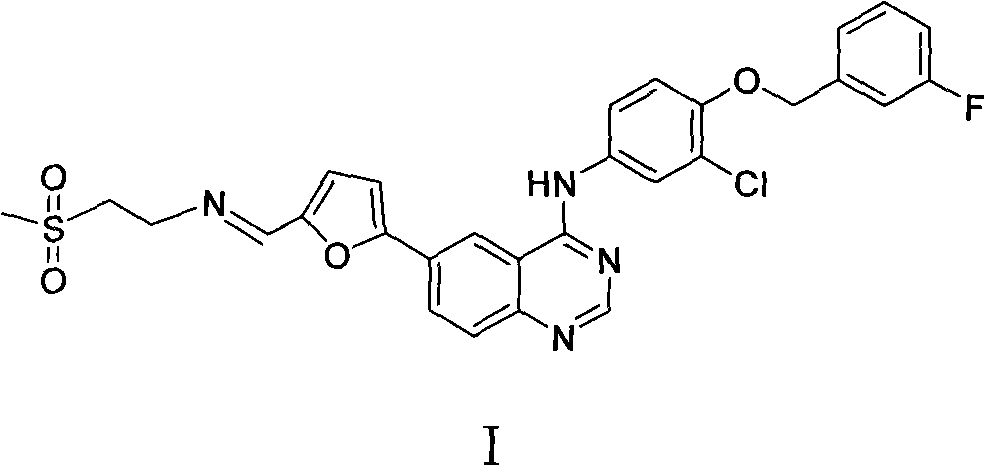
Lapatinib intermediate crystal form and preparation method thereof
ActiveCN102453025BOvercome the disadvantage of instabilityQuality improvementOrganic chemistryTyrosine-kinase inhibitorX-ray
The invention relates to a crystal form of a key intermediate compound I of a tyrosine kinase inhibitor lapatinib (a lapatinib imine crystal form for short) and a lapatinib imine p-toluenesulfonate crystal form (a crystal form of a compound II for short), and a preparation method for the crystal forms. The crystal shape of a lapatinib imine crystal is determined through powder X-ray diffraction detection; and the crystal shape of a lapatinib imine p-toluenesulfonate crystal is determined through powder X-ray diffraction detection. The two crystal forms have the advantages that: lapatinib imine can be separated from reaction liquid and purified, the crystal forms have positive significance for improving the quality of lapatinib, and the preparation process is simple and suitable for industrial production.
Owner:QILU PHARMA HAINAN
Water phase/organic phase two-phase extraction-chromatography purification method for gutta-percha
The invention provides a water phase / organic phase two-phase extraction-chromatography purification method for gutta-percha, and belongs to the technical field of gutta-percha purification. The method comprises the following steps: S1, obtaining a to-be-purified crude for gutta-percha solution; S2, performing water phase-organic phase two-phase extraction and purification; and S3, performing chromatographic column purification. According to the method, the eucommia ulmoides crude rubber is subjected to water phase / organic phase two-phase extraction and purification, and metal ions generated by hydrolysis in organic carboxylate are subjected to complexation reaction with a chelating agent in a water phase and exist in the water phase, so that the dissociation of the organic carboxylate is accelerated, and inorganic metal ions in the gutta-percha are more thoroughly removed; wherein organic lipids are hydrolyzed under the catalysis of alkali, and hydrolysate is transferred to a water phase, so that the impurities are removed, and the effective separation and purification of the gutta-percha are achieved. The primary column chromatography purification is carried out on the gutta-percha, and organic impurities such as proteins and phospholipids are effectively separated from the gutta-percha by utilizing polarity difference or an adsorption principle, so that the gutta-percha is purified.
