Lapatinib intermediate crystal form and preparation method thereof
A technology of lapatinib and patiniamine, applied in the chemical field, can solve problems such as ineffective progress of intermediates, obstacles to lapatinib research, cumbersome removal steps, etc., to achieve removal of impurities and suitable for industrialization The effect of stable production and reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Preparation of lapatinib amine crystal form
[0029] Add 100g of lapatinib crude product (solid) into 1L of tetrahydrofuran, heat to reflux to dissolve, filter with suction, cool the filtrate to 2-6°C for crystallization for 5 hours, filter with suction, wash the filter cake with 100ml of tetrahydrofuran to obtain a yellow solid , and dried at room temperature for 8-10 hours to obtain 83 g of lapatinib amine crystal form, with a yield of 83%.
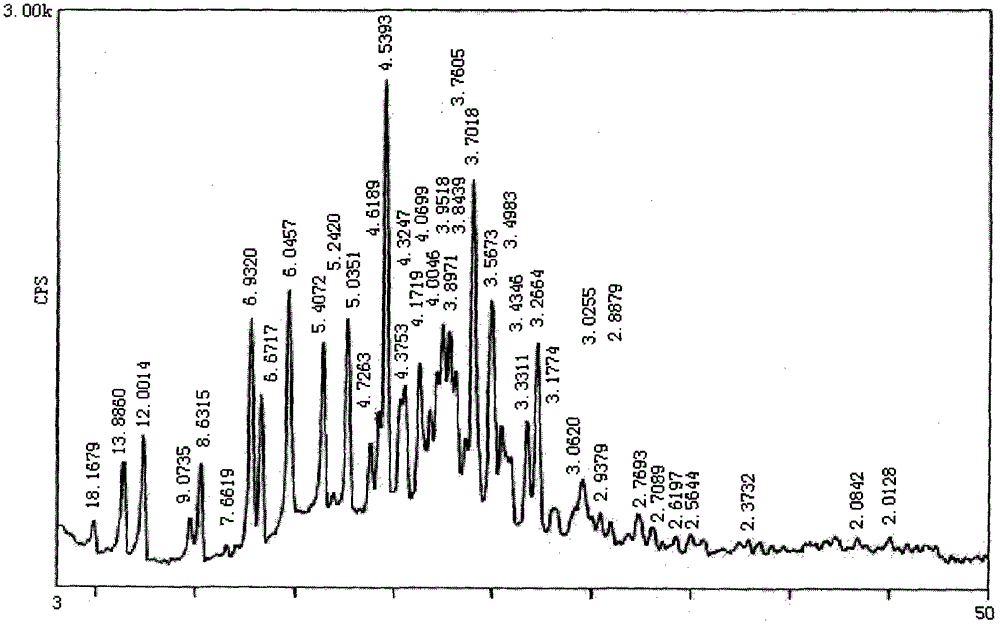
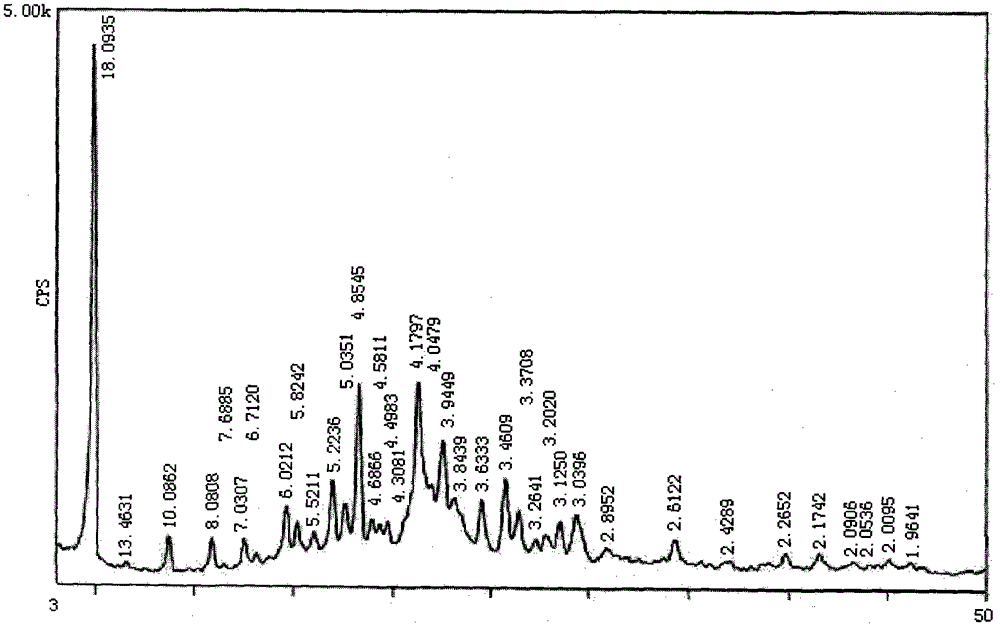
[0030] Powder X-ray diffraction determination of lapatinib amine crystal form:
[0031] Carry out powder X-ray diffraction measurement to the lapatinib amine that embodiment 1 obtains, measurement result is as table 1 and figure 1 shown.
[0032] Table 1
[0033]
[0034] Experimental study on the stability of lapatinib amine crystal form:
[0035] About 2 g of the crystal form of lapatinia amine obtained in Example 1 was put into a sample bottle, and stored at 2-6°C and 20-25°C. The purity of the original sample was take...
Embodiment 2
[0040] Preparation of lapatinib amine crystal form
[0041] Add 100g of lapatinib crude product (solid) into 1L of tetrahydrofuran, heat to reflux to dissolve, filter with suction, add 1L of ethanol to the filtrate, cool down to 2-6°C to crystallize for 5h, filter with suction, and filter the cake with 100ml of ethanol After washing, a yellow solid was obtained, which was dried at room temperature for 8-10 hours to obtain 89 g of lapatinib amine crystal form, with a yield of 89%. According to the XRD data, the obtained crystal form is the lapatinib amine crystal form described in the present invention.
Embodiment 3
[0043] Preparation of lapatinib amine crystal form
[0044] Add 50 g of lapatinib crude product (oil) into 0.6 L of ethyl acetate, heat to reflux to dissolve, filter with suction, add 1 L of ethanol to the filtrate, cool down to 2-6°C for crystallization for 5 hours, filter with suction, filter The cake was washed with 50 ml of ethyl acetate to obtain a yellow solid, which was dried at room temperature for 7-8 hours to obtain 40 g of the crystal form of lapatinib with a yield of 80%. According to the XRD data, the obtained crystal form is the lapatinib amine crystal form described in the present invention.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com