Owner:JISHOU UNIVERSITY
Method for determining asarinin in blood-nourishing and brain-refreshing water extract
ActiveCN110967416AMask chromatographic behaviorIncrease the difficultyComponent separationSolid phase extractionPharmacology
The invention relates to a method for determining asarinin in blood-nourishing and brain-refreshing water extract. The method comprises the following steps: 1) preparation of a reference substance solution: weighing a proper amount of asarinin reference substance, and adding methanol to dissolve to obtain the asarinin-containing reference substance solution; 2) preparation of a test solution: taking the blood nourishing and brain refreshing water extract, adding 40-60% (v / v) of methanol, ultrasonically dissolving, passing through a solid phase extraction column, washing with 50-60% of methanol, discarding, eluting with a pure methanol solution, collecting eluent, and adding methanol to a scale to obtain the test solution; and 3) detection: respectively taking the reference solution and thetest solution, injecting the reference solution and the test solution into an ultra-high performance liquid chromatograph, recording a chromatogram, calculating the asarinin content of the test solution by using an external standard method according to the peak area, and converting to obtain the asarinin content in the blood nourishing and brain refreshing aqueous extract.
Owner:TIANJIN TASLY MORDEN TCM RESOURCES
Purification method and device for continuous rectification separation of ibuprofen intermediate raw material
ActiveCN114773141AEffective separation and purificationImprove purification efficiencyChemical industryDistillation purification/separationN-butylbenzenePhysical chemistry
The invention belongs to the technical field of chemical separation and purification, and particularly relates to a purification method and device for continuous rectification separation of an ibuprofen intermediate raw material. The method comprises the following steps: carrying out primary rectification on a synthetic liquid to obtain a primary material and recycled 4-methyl-1-pentene; performing second-stage rectification on the first-stage material to obtain a second-stage material and a recycled toluene crude product; and carrying out third-stage rectification on the second-stage material to obtain isobutylbenzene and a recovered n-butylbenzene crude product. Results of the embodiment show that the purification method provided by the invention is high in product recovery rate and product purity, the purity of the isobutylbenzene is as high as 99.99 wt%, the recovery rate is as high as 99.9 wt%, the impurity content is not higher than 50ppm, the problem of separation of the isobutylbenzene from the 4-methyl-1-pentene, the toluene and the n-butylbenzene is solved, the steps are simple, and the purification method has the advantages of low cost and low energy consumption.
Owner:QINGDAO UNIV OF SCI & TECH
Extraction process of camel colostrum immune globulin IgA, IgG.
Owner:甘肃省华龙农业开发有限公司
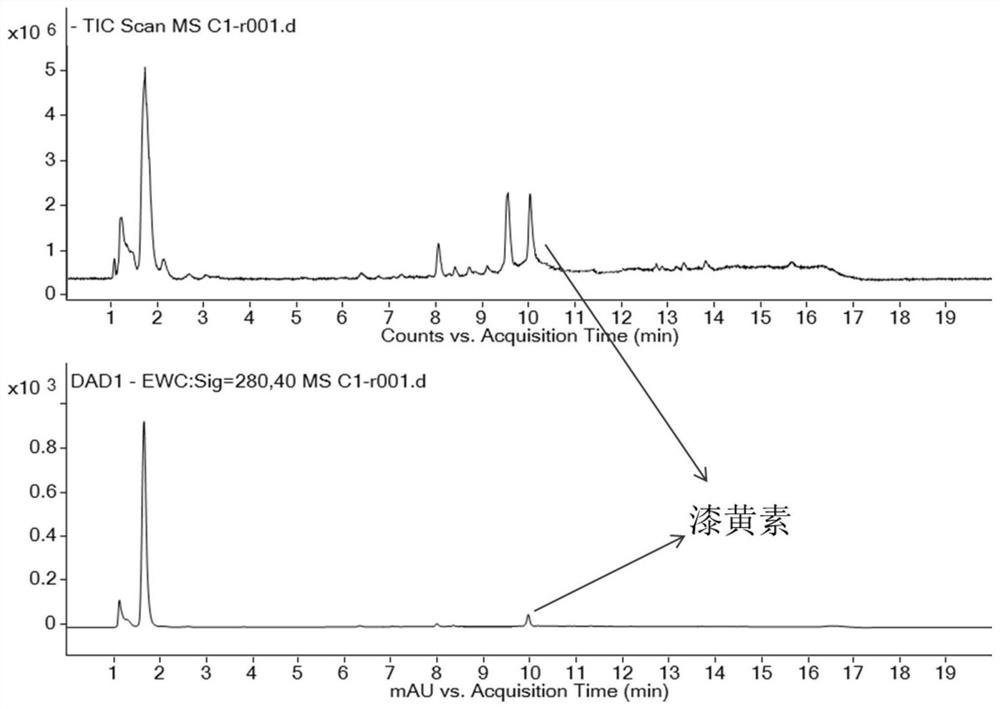
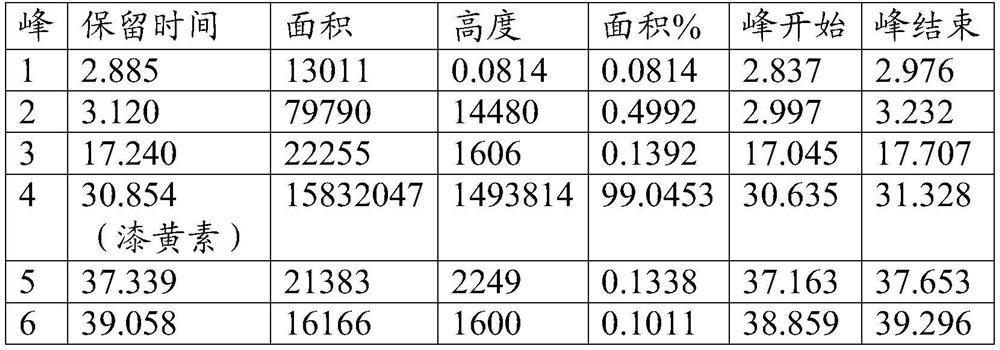
A method for extracting fisetin in emblica
ActiveCN109824643BFewer separation stepsHigh separation purityOrganic chemistryColumn chromatographyFisetin
The invention belongs to the technical field of plant extraction and discloses a method for extracting lactoflavin from phyllanthus emblica. According to the method for extracting the lactoflavin in the phyllanthus emblica, three methods of phyllanthus emblica extraction, normal phase silica gel column chromatography and a preparation liquid chromatograph are cooperatively used, so that the methodis a novel and effective separation and purification method, the anti-inflammatory active ingredient lactoflavin in the phyllanthus emblica is effectively separated and purified, separation steps arefew, the purity of the separated lactoflavin is high, and the separation purity of the lactoflavin can reach 95% or above. The lactoflavin is separated from phyllanthus emblica for the first time, and reference is provided for separation and purification of active ingredients in extracts of other natural products.
Owner:INFINITUS (CHINA) CO LTD
Detection method of by-products 4-methylimidazole and 2-acetyl-4-hydroxy-butylimidazole in caramel coloring
InactiveCN104297399BEffective separation and purificationAccurate and reliable measurement resultsComponent separationPhysical chemistryCombinatorial chemistry
A method for detecting byproducts 4-methylimidazole and 2-acetyl-4-hydroxy-butylimidazole in a caramel pigment comprises the following steps: 1, preparing a solution of a sample to be detected; 2, detecting the sample; and 3, analyzing result. The method has the advantages of rapid detection, high sensitivity, high accuracy and the like.
Owner:JIANGSU PROVINCIAL CENT FOR DISEASE PREVENTION & CONTROL
Filtration type gas separation and purification equipment with rapid moving filtering layer
InactiveCN1218768CEfficient separationStop passingDispersed particle filtrationFiltrationProduct gas
A filter-type gas separating-cleaning apparatus with quickly moving filter layer features that its filter cylinder rotating at high speed is composed of external filter net, supporting posts, internal filter net, shaft sleeve, shaft disk and supporting rings, and the gas containing solid particles and / or liquid drops is filtered by said filter cylinder. Its advantage is use of big-mesh filter material to remove fine solid particles and / or liquid drops, resulting in high filter speed, and easy cleaning.
Owner:暴辰生 +1
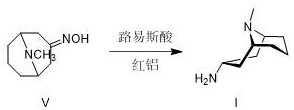
A kind of preparation method of granisetron intermediate
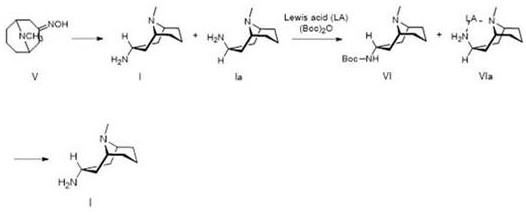
ActiveCN110804051BHigh purityEffective separation and purificationOrganic chemistryHydroxylamineMannich reaction
The invention discloses a preparation method of a granisetron intermediate. The preparation method comprises the following steps: step 1, carrying out a Mannich reaction on acetone dicarboxylic acid represented by a formula III to obtain pseudopelletierine represented by a formula IV; step 2, carrying out a reaction on the pseudopelletierine and hydroxylamine to prepare 3-pseudopelletierine oximerepresented by a formula V; and step 3, carrying out preparation by adopting one of the following schemes: (1) carrying out catalytic reduction on the 3-pseudopelletierine oxime through sodium bis(2-methoxyethoxy)aluminumhydride and Lewis acid to obtain a crude product of endo-3-amine-9-methyl-9-azabicyclo[3,3,1]nonane represented by a formula I, and directly using the crude product of endo-3-amine-9-methyl-9-azabicyclo[3,3,1]nonane to prepare granisetron, or purifying the crude product of endo-3-amine-9-methyl-9-azabicyclo[3,3,1]nonane for preparing granisetron; and (2) carrying out catalytichydrogenation reduction on the 3-pseudopelletierine oxime through Raney nickel to obtain a mixture of endo-3-amine-9-methyl-9-azabicyclo[3,3,1]nonane, purifying the mixture to obtain endo-3-amine-9-methyl-9-azabicyclo[3,3,1]nonane represented by the formula I, and using the endo-3-amine-9-methyl-9-azabicyclo[3,3,1]nonane used for preparing granisetron. The method has the advantages of mild reaction conditions, high reaction yield and low cost, and is suitable for industrial production.
Owner:杭州励德生物科技有限公司
A kind of purification method of whey protein
ActiveCN104513305BEconomical and efficient separation and purificationRapid separation and purificationAlbumin peptidesPeptide preparation methodsChromatographic separationAlpha globulin
Owner:北京济普霖生物技术有限公司 +1
Preparation method and application of anti-lipid peroxide
InactiveCN105820205AEffective separation and purificationSimple ingredientsOrganic active ingredientsDigestive systemSolubilitySolvent
The invention discloses a preparation method of anti-lipid peroxide. The preparation method comprises the following steps of: taking a dry monascus fermentation sample, adding an extraction solvent, obtaining a crude extract through extraction by adopting an extraction method, then eluting the crude extract by adopting column chromatography to obtain purified eluent, carrying out silica gel thin-layer chromatography on the purified eluent and combining the eluent with the same silica gel thin-layer chromatography result, thus obtaining anti-lipid peroxide. The preparation method has the beneficial effects that the method for preparing anti-lipid peroxide by using monascus as the raw material is provided for the first time; the crude extract of the monascus sample is firstly extracted and the best column chromatography eluent is further screened according to polar distribution and the solubility in different solvents to effectively separate and purify anti-lipid peroxide; the preparation method is simple, is easy to achieve, consumes short time, is accessible in raw materials and low in cost and achieves volume production of anti-lipid peroxide.
Owner:周礼红
A kind of active ingredient of Tibetan danshen tanshinone and its extraction method and application
InactiveCN108191947BEffective separation and purificationEasy to operateOrganic active ingredientsNervous disorderSalvia miltiorrhizaAlcohol ethyl
The invention discloses an active ingredient of Tibetan salvia tanshinone and its extraction method and application. The extraction method comprises the following steps: drying and crushing Tibetan salvia miltiorrhiza, then soaking in water, and adding 6-8 times the amount of 60-60 90% ethanol, ultrasonic extraction for 60-90min, extraction 1-2 times; Concentrate the ultrasonic extract, then extract with ethyl acetate, collect the extract, concentrate under reduced pressure to obtain a fat-soluble extract, and then extract the extract Dissolved to the corresponding concentration, separated and purified by medium-pressure preparative chromatography. The method of the invention is simple to operate, can effectively separate and purify target components, greatly improves its purity and extraction efficiency, and can fully exert its medicinal value.
Owner:CHENGDU UNIV
A simple and efficient method for two-liquid phase extraction, separation and purification and preparation of high-purity tea polyphenols
ActiveCN107954968BEasy extractionReduce extractionOrganic chemistryPlant ingredientsFluid phasePhenolic content in tea
The invention provides a simple and efficient method for two-liquid phase extraction, separation and purification, and preparation of high-purity tea polyphenols, which at least includes the steps of: preparing tea raw materials; tea raw materials, water phase, and organic phase in a mass-volume ratio of 1g:5~ 20mL: Mix 5-20mL; mix the tea raw materials with the water phase evenly, and keep the water phase and the organic phase separated; separate the organic phase after extraction, and use it as the crude extract; mix the crude extract with the water phase and the adsorbent according to the mass volume Mix 10mL: 10mL: 0.5~2g; mix the adsorbent with the water phase evenly, and keep the water phase and the organic phase separated; separate the organic phase again to obtain a refined extract. The invention selectively extracts tea polyphenols through a two-liquid phase system, and combines with a pretreatment adsorbent to reduce the extraction of impurities and improve the purity of finished products. At the same time, no toxic and harmful substances are introduced, which increases the safety of finished products. The reagent used in the invention is easy to obtain, can be recycled and re-entered into the process, has high utilization rate, and effectively reduces cost.
Owner:SOUTH CHINA NORMAL UNIVERSITY
Preparation method and application of anti-lipid peroxide
ActiveCN105853470AAchieve mass productionSimple preparation stepsAntinoxious agentsAntineoplastic agentsSolubilityElution
The invention discloses a preparation method of anti-lipid peroxide. The preparation method comprises the following steps that a dry monascus fermentation sample is taken, an extraction solvent is added into the sample, extracting is carried out through a lixiviating method so that a rough extraction product can be obtained, then, the rough extraction product is eluted through column chromatography so that purified eluant can be obtained, silica gel thin layer chromatography is conducted on the purified eluant, eluant with the same silica gel thin layer chromatography results is combined, column chromatography elution is carried out again, and then the anti-lipid peroxide is obtained. Monascus serves as a raw material to prepare the anti-lipid peroxide for the first time, the rough extraction product of the monascus sample is extracted firstly, optimal column chromatography eluant is screened out according to the activity distribution situation and the solubility in different solvents, and the anti-lipid peroxide is effectively separated and purified. The preparation method is simple and easy to realize, the consumed time is short, the raw material is easy to get and low in cost, and batch production of the anti-lipid peroxide is achieved.
Owner:周礼红
Isolation and Purification of Isoprenoids Produced by Arctic Marine Rhodococcus b7740
ActiveCN107021894BEffective separation and purificationUnique activityOrganic chemistryChromatographic separationMass Spectrometry-Mass Spectrometry
The invention discloses a separating and purifying method of Arctic marine rhodococcus B7740 produced isoprenoid. The method utilizes high-speed counter-current chromatography to separate and purify the Arctic marine rhodococcus B7740 produced isoprenoid. The method has the advantages that the isoprenoid produced by the Arctic marine rhodococcus B7740 is effectively separated and purified by the high-speed counter-current chromatography, so as to obtain three types of rare marine-derived carotenoids; the three types of rare marine-derived carotenoids are further identified by high-precision mass spectrometry.
Owner:HUAZHONG AGRI UNIV +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com